Abstract
In this work, CoPi and Co(OH)2 nanoparticles were deposited on the surface of Ta3N5 nanorod-arrays to yield a novel broad-spectrum response photocatalytic material for 304 stainless steel photocatalytic cathodic protection. The Ta3N5 nanorod-arrays were prepared by vapor-phase hydrothermal (VPH) and nitriding processes and characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and UV-Vis spectroscopy, respectively, to obtain morphologies, crystal structures, surface compositions, and light response range. In order to analyze the performance improvement mechanism of CoPi/Co(OH)2 on Ta3N5 nanorod-arrays, the electrochemical behavior of modified and unmodified Ta3N5 was obtained by measuring the open circuit potential and photocurrent in 3.5 wt% NaCl solution. The results revealed that the modified Ta3N5 material better protects 304 stainless steel at protection potentials reaching −0.45 V.
1. Introduction
Since Fujishima and Honda [1] first reported the photoelectric effect of TiO2 in 1972, photocatalytic materials have become increasingly important, with diverse applications in several industrial processes [2,3,4,5,6]. Research has shown that many common photocatalytic materials, such as TiO2 [5,7], ZnO [8,9], and SnO2 [10], have wide band gaps, which means that they have almost only ultraviolet light response and have a low utilization rate of sunlight in practical applications. What’s more, it is difficult for a single photocatalyst to function effectively independently, even for rather simple processes like decomposition of water. In the same vein, single photocatalyst materials do not function efficiently in electrochemical protection applications. Photocatalytic activity can often be improved by regulating crystal surface [11] and surface defects [12], as well as by noble metal deposition [13,14] or semiconductor combination [15,16], which often result in photocatalytic composite systems with improved performance. Another approach involves exploration, design, and development of novel photocatalytic materials.
Some recent studies have focused on developing a new class of photocatalytic materials that respond readily to a broad range of spectra and can make full use of visible light to achieve photocatalysis. A material like BiVO4, for instance, with a band gap of 2.4 eV, has a large visible region response and successfully degrades organics in visible light [17].
Ta3N5 is a novel photocatalytic material with a broad spectral response range [18,19,20,21]. Ta3N5, prepared by Hara et al. [22], could oxidize water into O2 efficiently, with maximum quantum yields of 10%. Luo et al. [23] reported that in the visible region nano Au/Ta3N5 composite showed a significantly enhanced photocatalytic activity for hydrogen evolution from water. Zhen et al. [24] reported that a Ta3N5 nanorod modified with Co(OH)x had a strong absorption in the visible light range up to 620 nm and could achieve high photon-to-current conversion efficiency. The use of catalyst promoters like Co(OH)x [25,26] and CoPi [27,28] has become an important and promising way to improve the photocatalytic capability of photocatalysts [29,30,31,32,33]. Such promoters function by restraining the recombination rate of interfacial photogenerated electron-hole pairs and play a useful role in providing ready sites for the oxidation half reaction [34].
In the 1990s, Tsujikawa and Yuan [5] first proposed a TiO2 photocatalytic coating technique for cathodic corrosion protection of carbon steel. Since then, the use of photocatalysts in cathodic protection has continued to attract attention within the scientific community and different photocatalytic materials have been investigated for metal corrosion protection efficacy. Sun et al. [35] reported that a C3N4-In2O3 nanocomposite with quasi-shell-core structure provides photoelectrochemical cathodic protection for the coupled 304 stainless steel (SS) under visible light. Wang et al. [36] observed that Bi2Se3/TiO2 nanocomposites successfully exhibited great photogenerated cathodic protection performance for 304 stainless steel.
However, we have not seen any reports on the use of Ta3N5 materials for photoelectrochemical cathodic protection of metals. Compared to the previous work on Ta3N5, it is necessary and meaningful to explore the cathodic protection performance of this material with a broad spectral response range.
In this work, a vapor-phase hydrothermal process and subsequent nitriding treatment were used to prepare Ta3N5 nanorod-array films, which were subsequently modified by addition of CoPi/Co(OH)2 and subjected to microstructure characterization. The change of photoelectrochemical cathodic protection properties of Ta3N5 material before and after modification were compared using electrochemical methods. This study provides a theoretical basis for the application of novel photocatalytic materials with broad spectral response ranges in cathodic protection.
2. Experimental Section
2.1. Specimen Preparation and Modification
Vapor-phase hydrothermal (VPH) is a process to grow metal oxides on the corresponding metal matrix, which has become a very attractive method in recent years [37,38]. It has been used in growth of ZnO nanotube and nanorod-array films on a zinc foil substrate [37] and growth of rutile nanorod and titanate nanotubes on a titanium foil substrate [38].
In this work, based on previous methods, one-dimensional Ta2O5 nanorod-arrays were synthesized on a tantalum foil substrate (10 mm × 15 mm) by the VPH process, and these arrays were subsequently converted to Ta3N5 nanorod-array films by NH3 nitriding treatment [24].
To obtain the Ta3N5 material modified by CoPi/Co(OH)2, double co-catalysts, CoPi and Co(OH)2, were deposited as follows: CoPi was loaded on the surface of Ta3N5 nanorod-array films by photoelectric chemical deposition [28], and then Co(OH)2 was modified by chemical deposition modification on top of CoPi [24].
2.2. Characterization of Ta3N5
The surface morphologies of the Ta3N5 nanorod-array films were observed and analyzed using an INSPECT F50 (FEI Co., Hillsboro, OR, USA) field emission scanning electron microscope (SEM), while the growth thickness of the films was also observed by cross-section morphologies at a working voltage of 25 kV. The crystalline structures were measured through a X’pert PRO (Panalytical, Almelo, The Netherlands) X-ray diffractometer (XRD) using Cu Kα radiation at 40 kV, with 2θ ranging from 10° to 90°. In order to identify if CoPi/Co(OH)2 was successfully loaded, the changes of surface elemental compositions were analyzed by ESCALAB250 (Thermo VG, Waltham, MA, USA) X-ray photoelectron spectroscopy (XPS) with Al Kα radiation. The ultraviolet-visible spectra of the Ta3N5 films were collected by a diffuse reflectance V-770 (JASCO, Tokyo, Japan) UV-Vis spectrophotometer in the wavelength range 200–900 nm.
2.3. Preparation of Ta3N5 Electrode
The Ta3N5 nanorod-array films were fabricated into electrodes before use in photoelectrochemical performance tests for metal protection. The Ta3N5 electrode and 304 stainless steel electrode were cut in the same size (10 mm × 15 mm). One side of each sample was exposed to the corrosive solution and the other sides covered with a resin-paraffin mixture (1:1). The samples were pasted and fixed to copper wires as electrodes. A 3.5 wt% NaCl solution was used as electrolyte solution in order to simulate the seawater environment.
2.4. Characterization of Photoelectrochemical Performance
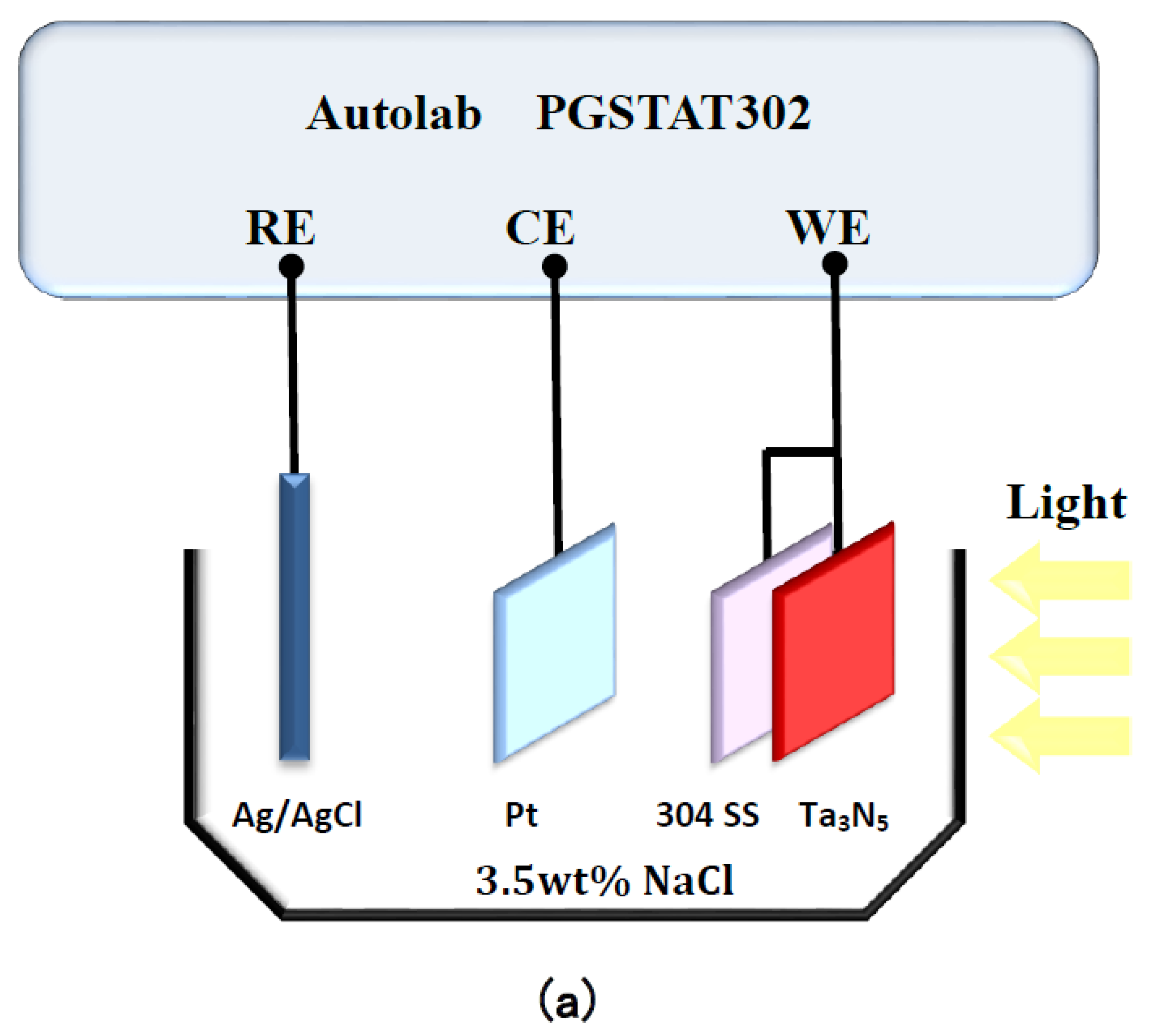
Characterization of photoelectrochemical performance was conducted using a PGSTAT302N potentiostat Autolab (Metrohm Autolab, Utrecht, The Netherlands). As shown in Figure 1a, a Pt electrode and a KCl-saturated silver/silver chloride electrode (Ag/AgCl) were connected as counter electrode and reference electrode, respectively. The Ta3N5 electrodes with and without CoPi/Co(OH)2 modification were coupled with 304 stainless steel and used as working electrodes. This connection method enabled detection of the open circuit potential (OCP) changes over time in the dark and under illumination, respectively.
