Selective Oxidation of Hydrogen Sulfide to Sulfur Using Vanadium Oxide Supported on Porous Clay Heterostructures (PCHs) Formed by Pillars Silica, Silica-Zirconia or Silica-Titania
Abstract
:1. Introduction
- (1)
- a cationic exchange of Na+ by a bulky cation, which produces an expansion of the interlayer spacing
- (2)
- the insertion of pillars by polymerization of alkoxides around the cationic micelle
- (3)
- calcination of the organic template, obtaining porous materials with higher specific surface area and more resistant than PILCs [23]. From this synthetic process, it is possible to modulate the pore volume by the use of an adequate cation to expand the interlayer spacing. In addition, the incorporation of heteroatoms such as Zr, Ti or Al in the pillars improves the thermal stability and provides interesting properties of these materials as catalyst, catalytic support [10,24,25] and adsorbent [26,27].
2. Materials and Methods
2.1. Synthesis of the PCH
2.2. Catalysts Characterization
2.3. Catalytic Tests
3. Characterization of the Catalysts
3.1. X-ray Diffraction
3.2. N2 Adsorption-Desorption at −196 °C
3.3. NH3-Thermoprogrammed Desorption
3.4. Raman Spectroscopy
3.5. Diffuse Reflectance UV-Vis
3.6. X-ray Photoelectronic Spectroscopy (XPS)
3.7. H2-Temperature Programmed Reduction
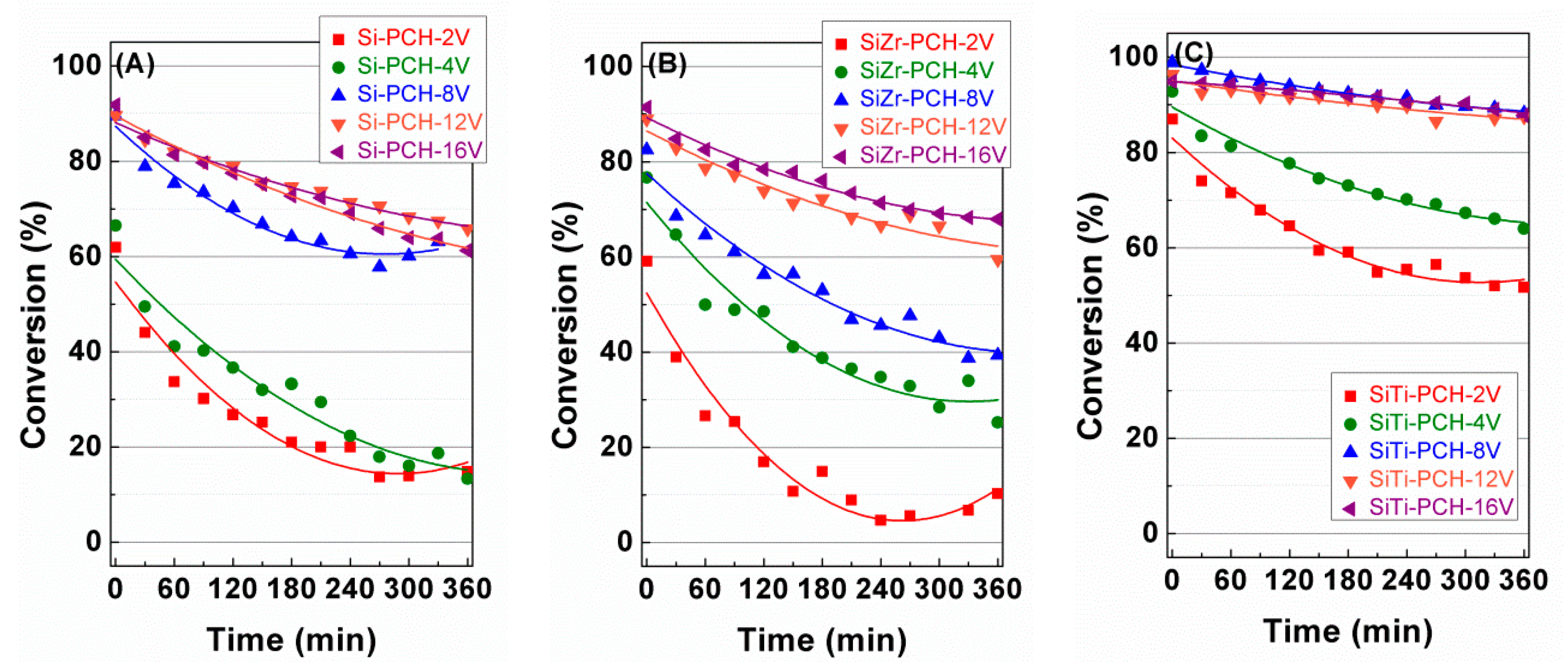
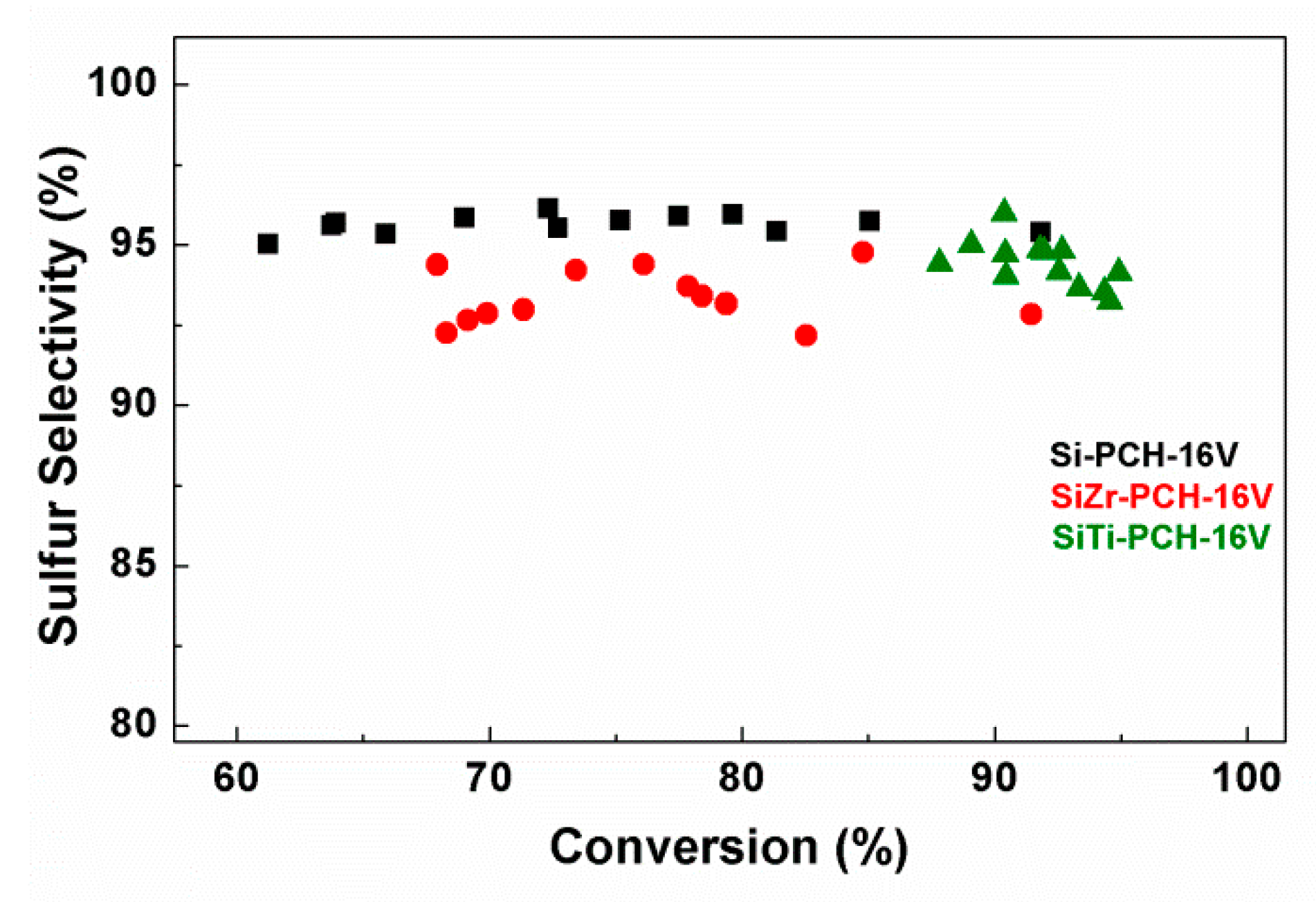
4. Catalytic Results
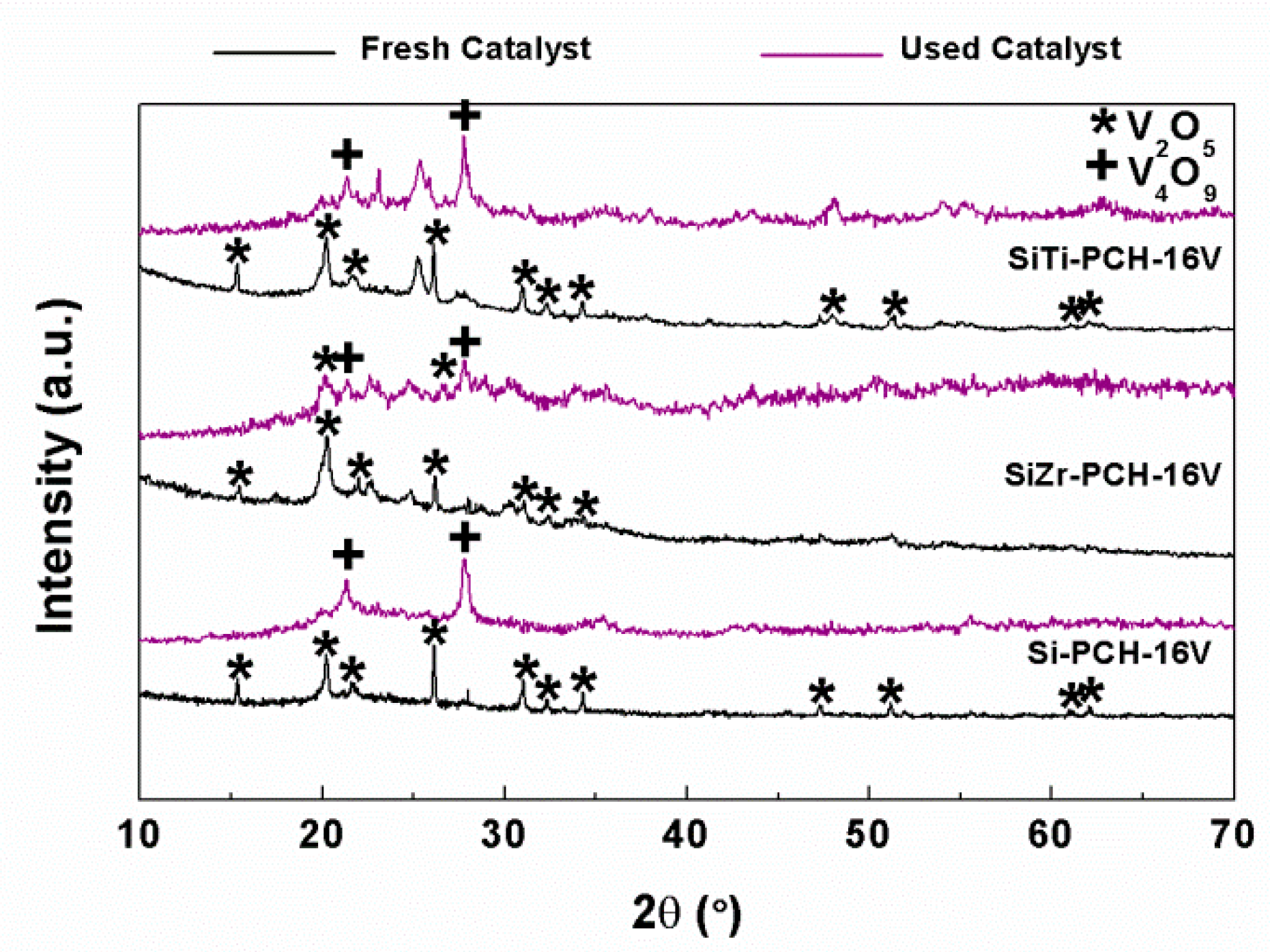
5. Evolution of the Active Phase
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hydrogen Sulfide: Human Health Aspects; Concise International Chemical Assessment Document 53; World Health Organization: Geneva, Switzerland, 2003.
