Recent Advances in Fluorescent Probes for Lipid Droplets
Abstract
1. Introduction
2. Common Methods and Probes in LDs Fluorescence Bioimaging
2.1. Fluorescence Immunostaining
2.2. Diazo Dyes
2.3. Nile Red
2.4. BODIPY 493/503
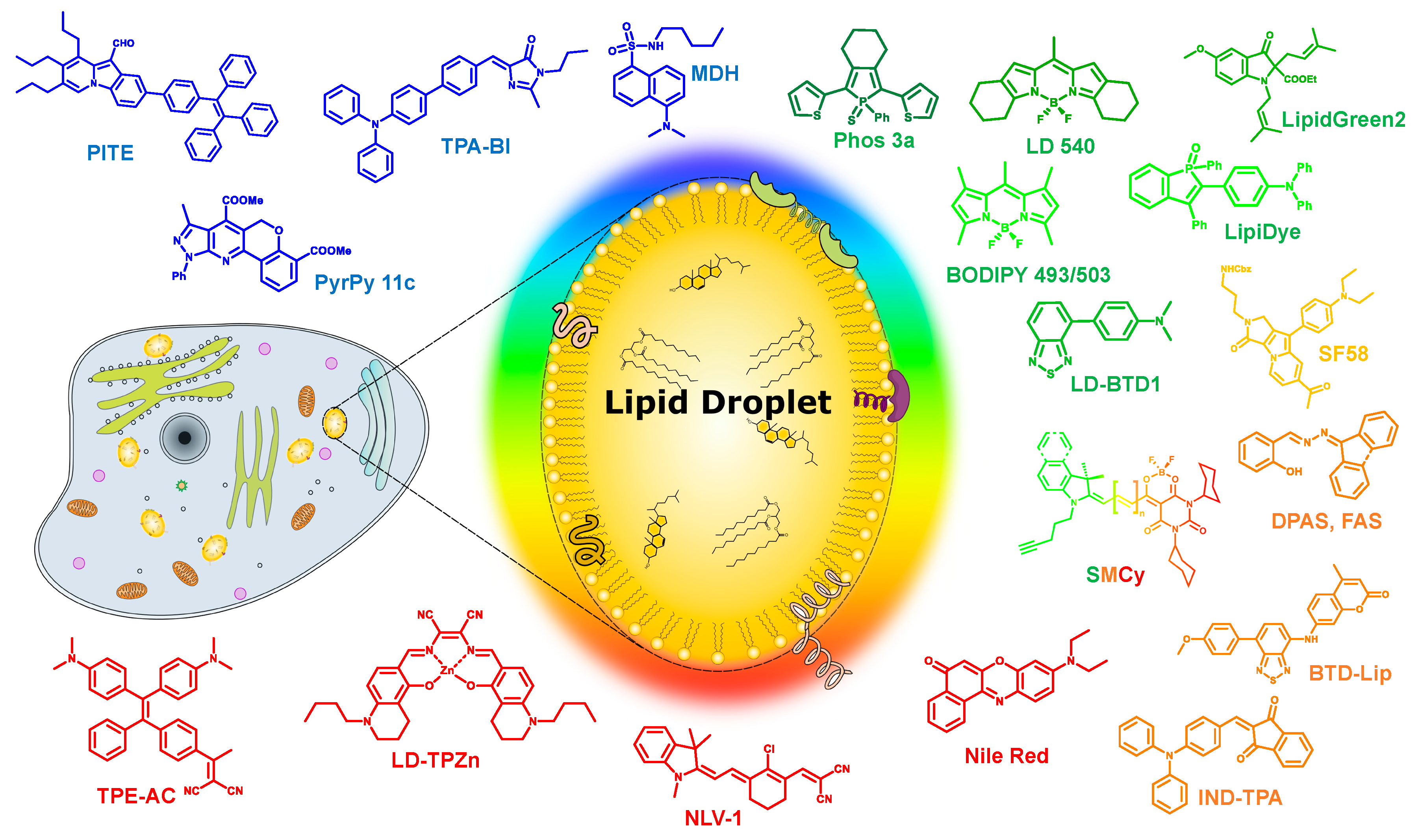
3. Recent Developments in LDs’ Selective Fluorescent Probes
3.1. Blue Emitting LD Probes (λem < 500 nm)
3.2. Green Emitting LD Probes (λem = 500–550 nm)
3.3. Orange Emitting LD Probes (550 nm < λem < 600 nm)
3.4. Red to Near-Infrared Emitting LD Probes (λem > 600 nm)
3.5. Families of LD Probes with Tunable Emission Color
4. Application of Fluorescent LD Probes
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martin, S.; Parton, R.G. Opinion: Lipid Droplets: A Unified View of a Dynamic Organelle. Nat. Rev. Mol. Cell Biol. 2006, 7, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Farese, R.V.; Walther, T.C. Lipid Droplets Finally Get a Little R-E-S-P-E-C-T. Cell 2009, 139, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The Biophysics and Cell Biology of Lipid Droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Blom, T.; Somerharju, P.; Ikonen, E. Synthesis and Biosynthetic Trafficking of Membrane Lipids. CSH Perspect. Biol. 2011, 3, a004713. [Google Scholar] [CrossRef] [PubMed]
- Olzmann, J.A.; Richter, C.M.; Kopito, R.R. Spatial Regulation of UBXD8 and P97/VCP Controls ATGL-Mediated Lipid Droplet Turnover. Proc. Natl. Acad. Sci. USA 2013, 110, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Onal, G.; Kutlu, O.; Gozuacik, D.; Dokmeci Emre, S. Lipid Droplets in Health and Disease. Lipids Health Dis. 2017, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Ohsaki, Y.; Suzuki, M.; Cheng, J. Chapter 13—Imaging Lipid Droplets by Electron Microscopy. In Methods in Cell Biology; Yang, H., Li, P., Eds.; Lipid Droplets; Academic Press: Cambridge, MA, USA, 2013; Volume 116, pp. 227–251. [Google Scholar]
- Horn, P.J.; Ledbetter, N.R.; James, C.N.; Hoffman, W.D.; Case, C.R.; Verbeck, G.F.; Chapman, K.D. Visualization of Lipid Droplet Composition by Direct Organelle Mass Spectrometry. J. Biol. Chem. 2011, 286, 3298–3306. [Google Scholar] [CrossRef] [PubMed]