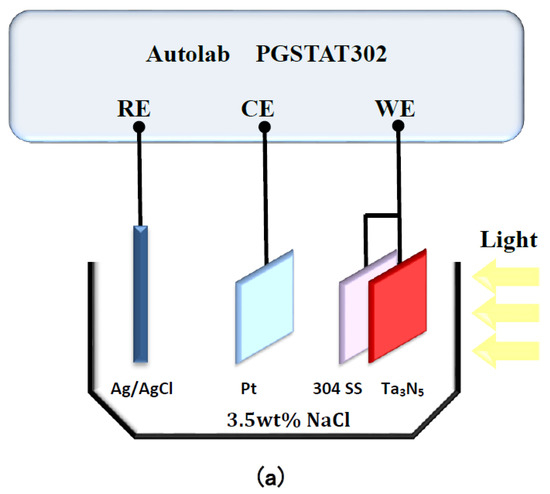
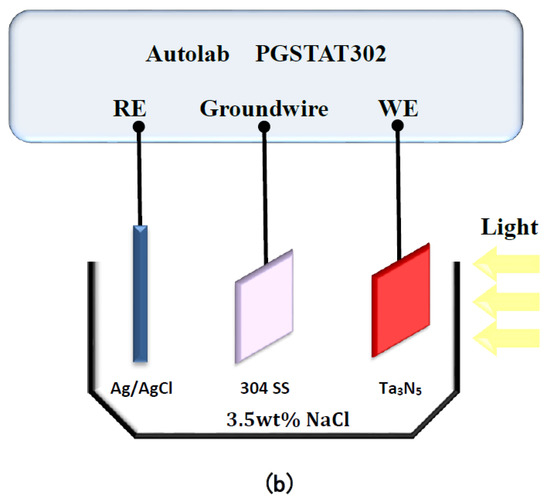
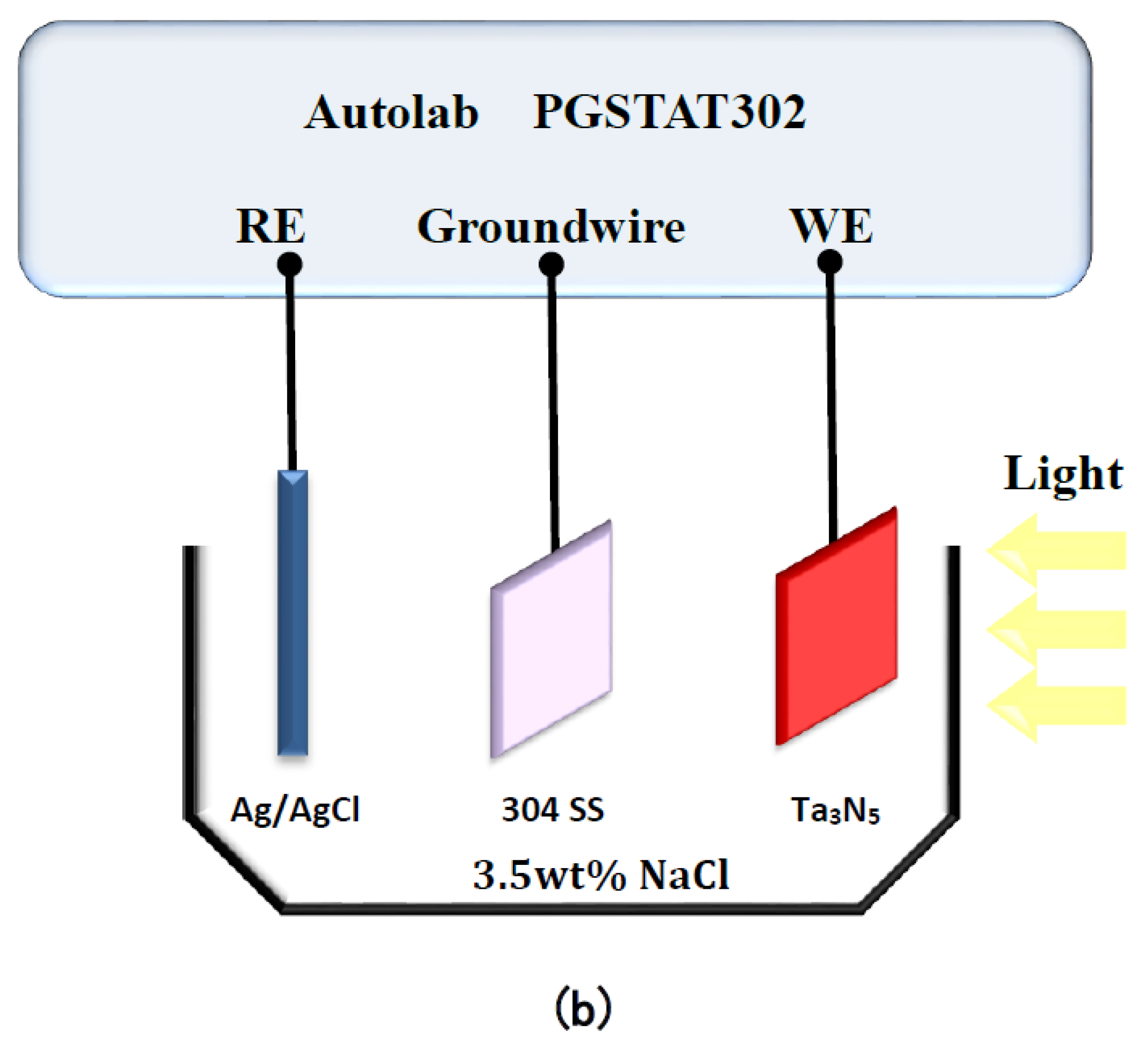
Figure 1.
Schematic diagram of the Autolab electrochemical test system for measuring (a) open circuit potentials and (b) photocurrent densities of Ta3N5 films.
The electrochemical noise module (ECN) was used to measure the photogenerated current of the two kinds of Ta3N5 electrodes as a function of time, in the dark and under illumination. As shown in Figure 1b, the working electrode Ta3N5 was placed opposite the 304 stainless steel counter electrode (connected to the ground wire in ECN measurements, so that electrons can flow from the Ta3N5 electrode to the 304 stainless steel electrode); the Ag/AgCl electrode was connected likewise as reference electrode. All the measured potentials were relative to the Ag/AgCl electrode (0.1981 V). The electrolyte, 3.5 wt% NaCl solution, was replaced for each new measurement. The light source was a 300 W PLS-SXE 300 Xe lamp (Beijing PerfectLight Co. Ltd., Beijing, China). The experimental temperature was at room temperature.