- García-Arriaga, V.; Álvarez-Ramírez, J.; Amaya, M.; Sosa, E. H2S and O2 influence on the corrosion of carbon steel immersed in a solution containing 3M diethanolamine. Corros. Sci. 2010, 52, 2268–2279. [Google Scholar] [CrossRef]
- Piéplu, A.; Saur, O.; Lavalley, J.C.; Legendre, O.; Nédez, C. Claus catalysis and H2S selective oxidation. Catal. Rev. Sci. Eng. 1998, 40, 409–450. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Qu, S.; Da, J.; Hao, Z. H2S-selective catalytic oxidation: Catalysts and processes. ACS Catal. 2015, 5, 1053–1067. [Google Scholar] [CrossRef]
- Davydov, A.A.; Marshneva, V.I.; Shepotko, M.L. Metal oxides in hydrogen sulfide oxidation by oxygen and sulfur dioxide: I. The comparison study of the catalytic activity. Mechanism of the interactions between H2S and SO2 on some oxides. Appl. Catal. A Gen. 2003, 244, 93–100. [Google Scholar] [CrossRef]
- Laperdrix, E.; Costentin, G.; Nguyen, N.; Studer, F.; Lavalley, J.C. Study of H2S selective oxidation on new model catalysts: Influence of composition. Catal. Today 2000, 61, 149–155. [Google Scholar] [CrossRef]
- Reyes-Carmona, A.; Soriano, M.D.; López Nieto, J.M.; Jones, D.; Jiménez-Jiménez, J.; Jiménez-López, A.; Rodríguez-Castellón, E. Iron-containing SBA-15 as catalyst for partial oxidation of hydrogen sulfide. Catal. Today 2013, 210, 117–123. [Google Scholar] [CrossRef]
- Soriano, M.D.; Jiménez-Jiménez, J.; Concepción, P.; Jiménez-López, A.; Rodríguez-Castellón, E.; López Nieto, J.M. Selective oxidation of H2S to sulfur over vanadia supported on mesoporous zirconium phosphate heterostructure. Appl. Catal. B Environ. 2009, 92, 271–279. [Google Scholar] [CrossRef]
- Holgado, J.P.; Soriano, M.D.; Jiménez-Jiménez, J.; Concepcion, P.; Jiménez-López, A.; Caballero, A.; Rodríguez-Castellón, E.; López Nieto, J.M. Operando XAS and Raman study on the structure of a supported vanadium oxide catalyst during the oxidation of H2S to sulphur. Catal. Today 2010, 155, 296–301. [Google Scholar] [CrossRef]
- Soriano, M.D.; Cecilia, J.A.; Natoli, A.; Jiménez-Jiménez, J.; López-Nieto, J.M.; Rodríguez-Castellón, E. Vanadium oxide supported on porous clay heterostructure for the partial oxidation of hydrogen sulphide to sulfur. Catal. Today 2015, 254, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, X.; Dou, G.; Wang, Z.; Li, L.; Wang, Y.; Wang, H.; Hao, Z. Selective catalytic oxidation of H2S over iron oxide supported on alumina-intercalated laponite clay catalysts. J. Hazard. Mater. 2016, 260, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Z.; Qiao, N.; Qu, S.; Hao, Z. Selective catalytic oxidation of H2S over well-mixed oxides derived from Mg2AlxV1−x layered double hydroxides. ACS Catal. 2014, 4, 1500–1510. [Google Scholar] [CrossRef]
- Barba, D.; Palma, V.; Ciambelli, P. Screening of catalysts for H2S abatement from biogas to feed molten carbonate fuel cells. Int. J. Hydrog. Energy 2013, 38, 328–335. [Google Scholar] [CrossRef]
