Unusually Small Thermal Expansion of Ordered Perovskite Oxide CaCu3Ru4O12 with High Conductivity
Abstract
:1. Introduction
2. Experimental
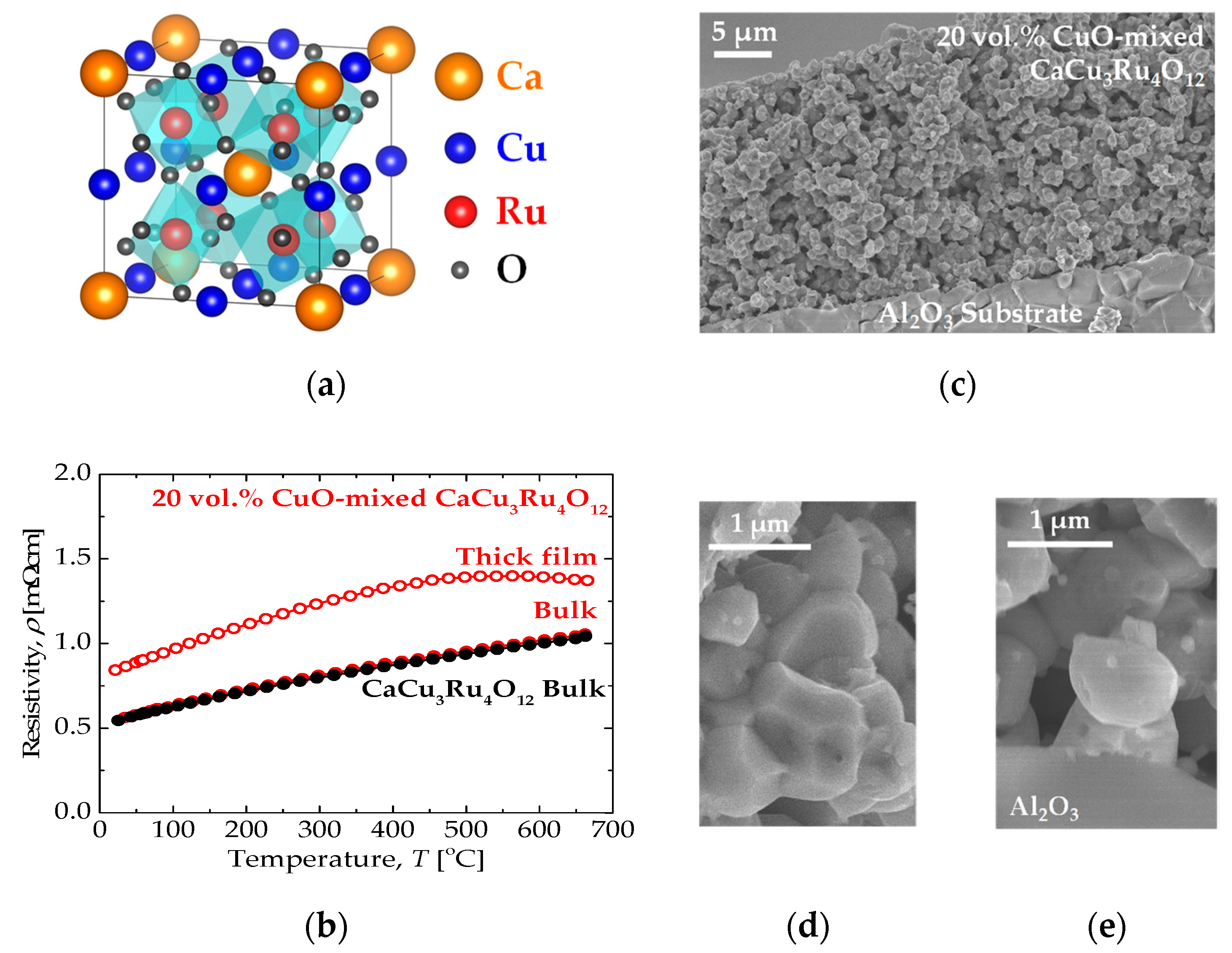
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gongora-Rubio, M.R.; Espinoza-Vallejos, P.; Sola-Laguna, L; Santiago-Avilés, J.J. Overview of low temperature co-fired ceramics tape technology for meso-system technology (MsST). Sens. Actuators A 2001, 89, 222–241. [Google Scholar] [CrossRef]
- Miyazaki, H.; Iwakiri, S.; Hirao, K.; Fukuda, S.; Izu, N.; Yoshizawa, Y.; Hyuga, H. Effect of high temperature cycling on both crack formation in ceramics and delamination of copper layers in silicon nitride active metal brazing substrates. Ceram. Int. 2017, 43, 5080–5088. [Google Scholar] [CrossRef]
- Jean, J.H.; Chang, C.R.; Chen, Z.C. Effect of Densification Mismatch on Camber Development during Cofiring of Nickel-Based Multilayer Ceramic Capacitors. J. Am. Ceram. Soc. 1997, 80, 2401–2406. [Google Scholar] [CrossRef]
- Niimi, H.; Mihara, K.; Sakabe, Y.; Kuwabara, M. Preparation of Multilayer Semiconducting BaTiO3 Ceramics Co-Fired with Ni Inner Electrodes. Jpn. J. Appl. Phys. 2007, 46, 6715–6718. [Google Scholar] [CrossRef]
- Park, J.H.; Akedo, J.; Sato, H. High-speed metal-based optical microscanners using stainless-steel substrate and piezoelectric thick films prepared by aerosol deposition method. Sens. Actuators A 2007, 135, 86–91. [Google Scholar] [CrossRef]
- Akedo, J.; Lebedev, M. Piezoelectric properties and poling effect of Pb(Zr, Ti)O3 thick films prepared for microactuators by aerosol deposition. Appl. Phys. Lett. 2000, 77, 1710–1712. [Google Scholar] [CrossRef]
- Iijima, Y.; Tanabe, N.; Kohno, O.; Ikeno, Y. In-plane aligned YBa2Cu3O7−x thin films deposited on polycrystalline metallic substrates. Appl. Phys. Lett. 1992, 60, 769–771. [Google Scholar] [CrossRef]
- Kakimoto, K.; Iijima, Y.; Saitoh, T. Fabrication of long-Y123 coated conductors by combination of IBAD and PLD. Physica C 2003, 392–396, 783–789. [Google Scholar] [CrossRef]
- Doi, T.; Hashimoto, M.; Horii, S.; Ichinose, A. Fabrication of YBa2Cu3O7 Superconducting Film on {100}<001> Textured Cu Tape via Conductive Buffer Layers. Mater. Trans. 2017, 58, 1493–1499. [Google Scholar] [CrossRef]
- Ichinose, A.; Horii, S.; Doi, T. Possibility of the material cost reduction forward developing low-cost 2nd generation superconducting wires. Jpn. J. Appl. Phys. 2017, 56, 103101. [Google Scholar] [CrossRef]
- Tucker, M.C.; Lau, G.Y.; Jacobson, C.P.; DeJonghe, L.C.; Visco, S.J. Performance of metal-supported SOFCs with infiltrated electrodes. J. Power Sources 2007, 171, 477–482. [Google Scholar] [CrossRef]
- Tucker, M.C. Progress in metal-supported solid oxide fuel cells: A review. J. Power Sources 2010, 195, 4570–4582. [Google Scholar] [CrossRef]