3. Results and Discussion
3.1. Micromorphologies of Ta3N5
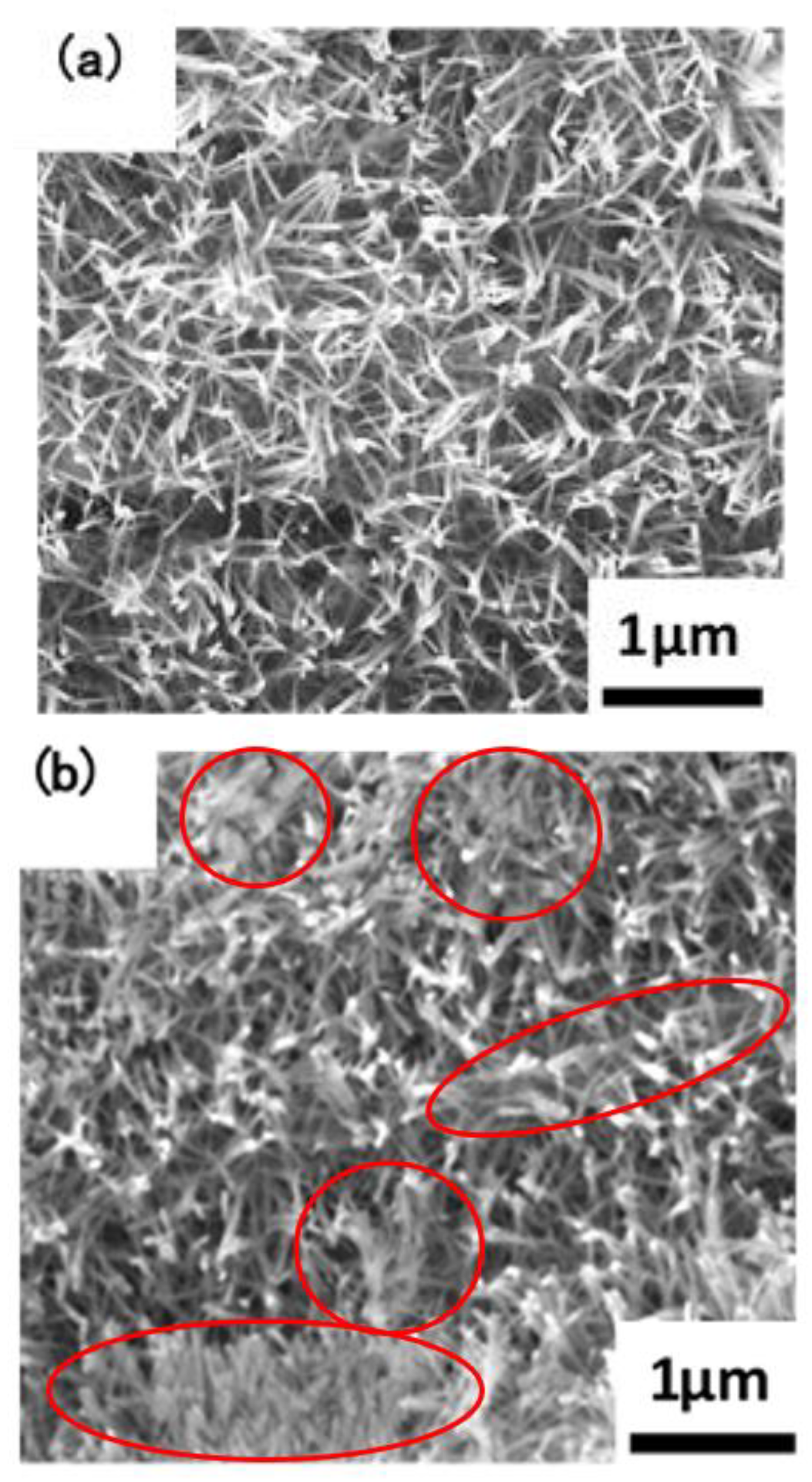
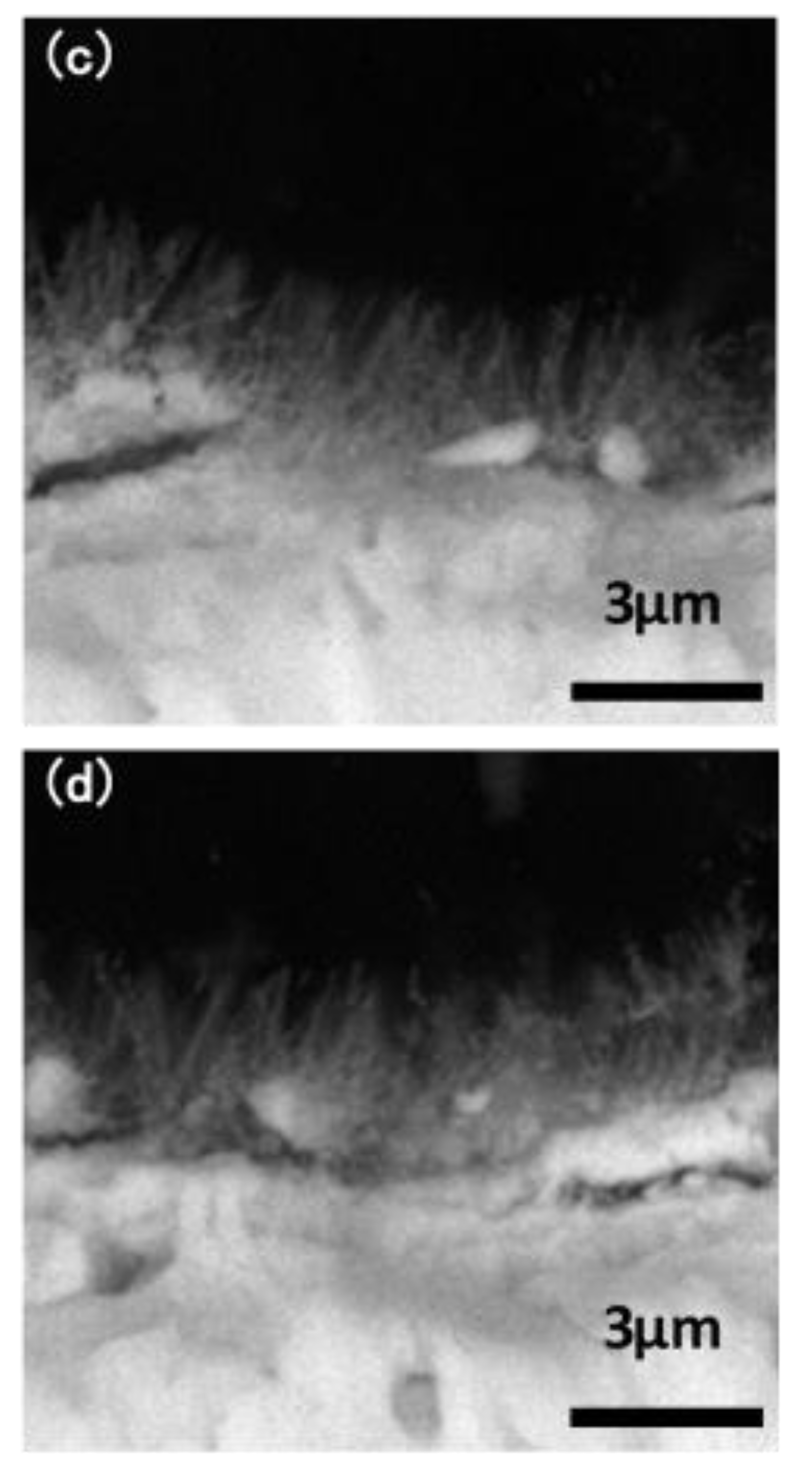
The macroscopic surface of the Ta3N5 material prepared by the VPH method and nitriding treatment was a uniform and wine-red film. The micromorphologies of the unmodified Ta3N5 (shown in Figure 2) grew in the form of a vimineous nanorod-array, continuously and closely connected to each other (Figure 2a). The corresponding cross-section morphology shows the nanorod-arrays to be almost vertical, with lengths of approximately 2–3 µm (Figure 2c).
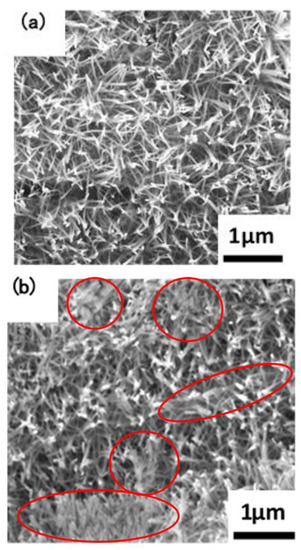
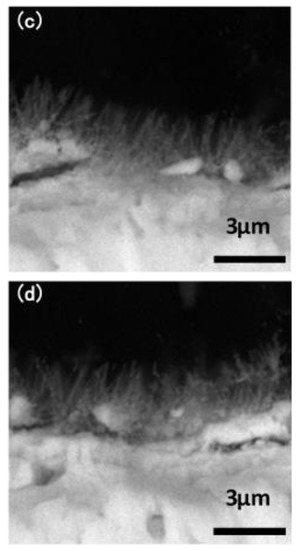
Figure 2.
SEM images of the microstructures of the prepared Ta3N5 nanorod-array films: (a) surface morphology of unmodified Ta3N5; (b) surface morphology of modified Ta3N5; (c) cross-section morphology of unmodified Ta3N5; (d) cross-section morphology of modified Ta3N5.
After surface modification with CoPi/Co(OH)2, the surface morphology shows evidence of agglomeration of some nanorods (Figure 2b), suggesting that CoPi/Co(OH)2 probably adsorbed on nanorod-arrays and caused them to bind together as shown in the marked areas. The corresponding cross-section morphology in Figure 2d shows similar features as the unmodified Ta3N5 nanorod-arrays in Figure 2c, and the nanorod agglomeration is not very clear.
Such nanorod-array structure should impart many merits like excellent conductivity and prominent quantum size effect, high specific surface area, and a large number of surface reaction sites, which will promote transport of electro-hole pairs. This means that solar energy conversion efficiency and light absorption ability will be enhanced, with the possibility of improved electrochemical protective performance.