- Li, K.T.; Huang, C.H. Hydrogen sulfide oxidation on RE (RE = Sm, Y, La)-V-Sb catalysts: Effects of RE size and electronegativity. Catal. Today 2011, 174, 25–30. [Google Scholar] [CrossRef]
- Vaccari, A. Clays and catalysis: A promising future. Appl. Clay Sci. 1999, 14, 161–198. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Kim, S.Y.; Kim, M.I.; Selvaraj, M.; Park, D.W. Design, synthesis and characterization of vanadia-doped iron-oxide pillared montmorillonite clay for the selective catalytic oxidation of H2S. Dalton Trans. 2011, 40, 3938–3945. [Google Scholar] [CrossRef] [PubMed]
- Bineesh, K.V.; Kim, D.K.; Cho, H.J.; Park, D.W. Synthesis of metal-oxide pillared montmorillonite clay for the selective catalytic oxidation of H2S. J. Ind. Eng. Chem. 2011, 16, 593–597. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Kim, S.Y.; Jerny, B.R.; Park, D.W. Synthesis, characterization and catalytic performance of vanadia-doped delaminated zirconia-pillared montmorillonite clay for the selective catalytic oxidation of hydrogen sulfide. J. Mol. Catal. A Chem. 2009, 308, 150–159. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Kim, M.J.; Lee, G.H.; Selvaraj, M.; Park, D.W. Catalytic performance of vanadia-doped alumina-pillared clay for selective oxidation of H2S. Appl. Clay Sci. 2013, 74, 127–134. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Kim, D.Y.; Kim, M.I.; Park, D.W. Selective catalytic oxidation of H2S over V2O5 supported on TiO2-pillared clay catalysts in the presence of water and ammonia. Appl. Clay Sci. 2001, 53, 204–211. [Google Scholar] [CrossRef]
- Bineesh, K.V.; Cho, D.R.; Kim, S.Y.; Jermy, B.R.; Park, D.W. Vanadia-doped titania-pillared montmorillonite clay for the selective catalytic oxidation of H2S. Catal. Commun. 2008, 9, 2040–2043. [Google Scholar] [CrossRef]
- Galarneau, A.; Barodawalla, A.; Pinnavaia, T.J. Porous clay heterostructures formed by gallery-templated synthesis. Nature 1995, 374, 529–531. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Franco, F. Montmorillonite based porous clay heterostructures: Influence of Zr in the structure and acidic properties. Microporous Mesoporous Mater. 2013, 176, 95–102. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Arango-Díaz, A.; Franco, F.; Jiménez-Jiménez, J.; Storaro, L.; Moretti, E.; Rodríguez-Castellón, E. CuO-CeO2 supported on montmorillonite-derived porous clay heterostructures (PCH) for preferential CO oxidation in H2-rich stream. Catal. Today 2015, 253, 126–136. [Google Scholar] [CrossRef]
- Chmielarz, L.; Piwowarska, Z.; Kustrowski, P.; Wegrzyn, A.; Gil, B.; Kowalczyk, A.; Dudek, B.; Dziembaj, R.; Michalik, M. Comparison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process. Appl. Clay Sci. 2011, 53, 164–173. [Google Scholar] [CrossRef]