- Nagaya, S.; Watanabe, T.; Tamada, T.; Naruse, M.; Kashima, N.; Katagiri, T.; Hirano, N.; Awaji, S.; Oguro, H.; Ishiyama, A. Development of High Strength Pancake Coil With Stress Controlling Structure by REBCO Coated Conductor. IEEE Trans. Appl. Supercond. 2013, 23, 4601204. [Google Scholar] [CrossRef]
- Gérard, B. Application of thermal spraying in the automobile industry. Surf. Coat. Technol. 2006, 201, 2028–2031. [Google Scholar] [CrossRef]
- Song, R.G. Hydrogen permeation resistance of plasma-sprayed Al2O3 and Al2O3–13wt.% TiO2 ceramic coatings on austenitic. Surf. Coat. Technol. 2003, 168, 191–194. [Google Scholar] [CrossRef]
- Itoh, T.; Miwa, T.; Tsuruta, A.; Akamatsu, T.; Izu, N.; Shin, W.; Park, J.; Hida, T.; Eda, T.; Setoguchi, Y. Development of an Exhaled Breath Monitoring System with Semiconductive Gas Sensors, a Gas Condenser Unit, and Gas Chromatograph Columns. Sensors 2016, 16, 1891. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Nakashima, T.; Akamatsu, T.; Izu, N.; Shin, W. Nonanal gas sensing properties of platinum, palladium, and gold-loaded tin oxide VOCs sensors. Sens. Actuators B 2013, 187, 135–141. [Google Scholar] [CrossRef]
- Takenaka, K. Negative thermal expansion materials: Technological key for control of thermal expansion. Sci. Technol. Adv. Mater. 2012, 13, 013001. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, H.; Trofimenko, N.; Tietz, F.; Stöver, D.; Ahmad-Khanlou, A. Correlation between thermal expansion and oxide ion transport in mixed conducting perovskite-type oxides for SOFC cathodes. Solid State Ion. 2000, 138, 79–90. [Google Scholar] [CrossRef]
- Labeau, M.; Bochu, B.; Joubert, J.C.; Chenavas, J. Synthèse et caractérisation cristallographique et physique d’une série de composés ACu3Ru4O12 de type perovskite. J. Solid State Chem. 1980, 33, 257–261. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Sleight, A.W. ACu3Ti4O12 and ACu3Ru4O12 perovskites: High dielectric constants and valence degeneracy. Solid State Sci. 2002, 4, 347–351. [Google Scholar] [CrossRef]
- Kobayashi, W.; Terasaki, I.; Takeya, J.; Tsukada, I.; Ando, Y. A Novel Heavy-Fermion State in CaCu3Ru4O12. J. Phys. Soc. Jpn. 2004, 73, 2373–2376. [Google Scholar] [CrossRef]
- Tran, T.T.; Takubo, K.; Mizokawa, T.; Kobayashi, W.; Terasaki, I. Electronic structure of CaCu3Ru4O12 studied by X-ray photoemission spectroscopy. Phys. Rev. B 2006, 73, 193105. [Google Scholar] [CrossRef]
- Juan, W.R.; Yuan, Z.Y.; Li, W.; Yong, L.; Jing, S.; Rui, X.; Feng, W.J. Growth and characterization of CaCu3Ru4O12 single crystal. Chin. Phys. B 2015, 24, 097501. [Google Scholar] [CrossRef]
- Sumi, H.; Yamaguchi, T.; Hamamoto, K.; Suzuki, T.; Fujishiro, Y.; Matsui, T.; Eguchi, K. AC impedance characteristics for anode-supported microtubular solid oxide fuel cells. Electrochim. Acta 2012, 67, 159–165. [Google Scholar] [CrossRef]
- Tsuruta, A.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. High electrical conductivity of composite ceramics consisting of insulating oxide and ordered perovskite conducting oxide. Phys. Status Solidi A 2017, 214, 1600968. [Google Scholar] [CrossRef]