3.2. Crystal Structure and Chemical Composition Analysis of Ta3N5
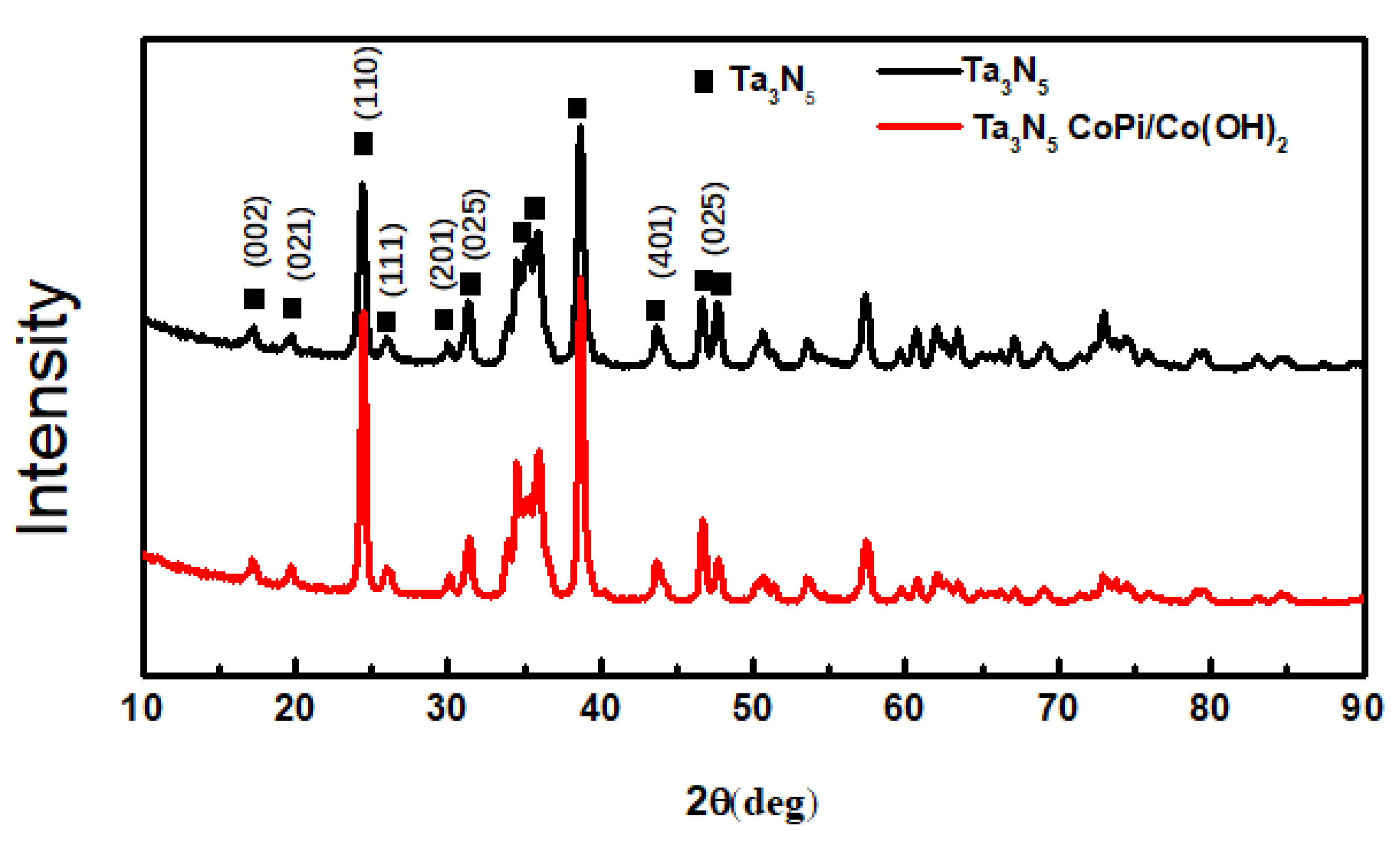
The XRD patterns of the as-prepared Ta3N5 material with and without CoPi/Co(OH)2 modification are shown in Figure 3. When 2θ is at 17.21°, 19.74°, 24.47°, 26.00°, 30.09°, 33.85°, 34.49°, 36.01°, 38.68°, 43.67°, 46.67°, and 47.75°, the diffraction peaks are basically consistent with the standard Tantalum Nitride Ta3N5 (JCPDS card no. 19-1291). These peaks match with the (002), (021), (110), (111), (201), (025,) (401), and (025) crystal planes completely. The other weaker peaks include the Ta matrix and a small amount of tantalum nitride compounds of other valences arising from the nitriding treatment. A comparison of the modified and unmodified Ta3N5 materials reveals that the diffraction peak position and peak intensity of the two curves almost coincide. Moreover, no peaks are observed for CoPi/Co(OH)2, indicating that their amounts may be too low to be detected by XRD. This could also imply that the CoPi/Co(OH)2 additives merely adhered to surface of the Ta3N5 nanorod-array, causing the observed agglomeration.
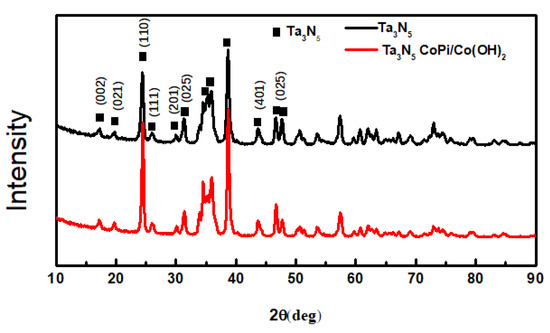
Figure 3.
X-ray diffraction patterns of the Ta3N5 material with and without CoPi/Co(OH)2 modification.
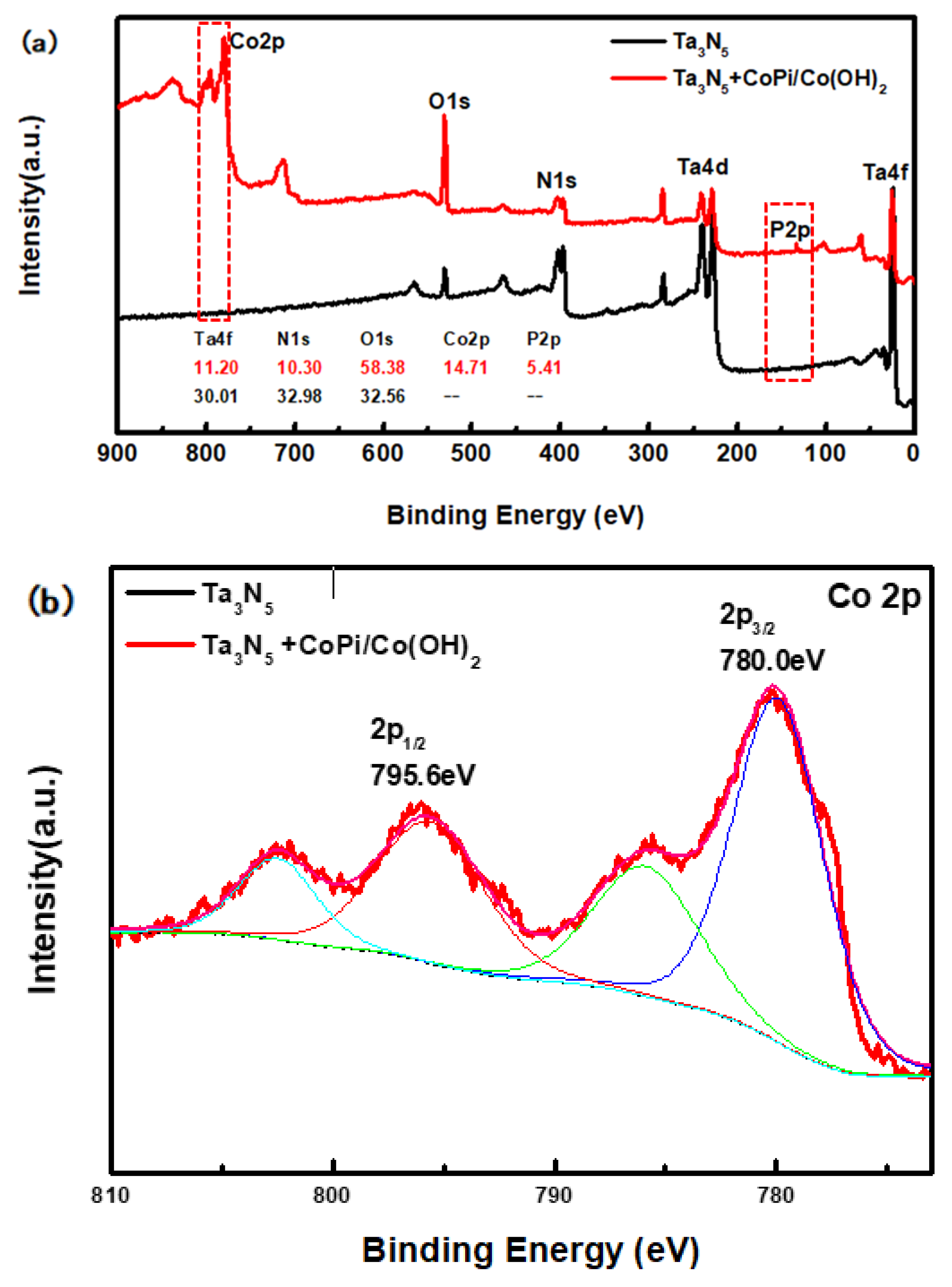
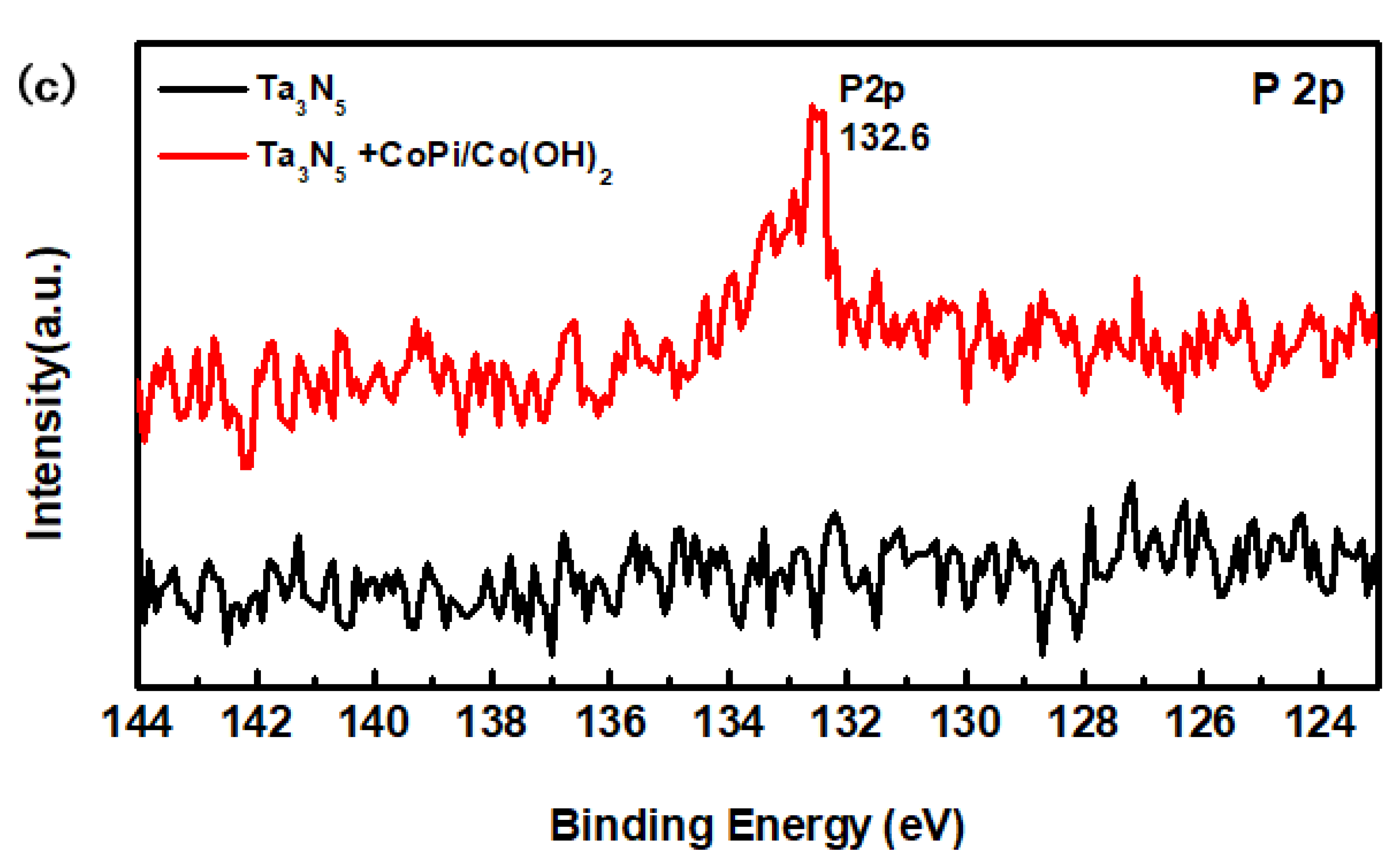
The compositions of the modified and unmodified Ta3N5 surfaces were further investigated by XPS, with special focus on the peaks and transformations of Co and P elements. The high-resolution complete survey XPS in Figure 4a shows Ta 4f, Ta 4d, N 1s, O 1s, Co 2p, and P 2p peaks, corresponding to the as-prepared samples. The major difference between the two curves is that the peak of the Co and P appeared only in the curve of the modified Ta3N5, indicating that CoPi/Co(OH)2 was indeed successfully deposited on the surface of the Ta3N5 nanorod-array in the modified sample.