- Abramczyk, H.; Surmacki, J.; Kopeć, M.; Olejnik, A.K.; Lubecka-Pietruszewska, K.; Fabianowska-Majewska, K. The Role of Lipid Droplets and Adipocytes in Cancer. Raman Imaging of Cell Cultures: MCF10A, MCF7, and MDA-MB-231 Compared to Adipocytes in Cancerous Human Breast Tissue. Analyst 2015, 140, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Yoon, J.; Heo, J.; Choi, C.; Park, Y. Three-Dimensional Label-Free Imaging and Quantification of Lipid Droplets in Live Hepatocytes. Sci. Rep. 2016, 6, 36815. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York City, NY, USA, 2006. [Google Scholar]
- Lavis, L.D. Teaching Old Dyes New Tricks: Biological Probes Built from Fluoresceins and Rhodamines. Annu. Rev. Biochem. 2017, 86, 825–843. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, J.; Du, J.; Peng, X. Fluorescent Probes for Sensing and Imaging within Specific Cellular Organelles. Acc. Chem. Res. 2016, 49, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Acc. Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Kuerschner, L.; Moessinger, C.; Thiele, C. Imaging of Lipid Biosynthesis: How a Neutral Lipid Enters Lipid Droplets. Traffic 2007, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Listenberger, L.L.; Brown, D.A. Fluorescent Detection of Lipid Droplets and Associated Proteins. Curr. Protoc. Cell Biol. 2007, 35, 24. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Hagiwara, H.; Fujimoto, T. Peculiar Distribution of Fodrin in Fat-Storing Cells. Exp. Cell Res. 1997, 234, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Koopman, R.; Schaart, G.; Hesselink, M.K. Optimisation of Oil Red O Staining Permits Combination with Immunofluorescence and Automated Quantification of Lipids. Histochem. Cell. Biol. 2001, 116, 63–68. [Google Scholar] [PubMed]
- Ohsaki, Y.; Shinohara, Y.; Suzuki, M.; Fujimoto, T. A Pitfall in Using BODIPY Dyes to Label Lipid Droplets for Fluorescence Microscopy. Histochem. Cell. Biol. 2010, 133, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Fujimoto, T. Deformation of Lipid Droplets in Fixed Samples. Histochem. Cell. Biol. 2002, 118, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile Red: A Selective Fluorescent Stain for Intracellular Lipid Droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Thermo Fisher Scientific. BODIPY 493/503. Available online: https://www.thermofisher.com/order/catalog/product/D3922 (accessed on 26 July 2018).