- Tsuruta, A.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. Element Strategy Using Ru-Mn Substitution in CuO-CaCu3Ru4O12 Composite Ceramics with High Electrical Conductivity. Crystals 2017, 7, 213. [Google Scholar] [CrossRef]
- Tsuruta, A.; Itoh, T.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. Trial of an All-Ceramic SnO2 Gas Sensor Equipped with CaCu3Ru4O12 Heater and Electrode. Materials 2018, 11, 981. [Google Scholar] [CrossRef] [PubMed]
- Brizé, V.; Lambert, C.A.; Wolfman, J.; Gervais, M.; Gervais, F. Synthesis and microstructural TEM investigation of CaCu3Ru4O12 ceramic and thin film. J. Solid State Chem. 2011, 184, 2719–2723. [Google Scholar] [CrossRef]
- Nomura, K. Crystal Structure and Proton Conduction Path of Perovskite-type Oxides by Using a Laboratory X-ray Diffractometer with a Parallel Beam Optics. J. Cryst. Soc. Jpn. 2008, 50, 155–160. [Google Scholar] [CrossRef]
- Izumi, F.; Momma, K. Three-dimensional visualization in powder diffraction. Solid State Phenom. 2007, 130, 15–20. [Google Scholar] [CrossRef]
- Ebbimghaus, S.G.; Weidenkaff, A.; Cava, R.J. Structural Investigations of ACu3Ru4O12 (A = Na, Ca, Sr, La, Nd)—A Comparison between XRD-Rietveld and EXAFS Results. J. Solid State Chem. 2002, 167, 126–136. [Google Scholar] [CrossRef]
- Rosen, B.W.; Hashin, Z. Effective thermal expansion coefficients and specific heats of composite materials. Int. J. Eng. Sci. 1970, 8, 157–173. [Google Scholar] [CrossRef]
- Inaba, H.; Tagawa, H. Semi-Empirical Estimation of Thermal Expansion Coefficients of Perovskite-Type Oxides. J. Ceram. Soc. Jpn. 1998, 106, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Mary, T.A.; Evans, J.S.O.; Vogt, T.; Sleight, A.W. Negative Thermal Expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 1996, 272, 90–92. [Google Scholar] [CrossRef]
- Long, Y.W.; Hayashi, N.; Saito, T.; Azuma, M.; Muranaka, S.; Shimakawa, Y. Temperature-induced A–B intersite charge transfer in an A-site-ordered LaCu3Fe4O12 perovskite. Nature 2009, 458, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Lee, J.; Lee, S.; Lim, T.; Park, S.; Snog, R.; Im, W.B.; Shin, D. La-doped SrTiO3 interconnect materials for anode-supported flat-tubular solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 4319–4327. [Google Scholar] [CrossRef]
- Blake, G.R.; Palstra, T.T.M.; Ren, Y.; Nugroho, A.A.; Menovsky, A.A. Neutron diffraction, x-ray diffraction, and specific heat studies of orbital ordering in YVO3. Phys. Rev. B 2002, 65, 174112. [Google Scholar] [CrossRef]
- Kennedy, B.J.; Hunter, B.A. High-temperature phases of SrRuO3. Phys. Rev. B 1998, 58, 653. [Google Scholar] [CrossRef]