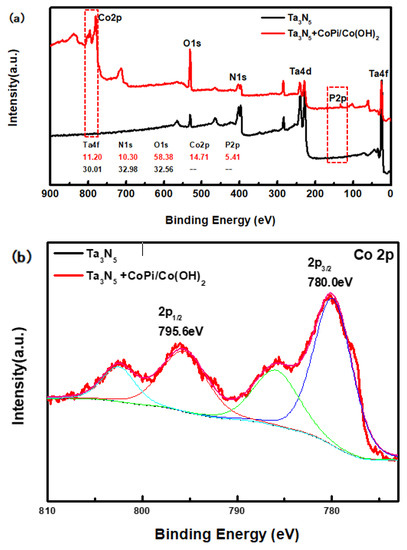
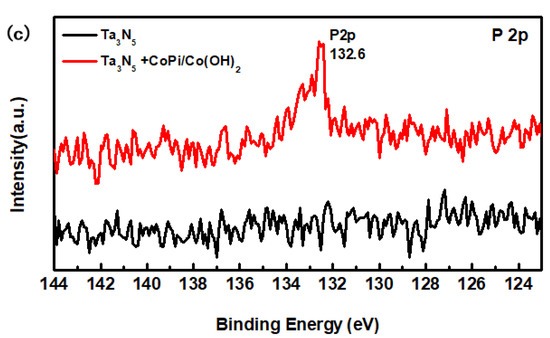
Figure 4.
X-ray photoelectron spectroscopy (XPS) spectra of the Ta3N5 material with and without CoPi/Co(OH)2 modification: (a) survey spectrum, (b) Co 2p peak, (c) P 2p peak.
From the high-resolution spectra of CoPi/Co(OH)2 for the modified Ta3N5 (Figure 4b), the binding energies of Co 2p3/2 and 2p1/2 (the red curve) are 780.0 eV and 795.6 eV, respectively. Furthermore, the peak of P 2p at 132.6 eV could be mainly attributed to the P element in CoPi in Figure 4c (the red curve). Both sets of results confirm the presence of CoPi/Co(OH)2 on the modified Ta3N5.
3.3. UV-Vis Absorption Properties of Ta3N5
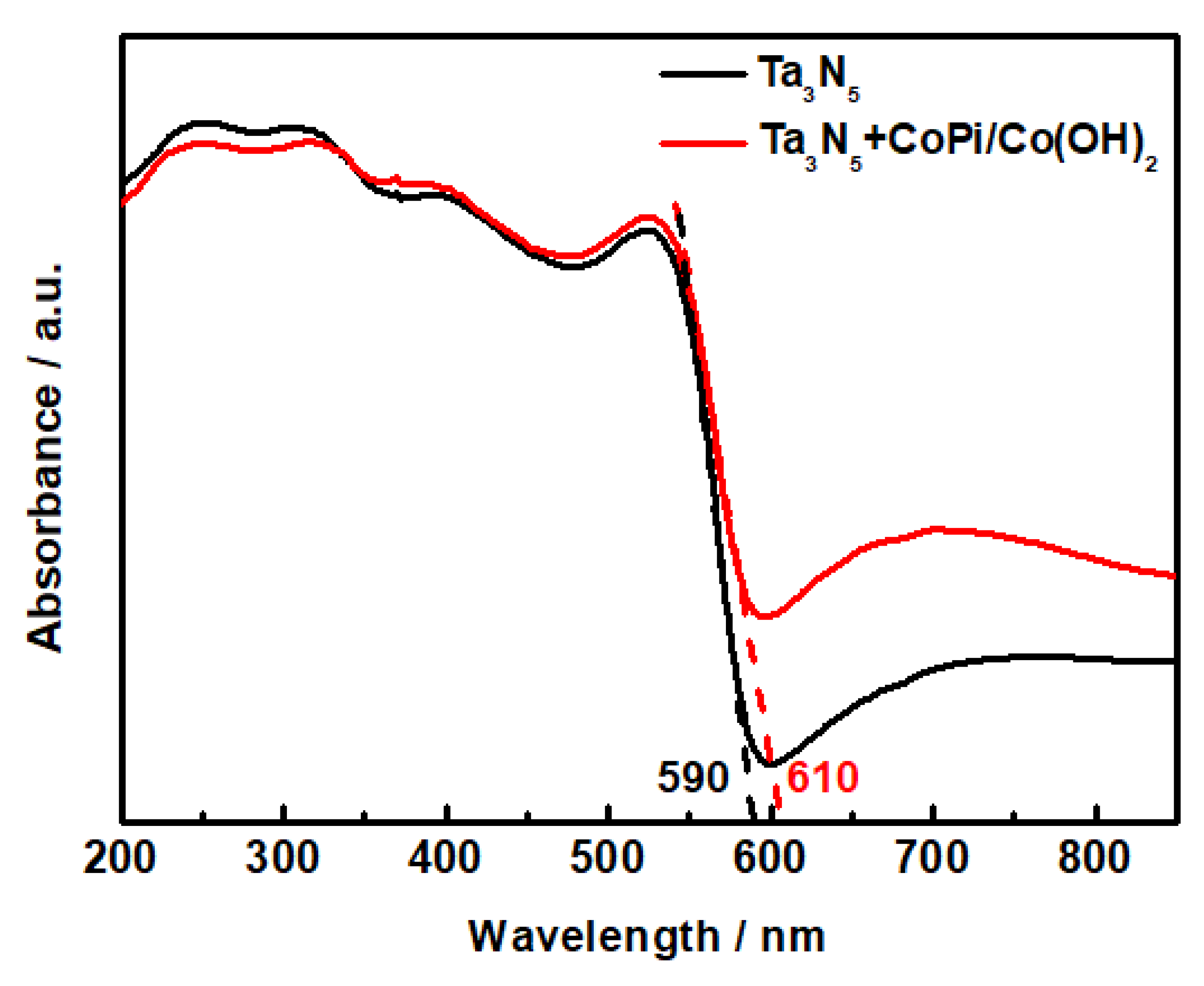
The UV-Vis diffuse reflectance spectrum of the Ta3N5 nanorod-array films with and without modification is shown in Figure 5. The absorption shoulders of both Ta3N5 curves are located deep into the visible region. The absorption spectra of the unmodified and CoPi/Co(OH)2 modified Ta3N5 are 590 nm and 610 nm, respectively, corresponding to band gap energies ΔEg = 2.10 eV and 2.03 eV according to the equation . The light absorption threshold of the CoPi/Co(OH)2 surface-modified Ta3N5 is wider than the unmodified one, hence the narrowed band gap would result in lower photogenerated electron transition energy, stronger photocatalytic activity, and better photoelectric chemical protection performance in theory.