- Collot, M.; Fam, T.K.; Ashokkumar, P.; Faklaris, O.; Galli, T.; Danglot, L.; Klymchenko, A.S. Ultrabright and Fluorogenic Probes for Multicolor Imaging and Tracking of Lipid Droplets in Cells and Tissues. J. Am. Chem. Soc. 2018, 140, 5401–5411. [Google Scholar] [CrossRef] [PubMed]
- Gocze, P.M.; Freeman, D.A. Factors Underlying the Variability of Lipid Droplet Fluorescence in MA-10 Leydig Tumor Cells. Cytometry 1994, 17, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Koreivienė, J. Microalgae Lipid Staining with Fluorescent BODIPY Dye. In SpringerLink; Methods in Molecular Biology; Humana Press: New York City, NY, USA, 2017; pp. 1–7. [Google Scholar]
- Yang, H.-J.; Hsu, C.-L.; Yang, J.-Y.; Yang, W.Y. Monodansylpentane as a Blue-Fluorescent Lipid-Droplet Marker for Multi-Color Live-Cell Imaging. PLoS ONE 2012, 7, e32693. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Ruiz, M.; Vargas, V.; Jara, P.; Tirapegui, C.; Carrasco, C.; Nuñez, M.; Lezana, N.; Galdámez, A.; Vilches-Herrera, M. Blue-Fluorescent Probes for Lipid Droplets Based on Dihydrochromeno-Fused Pyrazolo- and Pyrrolopyridines. Eur. J. Org. Chem. 2018, 34, 4795–4801. [Google Scholar] [CrossRef]
- Sk, B.; Thakre, P.K.; Tomar, R.S.; Patra, A. A Pyridoindole-Based Multifunctional Bioprobe: PH-Induced Fluorescence Switching and Specific Targeting of Lipid Droplets. Chem. Asian J. 2017, 12, 2501–2509. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Zhao, E.; Hong, Y.; Lam, J.W.Y.; Tang, B.Z. A Highly Selective AIE Fluorogen for Lipid Droplet Imaging in Live Cells and Green Algae. J. Mater. Chem. B 2014, 2, 2013–2019. [Google Scholar] [CrossRef]
- Jiang, M.; Gu, X.; Lam, J.W.Y.; Zhang, Y.; Kwok, R.T.K.; Wong, K.S.; Tang, B.Z. Two-Photon AIE Bio-Probe with Large Stokes Shift for Specific Imaging of Lipid Droplets. Chem. Sci. 2017, 8, 5440–5446. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; So, J.-H.; Jeon, J.H.; Choi, E.B.; Lee, Y.-R.; Chang, Y.-T.; Kim, C.-H.; Bae, M.A.; Ahn, J.H. Synthesis of a New Fluorescent Small Molecule Probe and Its Use for in Vivolipid Imaging. Chem. Commun. 2011, 47, 7500–7502. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.-S.; Jeon, J.H.; Pagire, H.S.; Lee, J.H.; Chung, H.-C.; Park, M.J.; So, J.-H.; Ryu, J.-H.; Kim, C.-H.; Ahn, J.H.; et al. Synthesis of LipidGreen2 and Its Application in Lipid and Fatty Liver Imaging. Mol. BioSyst. 2013, 9, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Spandl, J.; White, D.J.; Peychl, J.; Thiele, C. Live Cell Multicolor Imaging of Lipid Droplets with a New Dye, LD540. Traffic 2009, 10, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Umar, S.; Kar, P.; Singh, K.; Sachdev, M.; Goel, A. A New Type of Biocompatible Fluorescent Probe AFN for Fixed and Live Cell Imaging of Intracellular Lipid Droplets. Analyst 2015, 141, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Zhang, R.; Kwong, J.P.C.; Lam, J.W.Y.; Chen, C.; Wang, J.; Chen, Y.; Feng, X.; Kwok, R.T.K.; Sung, H.H.