Three-Dimensional Porous Ti3C2Tx-NiO Composite Electrodes with Enhanced Electrochemical Performance for Supercapacitors
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of Ti3AlC2
2.2. Synthesis of Ti3C2Tx MXene
2.3. Preparation of 3D Porous Ti3C2Tx-NiO Composites Electrodes
2.4. Material Characterization
2.5. Electrochemical Performance Tests
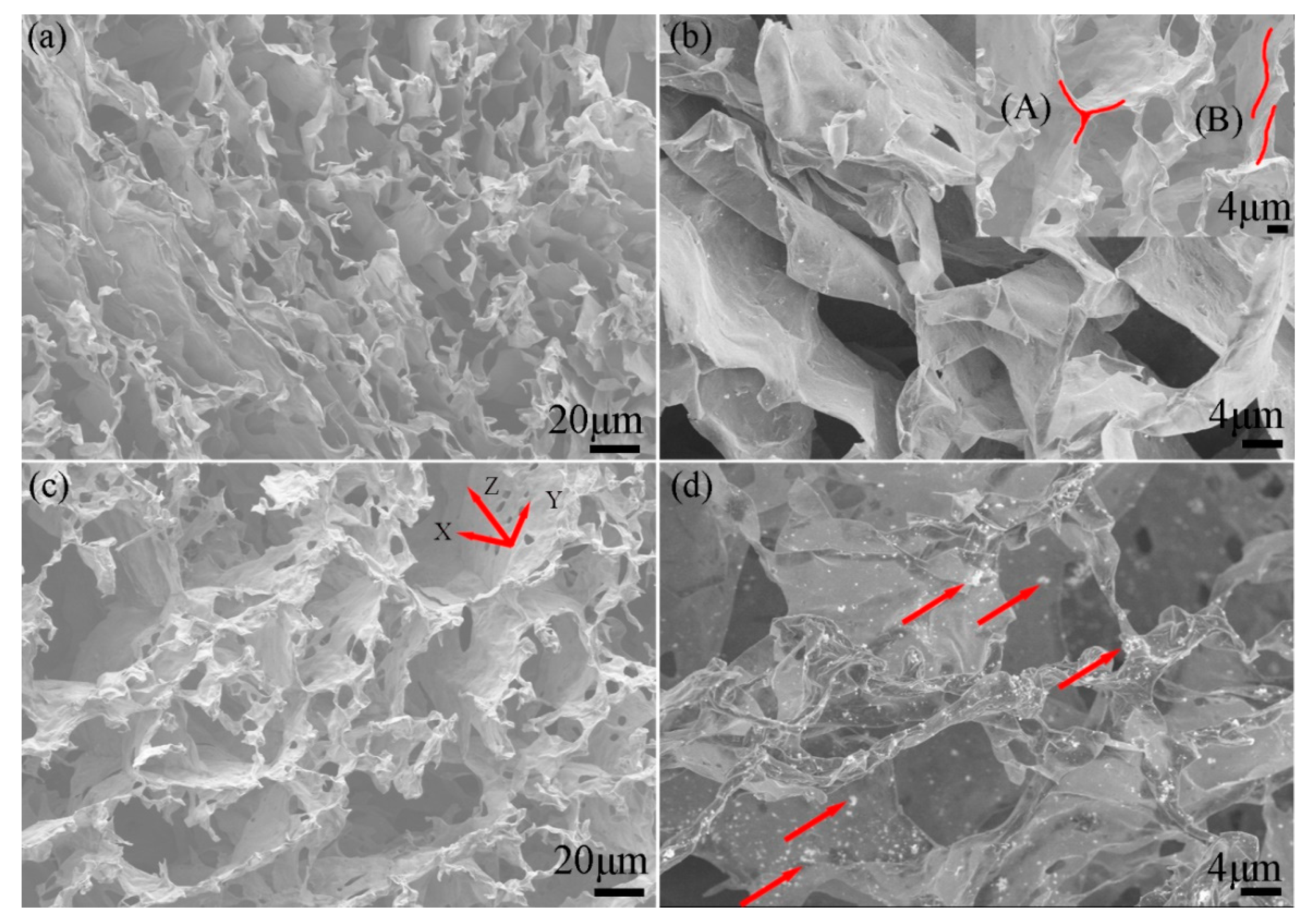
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.A.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kim, N.H.; Kuila, T.; Lau, K.T.; Lee, J.H. Electrochemical performance of a graphene-polypyrrole nanocomposite as a supercapacitor electrode. Nanotechnology 2011, 22, 295202. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, K.; Lu, H.; Yang, Y.; Nutt, S. Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J. Mater. Chem. 2009, 19, 7098–7105. [Google Scholar] [CrossRef]
- Lee, J.; Chae, H.; Won, Y.J.; Lee, K.; Lee, C.; Lee, H.H.; Kim, I.C.; Lee, J.M. Graphene oxide nanoplatelets composite membrane with hydrophilic and antifouling properties for wastewater treatment. J. Membr. Sci. 2013, 448, 223–230. [Google Scholar] [CrossRef]
- Lei, Y.; Chen, F.; Luo, Y.; Zhang, L. Synthesis of three-dimensional graphene oxide foam for the removal of heavy metal ions. Chem. Phys. Lett. 2014, 593, 122–127. [Google Scholar] [CrossRef]
- Kim, H.; Macosko, C.W. Processing-property relationships of polycarbonate/graphene composites. Polymer 2009, 50, 3797–3809. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Yao, J.; Park, J. Graphene nanosheets for enhanced lithium storage in lithium ion batteries. Carbon 2009, 47, 2049–2053. [Google Scholar] [CrossRef]
- Wang, H.; Cui, L.; Yang, Y.; Sanchez Casalongue, H.; Robinson, J.T.; Liang, Y.; Cui, Y.; Dai, H. Mn3O4−Graphene Hybrid as a High-Capacity Anode Material for Lithium Ion Batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Ying, G.; Dillon, A.D.; Fafarman, A.T.; Barsoum, M.W. Transparent, conductive solution processed spincast 2D Ti2CTx (MXene) films. Mater. Res. Lett. 2017, 5, 391–398. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A New Family of Promising Hydrogen Storage Medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, O.; Lukatskaya, M.R.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Amine-Assisted Delamination of Nb2C MXene for Li-Ion Energy Storage Devices. Adv. Mater. 2015, 27, 3501–3506. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.; Kota, S.; Dillion, A.D.; Fafarman, A.T.; Barsoum, M.W. Conductive Transparent V2CTx (MXene) Films. FlatChem 2018, 8, 25–30. [Google Scholar] [CrossRef]
- Qian, A.; Hyeon, S.E.; Seo, J.Y.; Chung, C. Capacitance changes associated with cation-transport in free-standing flexible Ti3C2Tx (T O, F, OH) MXene film electrodes. Electrochim. Acta 2018, 266, 86–93. [Google Scholar] [CrossRef]