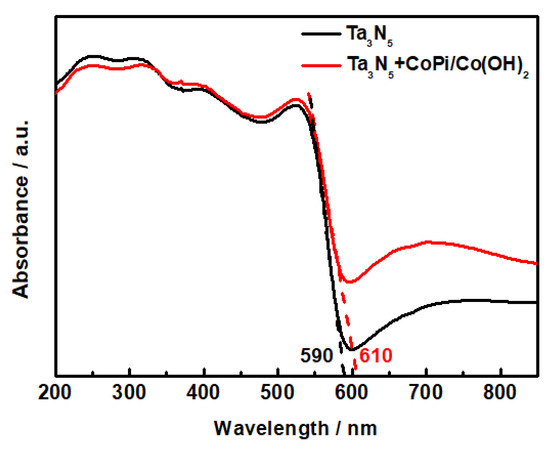
Figure 5.
UV-Vis absorption spectra of the Ta3N5 material with and without CoPi/Co(OH)2 modification.
Ta3N5 is a new type of photocatalytic material with a broad spectral response range, which has a much higher visible absorption value and narrower energy gap than that of the TiO2 photocatalyst. Hara et al. [22] reported the photocatalytic mechanism and energy band structure of TaON, Ta2O5, and Ta3N5 materials and ascribed the small energy gaps of TaON and Ta3N5 to the higher potential energy of the N 2p orbitals compared to the O 2p orbitals, resulting in the higher negative potential of Ta3N5 and the narrowing of the semiconductor energy gap.
3.4. Photoelectrochemical Cathodic Protection Performance of Ta3N5
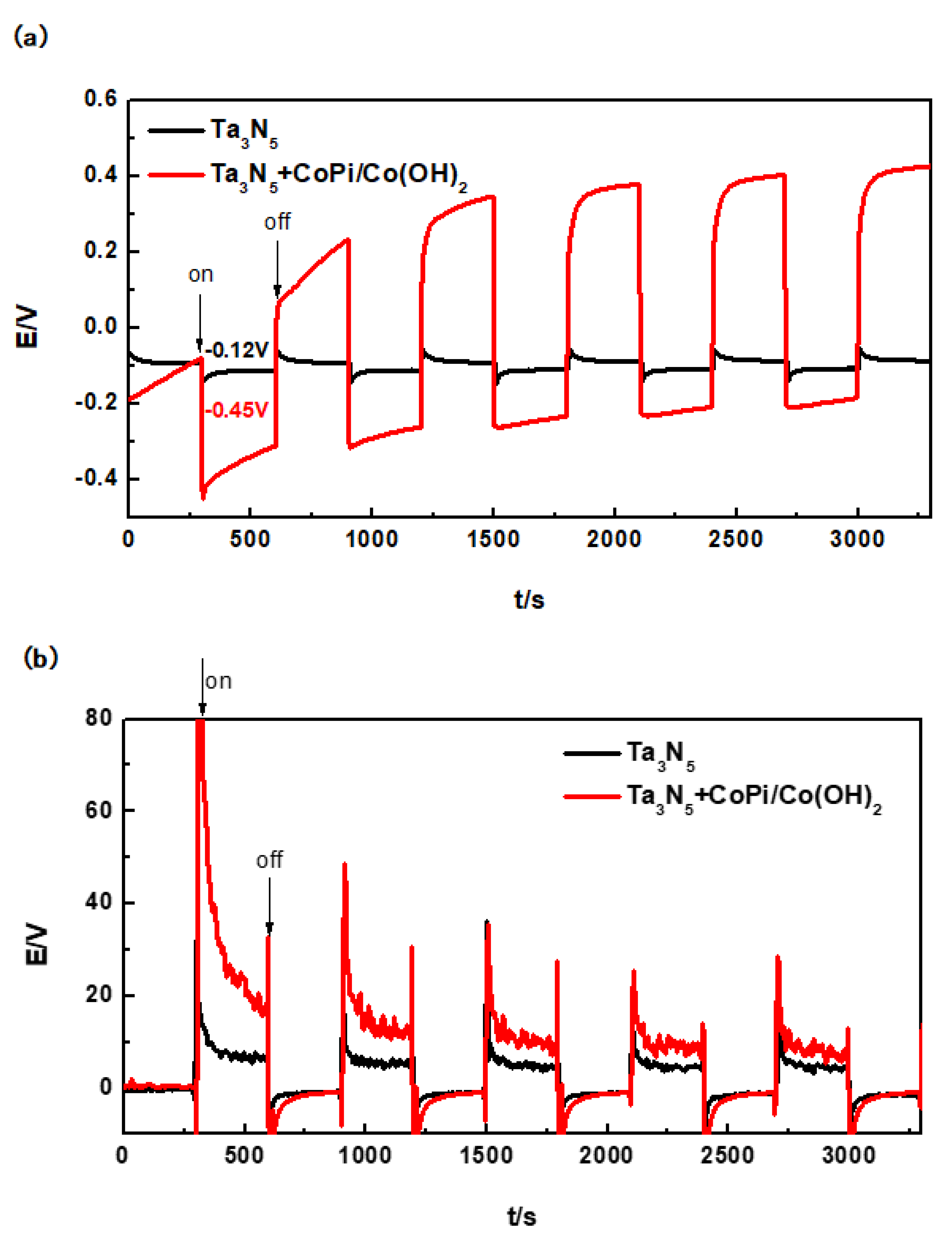
After coupling of the Ta3N5 electrode (with or without CoPi/Co(OH)2 modification) and the 304 stainless steel electrode, their open circuit potentials over time in 3.5 wt% NaCl solution in the dark and under the illumination were tested, as shown in Figure 6a. The duration of either cycle (in the dark and under the illumination) was 300 s, which means that a complete cycle lasted for 600 s.
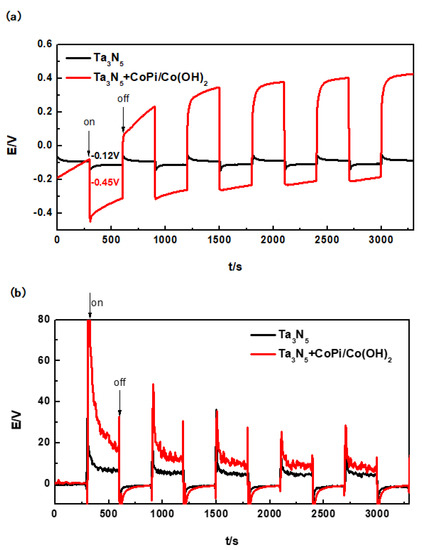
Figure 6.
Evolution of open circuit potentials (a) and photocurrent densities (b) of modified and unmodified Ta3N5 material with and without illumination.
The corrosion potential of the 304 stainless steel was initially determined to be approximately −0.17 V in 3.5 wt% NaCl solution by potentiodynamic polarization measurements. The unmodified Ta3N5 also has photocatalytic activity [23,24,39] and theoretically should generate some degree of protective potential. However, the actual results in Figure 6a show that the potential of the unmodified Ta3N5 could only reach to −0.12 V after the first illumination (300 s). It remained stable under illumination and did not change with the continued illumination. This means that the OCP of the unmodified Ta3N5 (−0.12 V) is more positive than that of 304 stainless steel (−0.17 V). Therefore, the unmodified Ta3N5 cannot achieve electrochemical protection of 304 stainless steel.
For the Ta3N5 modified with CoPi/Co(OH)2, the first photoinduced potential drop reached −0.45 V after 300 s, which is far lower (more cathodic) than the corrosion potential of 304 stainless steel, indicating that the generation and transfer of photogenerated electrons occurred instantaneously. Under this condition, there are no photogenerated holes to start reacting with OH− and H2O in solution, and the electrochemical protection of 304 stainless steel should be largely realizable. Although this potential increases slowly to −0.20 V with prolonged illumination for 3000 s (at the end of illumination cycle), it still remained more negative than the corrosion potential of 304 stainless steel. Our results therefore prove that Ta3N5 modified with CoPi/Co(OH)2 achieved photoelectrochemical cathodic protection of 304 stainless steel within the experimental time interval. However, the rising trend of its potential also indicates that CoPi/Co(OH)2 is probably not stable and its ability to consume holes decreases on the surface of Ta3N5 nanorods. Ta3N5 material is also unstable and easy to be oxidized by photogenerated holes due to the accumulation of holes, so that its photoelectrochemical protective performance is still unstable.
The minimum protection current density of 304 stainless steel is 15 µA/cm2 [29]. As shown in Figure 6b, the instantaneous photocurrent of unmodified Ta3N5 can reach to 32 µA/cm2 (300 s), but it remains stable at about 7 µA/cm2 (600 s). This illustrates that the stable current cannot reach to 15 µA/cm2, so that the 304 stainless steel cannot be protected, consistent with the change law of OCP.
There is an instantaneous rise to more than 80 µA/cm2 that rapidly drops to 15 µA/cm2 within one illumination cycle, and is just sufficient to protect 304 stainless steel within the first cycle. For subsequent cycles, the photocurrent density at the beginning of illumination consistently remained above 20 µA/cm2, and then dropped to around 10 µA/cm2 at the end of cycle. It is therefore obvious that CoPi/Co(OH)2 modification improved the photocurrent of Ta3N5 consistent with the OCP results, and can thus be further investigated as a method for improving the performance of photocatalytic materials. Although the CoPi/Co(OH)2 modified Ta3N5 does protect 304 stainless steel, the long-term performance would require further improvement.