-Y.; et al. Specific Two-Photon Imaging of Live Cellular and Deep-Tissue Lipid Droplets by Lipophilic AIEgens at Ultralow Concentration. Chem. Mater. 2018, 30, 4778–4787. [Google Scholar] [CrossRef]
- Appelqvist, H.; Stranius, K.; Börjesson, K.; Nilsson, K.P.R.; Dyrager, C. Specific Imaging of Intracellular Lipid Droplets Using a Benzothiadiazole Derivative with Solvatochromic Properties. Bioconjug. Chem. 2017, 28, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, E.; Wang, C.; Fukazawa, A.; Taki, M.; Sato, Y.; Sasaki, T.; Ueda, M.; Sasaki, N.; Higashiyama, T.; Yamaguchi, S. Environment-Sensitive Fluorescent Probe: A Benzophosphole Oxide with an Electron-Donating Substituent. Angew. Chem. Int. Edit. 2015, 127, 4622–4626. [Google Scholar] [CrossRef]
- High Sensitive Lipid Droplets Imaging Fluorescent Dye | LipiDye | Funakoshi Co., Ltd.: Tokyo, Japan. Available online: https://www.funakoshi.co.jp/exports_contents/80682 (accessed on 30 July 2018).
- Niko, Y.; Didier, P.; Mely, Y.; Konishi, G.; Klymchenko, A.S. Bright and Photostable Push-Pull Pyrene Dye Visualizes Lipid Order Variation between Plasma and Intracellular Membranes. Sci. Rep. 2016, 6, 18870. [Google Scholar] [CrossRef] [PubMed]
- Öberg, E.; Appelqvist, H.; Nilsson, K.P.R. Non-Fused Phospholes as Fluorescent Probes for Imaging of Lipid Droplets in Living Cells. Front. Chem. 2017, 5. [Google Scholar] [CrossRef] [PubMed]
- De Moliner, F.; King, A.; Dias, G.G.; de Lima, G.F.; de Simone, C.A.; da Silva Júnior, E.N.; Vendrell, M. Quinone-Derived π-Extended Phenazines as New Fluorogenic Probes for Live-Cell Imaging of Lipid Droplets. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, S.; Park, S.B. A Seoul-Fluor-Based Bioprobe for Lipid Droplets and Its Application in Image-Based High Throughput Screening. Chem. Commun. 2012, 48, 2331–2333. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Na, S.; Lee, S.; Jeon, N.L.; Park, S.B. Optimization of Seoul-Fluor-Based Lipid Droplet Bioprobes and Their Application in Microalgae for Bio-Fuel Study. Mol. Biosyst. 2013, 9, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gui, C.; Zhao, E.; Wang, J.; Li, X.; Qin, A.; Zhao, Z.; Yu, Z.; Tang, B.Z. Specific Fluorescence Probes for Lipid Droplets Based on Simple AIEgens. ACS Appl. Mater. Interfaces 2016, 8, 10193–10200. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.A.R.; Correa, J.R.; de Andrade, L.P.; Assumpção, J.A.F.; de Souza Cintra, G.A.; Freitas-Junior, L.H.; da Silva, W.A.; de Oliveira, H.C.B.; Neto, B.A.D. From Live Cells to Caenorhabditis Elegans: Selective Staining and Quantification of Lipid Structures Using a Fluorescent Hybrid Benzothiadiazole Derivative. ACS Omega 2018, 3, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Su, H.; Li, S.; Lin, Y.; Ling, X.; Qin, A.; Tang, B.Z. An Easily Accessible Aggregation-Induced Emission Probe for Lipid Droplet-Specific Imaging and Movement Tracking. Chem. Commun. 2017, 53, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ling, X.; Lin, Y.; Qin, A.; Gao, M.; Tang, B.Z. In Situ Generation of Photoactivatable Aggregation-Induced Emission Probes for Organelle-Specific Imaging. Chem. Sci. 2018, 9, 5730–5735. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhang, Y.; Yin, H.-Y.; Xu, G.; Zhang, J.-L. Precise Labeling and Tracking of Lipid Droplets in Adipocytes Using a Luminescent ZnSalen Complex. Chem. Asian J. 2017, 12, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Jana, N.R. Quantum Dot-Based Designed Nanoprobe for Imaging Lipid Droplet. J. Phys. Chem. C 2017, 121, 23727–23735. [Google Scholar] [CrossRef]
- Gao, M.; Su, H.; Lin, Y.; Ling, X.; Li, S.; Qin, A.; Tang, B.Z. Photoactivatable Aggregation-Induced Emission Probes for Lipid Droplets-Specific Live Cell Imaging. Chem. Sci. 2017, 8, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Gu, X.; Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Li, F.; Tang, B.Z. A Near-Infrared AIEgen for Specific Imaging of Lipid Droplets. Chem. Commun. 2016, 52, 5957–5960. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Su, H.; Kwok, R.T.K.; Shan, G.; Leung, A.C.S.; Lee, M.M.S.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; Tang, B.Z. Facile Synthesis of Red/NIR AIE Luminogens with Simple Structures, Bright Emissions, and High Photostabilities, and Their Applications for Specific Imaging of Lipid Droplets and Image-Guided Photodynamic Therapy. Adv. Funct. Mater. 2017, 27, 1704039. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhang, T.; Liu, H.; Chen, Y.; Kwok, R.T.K.; Ma, C.; Zhang, P.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Bright Near-Infrared Aggregation-Induced Emission Luminogens with Strong Two-Photon Absorption, Excellent Organelle Specificity, and Efficient Photodynamic Therapy Potential. ACS Nano 2018, 12, 8145–8159. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Yin, J.; Ma, Y.; Li, G.; Wang, Q.; Lin, W. A Novel NIR Probe for Detection of Viscosity in Cellular Lipid Droplets, Zebra Fishes and Living Mice. Sensor. Actuators B-Chem. 2018, 271, 321–328. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific, LipidTOX Neutral Lipid Stain. Available online: https://www.thermofisher.com/order/catalog/product/H34475 (accessed on 27 July 2018).