- Pinto, M.L.; Marques, J.; Pires, J. Porous clay heterostructures with zirconium for the separation of hydrocarbon mixtures. Sep. Purif. Technol. 2012, 98, 337–343. [Google Scholar] [CrossRef]
- Pires, J.; Bestilleiro, M.; Pinto, M.; Gil, A. Selective adsorption of carbon dioxide, methane and ethane by porous clays heterostructures. Sep. Purif. Technol. 2008, 61, 161–167. [Google Scholar] [CrossRef]
- Trillo, J.M.; Poyato, J.; Tobias, M.M.; Castro, M.A. Sorption of water vapour by M-Montmorillonite (M = Na, Li, La). Clay Miner. 1990, 25, 485–498. [Google Scholar] [CrossRef]
- Occelli, M.L. Surface properties and cracking activity of delaminated clay catalysts. Catal. Today 1988, 2, 339–355. [Google Scholar] [CrossRef]
- Saboya, R.M.A.; Cecilia, J.A.; García-Sancho, C.; Luna, F.M.T.; Rodríguez-Castellón, E.; Cavalcante, C.L. WO3-based catalysts supported on porous clay heterostructures (PCH) with Si–Zr pillars for synthetic esters production. Appl. Clay Sci. 2016, 124, 69–78. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: London, UK, 1999; Chapter 13; pp. 439–447. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Busca, G.; Centi, G.; Trifiro, F.; Lorenzelli, V. Surface acidity of vanadyl pyrophosphate, active phase in n-butane selective oxidation. J. Phys. Chem. 1986, 90, 1337–1344. [Google Scholar] [CrossRef]
- Cecilia, J.A.; García-Sancho, C.; Mérida-Robles, J.M.; Santamaría-González, J.; Moreno-Tost, R.; Maireles-Torres, P. V and V–P containing Zr-SBA-15 catalysts for dehydration of glycerol to acrolein. Catal. Today 2015, 254, 43–52. [Google Scholar] [CrossRef]
- Went, G.T.; Oyama, S.T.; Bell, A.T. Laser Raman spectroscopy of supported vanadium oxide catalysts. J. Phys. Chem. 1990, 94, 4240–4246. [Google Scholar] [CrossRef]
- Gao, X.; Wachs, I.E. Investigation of surface structures of supported vanadium oxide catalysts by UV−vis−NIR diffuse reflectance spectroscopy. J. Phys. Chem. B 2000, 104, 1261–1268. [Google Scholar] [CrossRef]
- Larina, T.V.; Cherepanova, S.V.; Rudina, N.A.; Kolesov, B.A.; Zagoruiko, A.N. Characterization of vanadia catalysts on structured micro-fibrous glass supports for selective oxidation of hydrogen sulfide. Catal. Sustain. Energy 2015, 2, 87–95. [Google Scholar] [CrossRef]
- Nilsson, R.; Lindbland, T.; Andersson, A. Ammoxidation of propane over antimony vanadium-oxide catalysts. J. Catal. 1994, 148, 501–513. [Google Scholar] [CrossRef]
- Shin, M.Y.; Nam, C.M.; Park, D.W.; Chung, J.S. Selective oxidation of H2S to elemental sulfur over VOx/SiO2 and V2O5 catalysts. Appl. Catal. A Gen. 2001, 211, 213–225. [Google Scholar] [CrossRef]
- Quijada, C.; Huerta, F.J.; Morallón, E.; Vázquez, J.L.; Berlouis, L.E.A. Electrochemical behaviour of aqueous SO2 at polycrystalline gold electrodes in acidic media: A voltammetric and in situ vibrational study: Part 1. Reduction of SO2: Deposition of monomeric and polymeric sulphur. Electrochim. Acta 2000, 45, 1847–1862. [Google Scholar] [CrossRef]