3.5. Mechanism Analysis of Photoelectrochemical Cathodic Protection
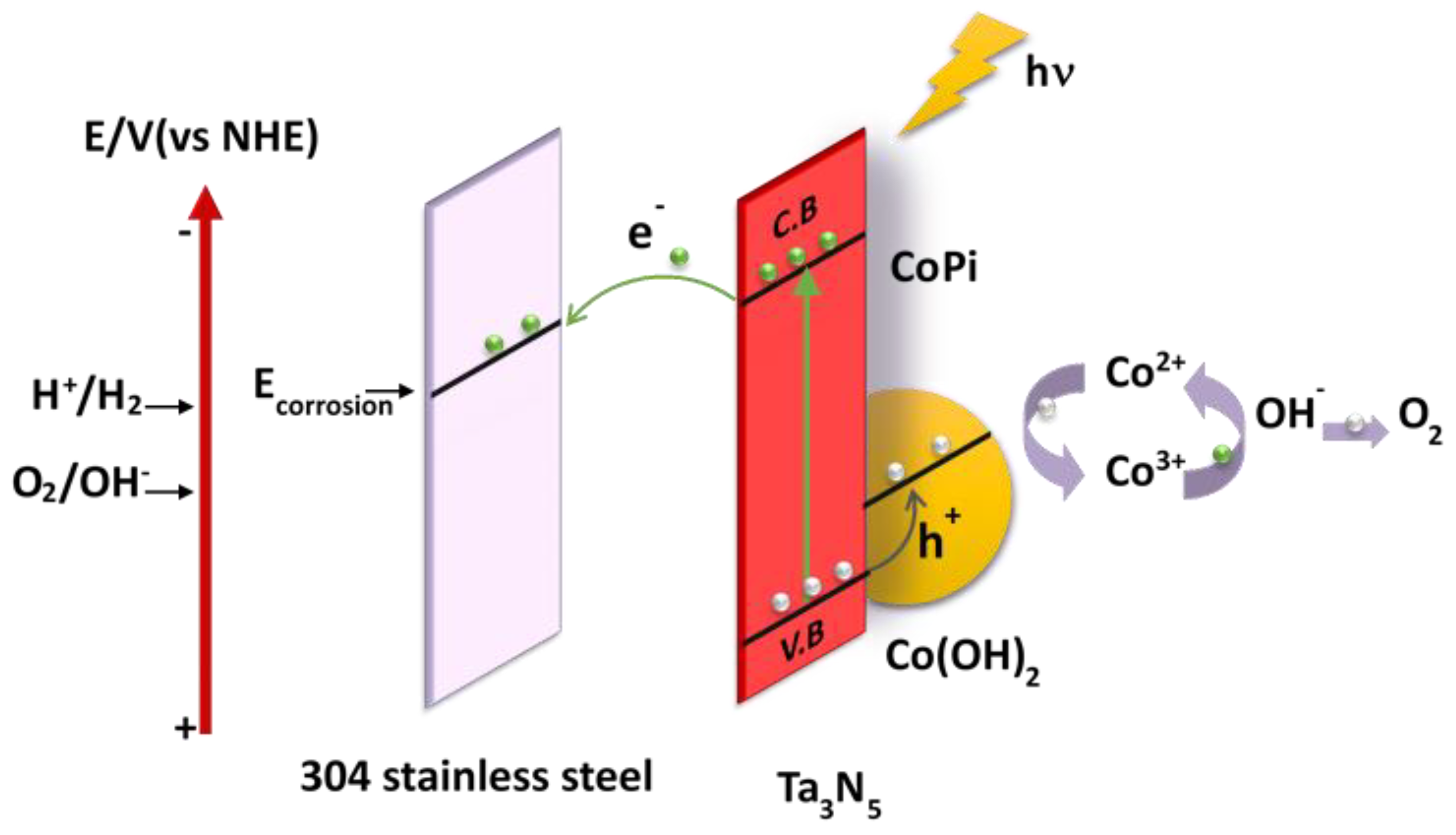
The proposed mechanism of photoelectrochemical cathodic protection for 304 stainless steel under illumination by Ta3N5 modified with CoPi/Co(OH)2 is schematically illustrated in Figure 7. Photogenerated electrons from the Ta3N5 valence band (VB) are excited to the conduction band (CB) under illumination, leaving photogenerated holes and electrons in the valence band and the conduction band, respectively. Subsequently, the photogenerated electrons transfer to the surface of the 304 stainless steel matrix to reduce oxygen, causing the surface potential of the 304 stainless steel to fall below its corrosion potential, thus realizing photoelectrochemical cathodic protection. At the same time, the photogenerated holes transfer from the VB to the CoPi/Co(OH)2 additive to oxidize Co2+ into Co3+ [28].
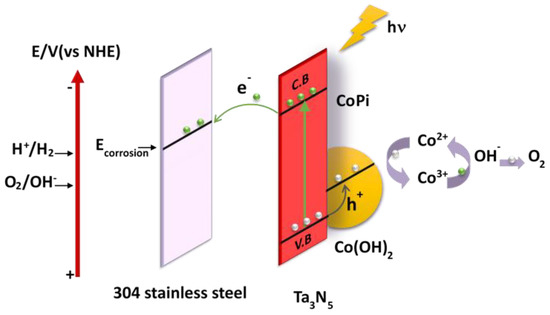
Figure 7.
Proposed mechanism of photoelectrochemical cathodic protection of 304 stainless steel by CoPi/Co(OH)2 modified Ta3N5 films under illumination.
CoPi is strongly adsorbed on the surface of Ta3N5 and provides more adsorption sites and reaction sites in the reaction process. Co(OH)2 is oxidized from Co2+ into Co3+, and then the Co3+ oxidizes H2O to O2. During this process, Co3+ is reduced to Co2+, which again awaits to be reoxidized by the next photogenerated hole. This cycle does not include Co2+ consumption. The valence transitions between Co2+ and Co3+ of the CoPi/Co(OH)2 co-catalysis is favorable for separation and migration of photogenerated charges [33,40,41], and as a result, more holes are consumed, photogenerated charge recombination and the reverse reaction is inhibited, and electron-hole pairs are increased [34,42,43]. Finally, the photostability and photogenerated electrons and holes utilization efficiency of Ta3N5 material are enhanced so that the protective effect on metals is improved.
4. Conclusions
The photoelectrochemical cathodic protection of 304 stainless steel in a 3.5 wt% NaCl solution by a novel broad-spectrum response material—CoPi/Co(OH)2 modified Ta3N5—was studied. The main conclusions are as follows: CoPi/Co(OH)2 modified Ta3N5 can attain a more negative corrosion potential and the minimum protection photocurrent density of 304 stainless steel and as such can theoretically achieve photoelectrochemical cathodic protection for 304 stainless steel. CoPi/Co(OH)2, as co-catalyst, can reduce the activation energy of Ta3N5, promote photogenerated charge separation, consume holes and provide more active sites for electrochemical reactions, thus improving the photoelectric property and greatly enhancing the electrochemical protection for metals. However, long-term performance would require further improvement due to the decline of protection current density at the end of the later illumination cycles.
Author Contributions
X.X.: literature search, data collection, data analysis, writing; L.L.: study design, data analysis, project administration; E.E.O.: writing—review and editing; Y.L.: resources; F.W.: resources.
Funding
This research was funded by the National Natural Science Foundation of China grant number 51622106 and 51871049. And the APC was funded by the National Natural Science Foundation of China grant number 51622106.