- Fujimoto, T.; Ohsaki, Y.; Cheng, J.; Suzuki, M.; Shinohara, Y. Lipid Droplets: A Classic Organelle with New Outfits. Histochem. Cell. Biol. 2008, 130, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Jacquier, N.; Choudhary, V.; Mari, M.; Toulmay, A.; Reggiori, F.; Schneiter, R. Lipid Droplets Are Functionally Connected to the Endoplasmic Reticulum in Saccharomyces Cerevisiae. J. Cell. Sci. 2011, 124, 2424–2437. [Google Scholar] [CrossRef] [PubMed]
- Layerenza, J.P.; González, P.; García de Bravo, M.M.; Polo, M.P.; Sisti, M.S.; Ves-Losada, A. Nuclear Lipid Droplets: A Novel Nuclear Domain. BBA-Mol. Cell Biol. Lipids 2013, 1831, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Nevo-Yassaf, I.; Lovelle, M.; Nahmias, Y.; Hirschberg, K.; Sklan, E.H. Live Cell Imaging and Analysis of Lipid Droplets Biogenesis in Hepatatis C Virus Infected Cells. Methods 2017, 127, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Tuohetahuntila, M.; Molenaar, M.R.; Spee, B.; Brouwers, J.F.; Wubbolts, R.; Houweling, M.; Yan, C.; Du, H.; VanderVen, B.C.; Vaandrager, A.B.; et al. Lysosome-Mediated Degradation of a Distinct Pool of Lipid Droplets during Hepatic Stellate Cell Activation. J. Biol. Chem. 2017, 292, 12436–12448. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Li, L.; Qiu, H.; Zhang, Z.; Wang, Y.; Sun, G. Application of the Fluorescent Dye BODIPY in the Study of Lipid Dynamics of the Rice Blast Fungus Magnaporthe Oryzae. Molecules 2018, 23, 1594. [Google Scholar] [CrossRef] [PubMed]
- Knight, M.; Braverman, J.; Asfaha, K.; Gronert, K.; Stanley, S. Lipid Droplet Formation in Mycobacterium Tuberculosis Infected Macrophages Requires IFN-γ/HIF-1α Signaling and Supports Host Defense. PLOS Pathog. 2018, 14, e1006874. [Google Scholar] [CrossRef] [PubMed]
- Masedunskas, A.; Chen, Y.; Stussman, R.; Weigert, R.; Mather, I.H.; Nusrat, A. Kinetics of Milk Lipid Droplet Transport, Growth, and Secretion Revealed by Intravital Imaging: Lipid Droplet Release Is Intermittently Stimulated by Oxytocin. MBoC 2017, 28, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J.G.; Moens, S.J.B.; Tiessens, F.; Bakker, G.J.; Dallinga-Thie, G.M.; Groen, A.K.; Nieuwdorp, M.; Stroes, E.S.G.; Kroon, J. Nile Red Quantifier: A Novel and Quantitative Tool to Study Lipid Accumulation in Patient-Derived Circulating Monocytes Using Confocal Microscopy. J. Lipid. Res. 2017, 58, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Held, P. Lipid Accumulation in HepG2 Cells Exposed to Free Fatty Acids. Available online: https://www.biotek.com/resources/application-notes/lipid-accumulation-in-hepg2-cells-exposed-to-free-fatty-acids/ (accessed on 14 August 2017).
- Helmchen, F.; Denk, W. Deep Tissue Two-Photon Microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Hu, F.; Shen, Y.; Chen, Z.; Yu, Y.; Lin, C.-C.; Wang, M.C.; Min, W. Live-Cell Imaging of Alkyne-Tagged Small Biomolecules by Stimulated Raman Scattering. Nat. Methods 2014, 11, 410–412. [Google Scholar] [CrossRef] [PubMed]