- Evans, J.C. The vibrational spectra and structure of the vanadyl ion in aqueous solution. Inorg. Chem. 1963, 2, 372–375. [Google Scholar] [CrossRef]







| Sample | SBET (m2 g−1) | t-Plotmicrop. (m2 g−1) | VP (cm3 g−1) | VPmicrop. (cm3 g−1) | Dp (nm) | µmol NH3 g−1 | µmol NH3 m−2 |
|---|---|---|---|---|---|---|---|
| Montmorillonite | 49 | 18 | 0.109 | 0.009 | 12.4 | 125 | 2.55 |
| Si-PCH | 643 | 460 | 0.773 | 0.280 | 5.5 | 308 | 0.48 |
| Si-PCH-2V | 555 | 367 | 0.773 | 0.219 | 5.6 | 517 | 0.93 |
| Si-PCH-4V | 483 | 316 | 0.751 | 0.191 | 7.0 | 671 | 1.39 |
| Si-PCH-8V | 374 | 224 | 0.612 | 0.136 | 7.6 | 711 | 1.90 |
| Si-PCH-12V | 241 | 83 | 0.549 | 0.047 | 10.5 | 587 | 2.44 |
| Si-PCH-16V | 147 | 43 | 0.400 | 0.021 | 12.8 | 417 | 2.84 |
| PCH-SiZr | 608 | 382 | 0.829 | 0.212 | 6.8 | 460 | 0.76 |
| SiZr-PCH-2V | 477 | 290 | 0.813 | 0.157 | 8.8 | 614 | 1.34 |
| SiZr-PCH-4V | 420 | 253 | 0.675 | 0.138 | 8.3 | 874 | 2.08 |
| SiZr-PCH-8V | 329 | 179 | 0.571 | 0.097 | 8.9 | 810 | 2.46 |
| SiZr-PCH-12V | 216 | 86 | 0.543 | 0.047 | 11.7 | 657 | 3.04 |
| SiZr-PCH-16V | 161 | 63 | 0.442 | 0.034 | 12.4 | 547 | 3.39 |
| SiTi-PCH | 562 | 287 | 0.796 | 0.164 | 6.8 | 395 | 0.70 |
| SiTi-PCH-2V | 472 | 230 | 0.836 | 0.130 | 8.8 | 559 | 1.18 |
| SiTi-PCH-4V | 405 | 170 | 0.750 | 0.097 | 8.5 | 599 | 1.48 |
| SiTi-PCH-8V | 262 | 54 | 0.607 | 0.033 | 9.2 | 799 | 3.04 |
| SiTi-PCH-12V | 158 | 17 | 0.544 | 0.008 | 15.1 | 677 | 4.28 |
| SiTi-PCH-16V | 109 | 11 | 0.440 | 0.004 | 17.5 | 603 | 5.53 |
| Sample | Atomic Concentrations | ||||||
|---|---|---|---|---|---|---|---|
| O 1s | Si 2p | Al 2p | Zr 3d | Ti 2p | V 2p | S 2p | |
| Si-PCH-2V | 66.30 | 30.85 | 2.19 | - | - | 0.67 | - |
| Si-PCH-4V | 66.39 | 30.75 | 1.95 | - | - | 0.90 | - |
| Si-PCH-8V | 67.14 | 30.04 | 1.68 | - | - | 1.15 | - |
| Si-PCH-12V | 65.78 | 31.11 | 1.35 | - | - | 1.76 | - |
| Si-PCH-16V | 65.53 | 30.96 | 1.70 | - | - | 1.81 | - |
| Si-PCH-16V-u | 64.93 | 28.89 | 2.26 | - | - | 3.25 | 0.67 |
| SiZr-PCH-2V | 67.03 | 28.18 | 2.51 | 1.81 | - | 0.40 | - |
| SiZr-PCH-4V | 66.86 | 27.47 | 2.73 | 1.90 | - | 1.04 | - |
| SiZr-PCH-8V | 66.35 | 26.86 | 3.13 | 1.83 | - | 1.89 | - |
| SiZr-PCH-12V | 66.55 | 26.49 | 3.16 | 1.54 | - | 2.26 | - |
| SiZr-PCH-16V | 65.71 | 27.45 | 2.93 | 1.23 | - | 2.76 | - |
| SiZr-PCH-16V-u | 66.59 | 25.14 | 2.67 | 1.26 | - | 2.83 | 1.51 |
| SiTi-PCH-2V | 66.59 | 28.88 | 3.10 | - | 0.98 | 0.53 | |
| SiTi-PCH-4V | 67.67 | 28.44 | 3.12 | - | 0.81 | 0.56 | |
| SiTi-PCH-8V | 67.31 | 27.49 | 3.09 | - | 0.71 | 1.40 | |
| SiTi-PCH-12V | 66.30 | 27.76 | 3.07 | - | 0.48 | 2.38 | |
| SiTi-PCH-16V | 65.97 | 27.94 | 2.63 | - | 0.45 | 3.02 | |
| SiTi-PCH-16V-u | 65.35 | 26.61 | 2.69 | - | 0.51 | 3.12 | 1.72 |
| Sample | Superficial Atomic Ratio | Binding Energy (eV) | ||||