Acknowledgments
This investigation was supported by the National Natural Science Foundation of China under the Contract No. 51622106 and 51871049.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fujishima, H. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Chen, Z.; Bu, Y.; Yu, J.; Hou, B. Effect of ZnO on the corrosion of zinc, Q235 carbon steel and 304 stainless steel under white light illumination. Corros. Sci. 2014, 82, 77–84. [Google Scholar] [CrossRef]
- Song, S.; Chen, Z. Effect of UV Illumination on the NaCl-Induced Atmospheric Corrosion of Pure Zinc. J. Electrochem. Soc. 2014, 161, C288–C293. [Google Scholar] [CrossRef]
- Hasanov, R.; Bilge, S.; Bilgiç, S.; Gece, G.; Kılıç, Z. Experimental and theoretical calculations on corrosion inhibition of steel in 1M H2SO4 by crown type polyethers. Corros. Sci. 2010, 52, 984–990. [Google Scholar] [CrossRef]
- Yuan, J.; Tsujikawa, S. Photo-Effects of Sol-Gel Derived TiO2 Coating on Carbon Steel in Alkaline Solution. Zairyo-to-Kankyo 1995, 44, 534–542. [Google Scholar] [CrossRef]
- Tatsuma, T.; Saitoh, S.; Ohko, Y.; Fujishima, A. TiO2-WO3 photoelectrochemical anticorrosion system with an energy storage ability. Chem. Mater. 2001, 13, 2838–2842. [Google Scholar] [CrossRef]
- Wei, N.; Liu, Y.; Zhang, T.; Liang, J.; Wang, D. Hydrogenated TiO2 nanotube arrays with enhanced photoelectrochemical property for photocathodic protection under visible light. Mater. Lett. 2016, 185, 81–84. [Google Scholar] [CrossRef]
- Lin, Z.; Lai, Y.; Hu, R.; Li, J.; Du, R.; Lin, C. A highly efficient ZnS/CdS@TiO2 photoelectrode for photogenerated cathodic protection of metals. Electrochim. Acta 2010, 55, 8717–8723. [Google Scholar] [CrossRef]
- Brzezinska, M.; Garcia-Munoz, P.; Ruppert, A.M.; Keller, N. Photoactive ZnO Materials for Solar Light-Induced CuxO-ZnO Catalyst Preparation. Materials (Basel) 2018, 11, 2260. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.-F.; Liu, Q.; Gao, Y.-B.; Du, R.-G.; Lin, C.-J. SnO2 nanoparticle films prepared by pulse current deposition for photocathodic protection of stainless steel. J. Electrochem. Soc. 2015, 162, C161–C166. [Google Scholar] [CrossRef]
- Pan, J.; Liu, G.; Lu, G.Q.; Cheng, H.M. On the true photoreactivity order of {001}, {010}, and {101} facets of anatase TiO2 crystals. Angew. Chem. 2011, 123, 2181–2185. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Yang, J.; Yan, H.; Wang, X.; Wen, F.; Wang, Z.; Fan, D.; Shi, J.; Li, C. Roles of cocatalysts in Pt–PdS/CdS with exceptionally high quantum efficiency for photocatalytic hydrogen production. J. Catal. 2012, 290, 151–157. [Google Scholar] [CrossRef]
- Bu, Y.; Chen, Z.; Li, W. Dramatically enhanced photocatalytic properties of Ag-modified graphene–ZnO quasi-shell–core heterojunction composite material. RSC Adv. 2013, 3, 24118–24125. [Google Scholar] [CrossRef]
- Xie, Y.P.; Yu, Z.B.; Liu, G.; Ma, X.L.; Cheng, H.-M. CdS–mesoporous ZnS core–shell particles for efficient and stable photocatalytic hydrogen evolution under visible light. Energy Environ. Sci. 2014, 7, 1895–1901. [Google Scholar] [CrossRef]
- Wang, X.; Wei, Q.; Zhang, L.; Sun, H.; Li, H.; Zhang, Q. CdTe/TiO2 nanocomposite material for photogenerated cathodic protection of 304 stainless steel. Mater. Sci. Eng. B 2016, 208, 22–28. [Google Scholar] [CrossRef]
- Wang, D.; Li, R.; Zhu, J.; Shi, J.; Han, J.; Zong, X.; Li, C. Photocatalytic Water Oxidation on BiVO4with the Electrocatalyst as an Oxidation Cocatalyst: Essential Relations between Electrocatalyst and Photocatalyst. J. Phys. Chem. C 2012, 116, 5082–5089. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Wang, H.; Chen, Z.; Hu, J.; Xu, K.; Liu, J. Ta3N5-Pt nonwoven cloth with hierarchical nanopores as efficient and easily recyclable macroscale photocatalysts. Sci. Rep. 2014, 4, 3978. [Google Scholar] [CrossRef]
- Wu, X.C.; Tao, Y.R.; Li, L.; Bando, Y.; Golberg, D. Centimeter-long Ta3N5 nanobelts: Synthesis, electrical transport, and photoconductive properties. Nanotechnology 2013, 24, 175701. [Google Scholar] [CrossRef]
- Yokoyama, D.; Hashiguchi, H.; Maeda, K.; Minegishi, T.; Takata, T.; Abe, R.; Kubota, J.; Domen, K. Ta3N5 photoanodes for water splitting prepared by sputtering. Thin Solid Films 2011, 519, 2087–2092. [Google Scholar] [CrossRef]
- Higashi, M.; Domen, K.; Abe, R. Fabrication of efficient TaON and Ta3N5 photoanodes for water splitting under visible light irradiation. Energy Environ. Sci. 2011, 4, 4138–4147. [Google Scholar] [CrossRef]
- Hara, M.; Hitoki, G.; Takata, T.; Kondo, J.N.; Kobayashi, H.; Domen, K. TaON and Ta3N5 as new visible light driven photocatalysts. Catal. Today 2003, 78, 555–560. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, X.; Tang, X.; Luo, Y.; Zeng, Q.; Deng, X.; Ding, S.; Sun, Y. Gold nanoparticles embedded in Ta2O5/Ta3N5 as active visible-light plasmonic photocatalysts for solar hydrogen evolution. J. Mater. Chem. A 2014, 2, 14927–14939. [Google Scholar] [CrossRef]
- Zhen, C.; Wang, L.; Liu, G.; Lu, G.Q.; Cheng, H.M. Template-free synthesis of Ta3N5 nanorod arrays for efficient photoelectrochemical water splitting. Chem. Commun. 2013, 49, 3019–3021. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, S.; Ge, L.; Han, C.; Gao, Y.; Dai, D. In-situ synthesis of novel plate-like Co(OH) 2 co-catalyst decorated TiO 2 nanosheets with efficient photocatalytic H 2 evolution activity. Int. J. Hydrogen Energy 2017, 42, 22877–22886. [Google Scholar] [CrossRef]
- Liu, H.; Xue, Q.; Zhao, J.; Zhang, Q. Enhanced supercapacitive performance of binary cooperative complementary Co(OH)2/Mn3O4 nanomaterials directly synthesized through ion diffusion method controlled by ion exchange membrane. Electrochim. Acta 2018, 260, 330–337. [Google Scholar] [CrossRef]
- An, Y.; Xu, B.; Liu, Y.; Wang, Z.; Wang, P.; Dai, Y.; Qin, X.; Zhang, X.; Huang, B. Photocatalytic Overall Water Splitting over MIL-125(Ti) upon CoPi and Pt Co-catalyst Deposition. ChemistryOpen 2017, 6, 701–705. [Google Scholar] [CrossRef]
- Wang, P.; Xu, S.; Xia, Y.; Wang, X.; Yu, H.; Yu, J. Synergistic effect of CoPi-hole and Cu(ii)-electron cocatalysts for enhanced photocatalytic activity and photoinduced stability of Ag3PO4. Phys. Chem. Chem. Phys. 2017, 19, 10309–10316. [Google Scholar] [CrossRef]
- Xie, X.; Liu, L.; Chen, R.; Liu, G.; Li, Y.; Wang, F. Long-Term Photoelectrochemical Cathodic Protection by Co(OH)2-Modified TiO2 on 304 Stainless Steel in Marine Environment. J. Electrochem. Soc. 2018, 165, H3154–H3163. [Google Scholar] [CrossRef]
- Wang, L.; Dionigi, F.; Nguyen, N.T.; Kirchgeorg, R.; Gliech, M.; Grigorescu, S.; Strasser, P.; Schmuki, P. Tantalum Nitride Nanorod Arrays: Introducing Ni–Fe Layered Double Hydroxides as a Cocatalyst Strongly Stabilizing Photoanodes in Water Splitting. Chem. Mater. 2015, 27, 2360–2366. [Google Scholar] [CrossRef]
- Hutchings, G.S.; Zhang, Y.; Li, J.; Yonemoto, B.T.; Zhou, X.; Zhu, K.; Jiao, F. In situ formation of cobalt oxide nanocubanes as efficient oxygen evolution catalysts. J. Am. Chem. Soc. 2015, 137, 4223–4229. [Google Scholar] [CrossRef] [PubMed]
- Irshad, A.; Munichandraiah, N. An oxygen evolution Co-Ac catalyst--the synergistic effect of phosphate ions. Phys. Chem. Chem. Phys. 2014, 16, 5412–5422. [Google Scholar] [CrossRef] [PubMed]
- Qorbani, M.; Naseri, N.; Moshfegh, A.Z. Hierarchical Co3O4/Co(OH)2Nanoflakes as a Supercapacitor Electrode: Experimental and Semi-Empirical Model. ACS Appl. Mater. Interfaces 2015, 7, 11172–11179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zang, S.; Wang, X. Layered Co(OH)2 Deposited Polymeric Carbon Nitrides for Photocatalytic Water Oxidation. ACS Catal. 2015, 5, 941–947. [Google Scholar] [CrossRef]
- Sun, M.; Chen, Z.; Bu, Y. Enhanced photoelectrochemical cathodic protection performance of the C3N4@In2O3 nanocomposite with quasi-shell–core structure under visible light. J. Alloys Compd. 2015, 618, 734–741. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Wang, N.; Ning, X.; Li, H.; Lu, D.; Liu, X.; Zhang, Q.; Huang, Y. Bi2Se3 Sensitized TiO2 Nanotube Films for Photogenerated Cathodic Protection of 304 Stainless Steel Under Visible Light. Nanoscale Res. Lett. 2018, 13, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Liu, P.; Li, Y.; Yang, D.; Yang, H.; Zhao, H. A new vapor-phase hydrothermal method to concurrently grow ZnO nanotube and nanorod array films on different sides of a zinc foil substrate. Chemistry 2012, 18, 5165–5169. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhang, H.; Liu, H.; Wang, Y.; Yao, X.; Zhu, G.; Zhang, S.; Zhao, H. A Facile Vapor-Phase Hydrothermal Method for Direct Growth of Titanate Nanotubes on a Titanium Substrate via a Distinctive Nanosheet Roll-Up Mechanism. J. Am. Chem. Soc. 2011, 133, 19032–19035. [Google Scholar] [CrossRef]
- Feng, X.; Latempa, T.J.; Basham, J.I.; Mor, G.K.; Varghese, O.K.; Grimes, C.A. Ta3N5 nanotube arrays for visible light water photoelectrolysis. Nano Lett. 2010, 10, 948–952. [Google Scholar] [CrossRef]
- Nellist, M.R.; Laskowski, F.A.L.; Qiu, J.; Hajibabaei, H.; Sivula, K.; Hamann, T.W.; Boettcher, S.W. Potential-sensing electrochemical atomic force microscopy for in operando analysis of water-splitting catalysts and interfaces. Nat. Energy 2018, 3, 46. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Shen, Y.; Liang, Y.; Li, H. Synthesis and characterization of Co(OH)2/TiO2 nanotube composites as supercapacitor materials. J. Solid State Electrochem. 2006, 11, 853–858. [Google Scholar] [CrossRef]
- Hou, J.; Wang, Z.; Yang, C.; Cheng, H.; Jiao, S.; Zhu, H. Cobalt-bilayer catalyst decorated Ta3N5 nanorod arrays as integrated electrodes for photoelectrochemical water oxidation. Energy Environ. Sci. 2013, 6, 3322–3330. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).