| Dye | λex/λem, nm | ε, M−1 cm−1 | QY | Excitation Mode | Application | Reference |
|---|---|---|---|---|---|---|
| Commonly used | ||||||
| Nile Red | 553/635 (MeOH) | 44,000 | n.r. | 1P | Cells | [21] |
| BODIPY 493/503 | 493/503 (MeOH) | 90,000 | n.r. | 1P | Cells | [22] |
| Blue | ||||||
| PITE | 313 a/487 (MeCN) | n.r. | 0.48 (MeCN) | 1P | Mammalian and bacterial cells | [28] |
| MDH | 405/570 (MeOH) | n.r. | n.r. | 1P, 2P (δ2PA n.r.) | Cells | [26] |
| TPA-BI | 447/619 (MeCN) | 34,000 | 0.22 (water 70%) | 1P, 2P (δ2PA = 213 GM at 840 nm) | 1PE and 2PE imaging in cells | [31] |
| TPE-AmAl | 400/470 (THF) | n.r. | 0.22 (solid state) | 1P | Cells and algae | [30] |
| PyrPy 10d | 356/449 (CHCl3) | 12,400 | 0.322 (CHCl3) | 1P | Cells | [27] |
| PyrPy 11c | 344/447 (CHCl3) | 13,200 | 0.029 (CHCl3) | 1P | Cells | [27] |
| Green | ||||||
| BODIPY 505/515 | 505/515 (MeOH) | 94,000 | n.r. | 1P | Cells | [22] |
| LipidGreen | 485/515 (PBS) | n.r. | n.r. | 1P | See footnote 1 | [32] |
| LipidGreen2 | 456/534 (PBS) | n.r. | n.r. | 1P | See footnote 2 | [33] |
| LD540 | 540/545.5 (oil) | n.r. | n.r. | 1P | Multicolor imaging in cells | [34] |
| LD-BTD1 | 420/511 (hexane) | 7100 | 0.66 (hexane) | 1P | Cells | [37] |
| AF8 | 380/479 (DMSO) | n.r. | 0.31 (DMSO) | 1P | Cells | [35] |
| AFN | 428/592 (DMSO) | n.r. | 0.17 (DMSO) | 1P | Cells | [35] |
| AF10 | 356/477 (DMSO) | n.r. | 0.18 (DMSO) | 1P | Cells | [35] |
| NAP-Ph | 409/523 (water) | n.r. | 0.018 (water) | 1P, 2P (δ2PA = 100 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| NAP-Br | 409/525 (water) | n.r. | 0.014 (water) | 1P, 2P (δ2PA = ~50 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| NAP-CF3 | 425/560 (water) | n.r. | 0.016 (water) | 1P, 2P (δ2PA = ~50 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| NAP-Py | 413/541 (water) | n.r. | 0.015 (water) | 1P, 2P (δ2PA = 45 GM at 860 nm) | 1PE and 2PE imaging in cells and tissues | [36] |
| LipiDye | 415 (toluene) | 18,700 | 0.94 (toluene) | 1P | Cells | [38,39] |
| PA | 434/521 (toluene) | 25,000 | 0.95 (toluene) | 1P, 2P (δ2PA =35 GM at 820 nm) | Lipid order in cells | [40] |
| Phos 2a | 439/554 (DMSO) | n.r. | n.r. | 1P | Cells | [41] |
| Phos 3a | 429/512 (DMSO) | n.r. | n.r. | 1P | Cells | [41] |
| Phos 2b | 437/554 (DMSO) | n.r. | n.r. | 1P | Not applicable for cells | [41] |
| Phos 3b | 374/550 (DMSO) | n.r. | n.r. | 1P | Not applicable for cells | [41] |
| P1 | 428/500 (Dioxane) | n.r. | 0.22 | 1P | Cells | [42] |
| Orange | ||||||
| SF44 | 455/626 (MeCN) | n.r. | 0.09 (MeCN) | 1P | Cells, HTS for LD modulator | [43] |
| SF58 | 440/623 (MeCN) | n.r. | 0.09 (MeCN) | 1P | Cells and algae | [44] |
| FAS | 322/600 (water) b | n.r. | 0.021 (solid state) | 1P | Cells | [45] |
| DPAS | 301/550 (water) b | n.r. | 0.03 (solid state | 1P | Cells | [45] |
| BTD-Lip | 455/624 (MeCN) | 7586 | 0.2 (MeCN) | 1P | Cells and worms | [46] |
| IND-TPA | 478/594 (THF) | n.r. | 0.069 (THF) | 1P, 2P (δ2PA = 119 GM at 920 nm) | 1PE and 2PE imaging in cells | [47] |