|---|---|---|---|---|---|---|
| V/(Si + Al + Zr + Ti) | V/S | V5+ | V4+ | SO42− | S0 | |
| Si-PCH-2V | 0.020 | - | 517.5 (78.3%) | 516.3 (21.7%) | - | - |
| Si-PCH-4V | 0.028 | - | 517.7 (80.5%) | 516.5 (19.5%) | - | - |
| Si-PCH-8V | 0.036 | - | 517.6 (82.4%) | 516.1 (17.6%) | - | - |
| Si-PCH-12V | 0.054 | - | 517.1 (86.2%) | 516.0 (13.8%) | - | - |
| Si-PCH-16V | 0.055 | - | 517.1 (87.2%) | 516.1 (12.8%) | - | - |
| Si-PCH-16V-u | 0.104 | 4.850 | 517.4 (60.4%) | 516.3 (39.6%) | 168.7 (46.5%) | 163.9 (54.5%) |
| SiZr-PCH-2V | 0.012 | - | 517.5 (84.8%) | 516.2 (15.2%) | - | - |
| SiZr-PCH-4V | 0.032 | - | 517.3 (83.1%) | 516.1 (16.9%) | - | - |
| SiZr-PCH-8V | 0.059 | - | 517.7 (86.6%) | 516.3 (13.4%) | - | - |
| SiZr-PCH-12V | 0.072 | - | 517.6 (91.9%) | 516.1 (8.1%) | - | - |
| SiZr-PCH-16V | 0.087 | - | 517.6 (92.0%) | 516.3 (8.0%) | - | - |
| SiZr-PCH-16V-u | 0.097 | 1.874 | 517.7 (62.8%) | 516.5 (37.2%) | 168.4 (27.4%) | 163.6 (72.6%) |
| SiTi-PCH-2V | 0.016 | - | 517.3 (82.6%) | 516.0 (17.4%) | - | - |
| SiTi-PCH-4V | 0.017 | - | 517.4 (88.4%) | 516.1 (11.6%) | - | - |
| SiTi-PCH-8V | 0.044 | - | 517.5 (88.0%) | 516.3 (12.0%) | - | - |
| SiTi-PCH-12V | 0.076 | - | 517.6 (88.8%) | 516.3 (11.2%) | - | - |
| SiTi-PCH-16V | 0.097 | - | 517.5 (88.8%) | 516.2 (11.2%) | - | - |
| SiTi-PCH-16V-u | 0.105 | 1.814 | 517.7 (62.8%) | 516.6 (37.2%) | 168.6 (29.0%) | 163.7 (71.0%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cecilia, J.A.; Soriano, M.D.; Natoli, A.; Rodríguez-Castellón, E.; López Nieto, J.M. Selective Oxidation of Hydrogen Sulfide to Sulfur Using Vanadium Oxide Supported on Porous Clay Heterostructures (PCHs) Formed by Pillars Silica, Silica-Zirconia or Silica-Titania. Materials 2018, 11, 1562. https://doi.org/10.3390/ma11091562
Cecilia JA, Soriano MD, Natoli A, Rodríguez-Castellón E, López Nieto JM. Selective Oxidation of Hydrogen Sulfide to Sulfur Using Vanadium Oxide Supported on Porous Clay Heterostructures (PCHs) Formed by Pillars Silica, Silica-Zirconia or Silica-Titania. Materials. 2018; 11(9):1562. https://doi.org/10.3390/ma11091562
Chicago/Turabian StyleCecilia, Juan Antonio, M. Dolores Soriano, Alejandro Natoli, Enrique Rodríguez-Castellón, and José Manuel López Nieto. 2018. "Selective Oxidation of Hydrogen Sulfide to Sulfur Using Vanadium Oxide Supported on Porous Clay Heterostructures (PCHs) Formed by Pillars Silica, Silica-Zirconia or Silica-Titania" Materials 11, no. 9: 1562. https://doi.org/10.3390/ma11091562
APA StyleCecilia, J. A., Soriano, M. D., Natoli, A., Rodríguez-Castellón, E., & López Nieto, J. M. (2018). Selective Oxidation of Hydrogen Sulfide to Sulfur Using Vanadium Oxide Supported on Porous Clay Heterostructures (PCHs) Formed by Pillars Silica, Silica-Zirconia or Silica-Titania. Materials, 11(9), 1562. https://doi.org/10.3390/ma11091562