- Wang, X.; Han, Y.; Song, X.; Liu, W.; Jin, Y.; Liu, W.; Cui, H. An insight into the effects of transition metals on the thermal expansion of complex perovskite compounds: An experimental and density functional theory investigation. Phys. Chem. Chem. Phys. 2018, 20, 17781–17789. [Google Scholar] [CrossRef] [PubMed]
- Alonso, J.A.; Martínez-Lope, M.J.; Casais, M.T.; García-Muñoz, J.L.; Fernández-Díaz, M.T.; Aranda, M.A.G. High-temperature structural evolution of RNiO3 (R = Ho, Y, Er, Lu) perovskites: Change disproportionation and electronic localization. Phys. Rev. B 2001, 64, 094102. [Google Scholar] [CrossRef]




| Material | CTE (×10−6/K) | Conduction Behavior | B-Site Cation | Electron Orbital of B-Site Cation | Number of D-Electron | |
|---|---|---|---|---|---|---|
| MgTiO3 | [34] | 10.1 | Insulator | Ti4+ | 3d0 | 0 |
| CaTiO3 | [34] | 11.6 | Insulator | Ti4+ | 3d0 | 0 |
| BaTiO3 | [34] | 12.1 | Insulator | Ti4+ | 3d0 | 0 |
| Sr0.8La0.2TiO3 | [37] | 12.5 | Conductor | Ti4+ | 3d0 | 0 |
| LiNbO3 | [34] | 13.7 | Insulator | Nb5+ | 4d0 | 0 |
| CaHfO3 | [34] | 9.6 | Insulator | Hf4+ | 5d0 | 0 |
| LiTaO3 | [34] | 13.3 | Insulator | Ta5+ | 5d0 | 0 |
| KTaO3 | [34] | 7.01 | Insulator | Ta5+ | 5d0 | 0 |
| YVO3 | [38] | 6.4 | Insulator | V3+ | 3d2 | 2 |
| LaCrO3 | [34] | 9.2 | Insulator | Cr3+ | 3d3 | 3 |
| YMnO3 | [34] | 11.2 | Insulator | Mn3+ | 3d4 | 4 |
| LaMnO3 | [34] | 10.9 | Insulator | Mn3+ | 3d4 | 4 |
| SrRuO3 | [39] | 12.7 | Conductor | Ru4+ | 4d4 | 4 |
| CaCu3Ru4O12 | 8.9 | Conductor | Ru4+ | 4d4 | 4 | |
| LaFeO3 | [34] | 9.7 | Insulator | Fe3+ | 3d5 | 5 |
| La0.6Sr0.4Fe0.2Co0.8O3−x | [19] | 21.4 | Conductor | Fe3+, Co3+ | 3d5, 3d6 | 5.8 |
| SrCoO3 | [34] | 15.6 | Conductor | Co4+ | 3d5 | 5 |
| LaCoO3 | [34] | 23.1 | Conductor | Co3+ | 3d6 | 6 |
| LaCo0.5Ni0.5O3 | [40] | 15.1 | Conductor | Co3+, Ni3+ | 3d6, 3d7 | 6.5 |
| ErNiO3 | [41] | 8.1 | Insulator | Ni3+ | 3d7 | 7 |
| CaSnO3 | [34] | 9.2 | Insulator | Sn4+ | 4d10 | 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuruta, A.; Nomura, K.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. Unusually Small Thermal Expansion of Ordered Perovskite Oxide CaCu3Ru4O12 with High Conductivity. Materials 2018, 11, 1650. https://doi.org/10.3390/ma11091650
Tsuruta A, Nomura K, Mikami M, Kinemuchi Y, Terasaki I, Murayama N, Shin W. Unusually Small Thermal Expansion of Ordered Perovskite Oxide CaCu3Ru4O12 with High Conductivity. Materials. 2018; 11(9):1650. https://doi.org/10.3390/ma11091650
Chicago/Turabian StyleTsuruta, Akihiro, Katsuhiro Nomura, Masashi Mikami, Yoshiaki Kinemuchi, Ichiro Terasaki, Norimitsu Murayama, and Woosuck Shin. 2018. "Unusually Small Thermal Expansion of Ordered Perovskite Oxide CaCu3Ru4O12 with High Conductivity" Materials 11, no. 9: 1650. https://doi.org/10.3390/ma11091650
APA StyleTsuruta, A., Nomura, K., Mikami, M., Kinemuchi, Y., Terasaki, I., Murayama, N., & Shin, W. (2018). Unusually Small Thermal Expansion of Ordered Perovskite Oxide CaCu3Ru4O12 with High Conductivity. Materials, 11(9), 1650. https://doi.org/10.3390/ma11091650