| BZT 3a | 309/576 (water) | n.r. | n.r. | 1P, 2P at 780 nm | 1PE and 2PE imaging in cells | [49] |
| BZT 4a | -/570 (solid) | n.r. | 0.4 (solid state) | 1P, 2P at 780 nm | 1PE and 2PE imaging in cells | [49] |
| Red to NIR | ||||||
| LD-TPZn | 599/630 (DMSO) | 10,600 | 0.44 (DMSO) | 1P, 2P (δ2PA = 110 GM at 880 nm) | 1PE and 2PE imaging in cells | [50] |
| LQD | 495/600 (colloidal) | n.r. | n.r. | 1P | Cells | [51] |
| PhotoAFN 2a | 409/624 (THF) | n.r. | 0.006 (THF) | 1P | Cells | [52] |
| PhotoAFN 2b | 402/617 (THF) | n.r. | 0.005 (THF) | 1P | Cells | [52] |
| PhotoAFN 2c | 400/610 (THF) | n.r. | 0.008 (THF) | 1P | Cells | [52] |
| TPE-AC | 455/724 (THF) | n.r. | 0.05 (solid state) | 1P | Cells | [53] |
| TPMN | 441/635 (MeCN) | n.r. | 0.0021 (MeCN) | 1P | Cells and zebrafish | [54] |
| TTMN | 483/664 (MeCN) | n.r. | 0.0032 (MeCN) | 1P | Cells and zebrafish | [54] |
| MeTTMN | 441/635 (MeCN) | n.r. | 0.0021 (MeCN) | 1P | Cells and zebrafish | [54] |
| MeOTTMN | 499/-(MeCN) | n.r. | n.r. | 1P | Cells | [54] |
| DCMa | 478/665 (water) | n.r. | 0.296 (solid state) | 1P, 2P (δ2PA = 394 GM at 940 nm) | 1PE and 2PE imaging in cells | [54] |
| DCIs | 510/709 (water) | n.r. | 0.135 (solid state) | 1P, 2P (δ2PA = 548 GM at 980 nm) | 1PE and 2PE imaging in cells | [55] |
| DCFu | 538/755 (water) | n.r. | 0.017 (solid state) | 1P, 2P (δ2PA = 887 GM at 1020 nm) | 1PE and 2PE imaging in cells | [55] |
| NLV-1 | 680/719 (glycerol) | n.r. | 0.204 (glycerol) | 1P | See footnote 3 | [56] |
| Multicolor | ||||||
| LipidTox™ | Analysis of steatosis | [57] | ||||
| green | 495/505 | n.r. | n.r. | 1P | [57] | |
| red | 577/609 | n.r. | n.r. | 1P | [57] | |
| deep red | 637/655 | n.r. | n.r. | 1P | [57] | |
| SMCy family | See footnote 4 | [23] | ||||
| SMCy3 | 512/541 (oil) | 82,900 | 0.21 (oil) | 1P, 2P (δ2PA = 178 GM at 690 nm, DMSO) | [23] | |
| SMCy3.5 | 530/559 (oil) | 100,000 | 0.4 (oil) | 1P, 2P (δ2PA = 2400 GM at 760 nm, DMSO) | [23] | |
| SMCy5 | 618/648 (oil) | 256,000 | 0.6 (oil) | 1P, 2P (δ2PA = 6250 GM at 740 nm, DMSO) | [23] | |
| SMCy5.5 | 638/662 (oil) | 169,000 | 0.74 (oil) | 1P, 2P (δ2PA = 13330 GM at 770 nm and 10400 GM at 820 nm, DMSO) | [23] | |
| SMCy7 | 692/744 (oil) | 44,000 | 0.42 (oil) | 1P | [23] | |
| SMCy7.5 | 716/753 (oil) | 103,000 | 0.19 (oil) | 1P | [23] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fam, T.K.; Klymchenko, A.S.; Collot, M. Recent Advances in Fluorescent Probes for Lipid Droplets. Materials 2018, 11, 1768. https://doi.org/10.3390/ma11091768
Fam TK, Klymchenko AS, Collot M. Recent Advances in Fluorescent Probes for Lipid Droplets. Materials. 2018; 11(9):1768. https://doi.org/10.3390/ma11091768
Chicago/Turabian StyleFam, Tkhe Kyong, Andrey S. Klymchenko, and Mayeul Collot. 2018. "Recent Advances in Fluorescent Probes for Lipid Droplets" Materials 11, no. 9: 1768. https://doi.org/10.3390/ma11091768
APA StyleFam, T. K., Klymchenko, A. S., & Collot, M. (2018). Recent Advances in Fluorescent Probes for Lipid Droplets. Materials, 11(9), 1768. https://doi.org/10.3390/ma11091768