- Lin, Z.; Barbara, D.; Taberna, P.; Van Aken, K.L.; Anasori, B.; Gogotsi, Y.; Simon, P. Capacitance of Ti3C2Tx MXene in ionic liquid electrolyte. J. Power Sources 2016, 326, 575–579. [Google Scholar] [CrossRef]
- Sun, H.; Xu, Z.; Gao, C. Multifunctional, Ultra-Flyweight, Synergistically Assembled Carbon Aerogels. Adv. Mater. 2013, 25, 2554–2560. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.F.; Zhang, X.D.; Liang, H.W.; Kong, M.; Guan, Q.F.; Chen, P.; Wu, Z.-Y.; Yu, S.-H. Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 2012, 6, 7092–7102. [Google Scholar] [CrossRef]
- Du, R.; Tian, X.; Yao, J.; Sun, Y.; Jin, J.; Zhang, Y.; Liu, Y. Controlled synthesis of three-dimensional reduced graphene oxide networks for application in electrode of supercapacitor. Diam. Relat. Mater. 2016, 70, 186–193. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.; Lee, S.; Kuo, W. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908–7912. [Google Scholar] [CrossRef]
- Qian, Y.; Ismail, I.M.; Stein, A. Ultralight, high-surface-area, multifunctional graphene-based aerogels from self-assembly of graphene oxide and resol. Carbon 2014, 68, 221–231. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, M.; Zhang, Q.; Dong, F.; Zhou, Y. Multifunctional g-C3N4/graphene oxide wrapped sponge monoliths as highly efficient adsorbent and photocatalyst. Appl. Catal. B Environ. 2018, 235, 17–25. [Google Scholar] [CrossRef]
- Zhao, J.; Ren, W.; Cheng, H. Graphene sponge for efficient and repeatable adsorption and desorption of water contaminations. J. Mater. Chem. 2012, 22, 20197–20202. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, N.; Ma, Y.; Wang, S.; Liu, W.; Luo, C.; Zhang, H.; Cheng, F.; Rao, J.; Hu, X.; et al. Highly Self-Healable 3D Microsupercapacitor with MXene–Graphene Composite Aerogel. ACS Nano 2018, 12, 4224–4232. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Tang, X.; Guo, X.; Choi, S.; Wang, C.; Gogotsi, Y.; Wang, G. Porous Cryo-Dried MXene for Efficient Capacitive Deionization. Joule 2018, 2, 778–787. [Google Scholar] [CrossRef]
- Yang, H.Z.; Zou, J.P. Controllable preparation of hierarchical NiO hollow microspheres with high pseudo-capacitance. Trans. Nonferr. Met. Soc. China 2018, 28, 1808–1818. [Google Scholar] [CrossRef]
- Bi, R.R.; Wu, X.L.; Cao, F.F.; Jiang, L.Y.; Guo, Y.G.; Wan, L.J. Highly Dispersed RuO2 Nanoparticles on Carbon Nanotubes: Facile Synthesis and Enhanced Supercapacitance Performance. J. Phys. Chem. C 2010, 114, 2448–2451. [Google Scholar] [CrossRef]
- Eskusson, J.; Rauwel, P.; Nerut, J.; Jänes, A. A Hybrid Capacitor Based on Fe3O4-Graphene Nanocomposite/Few-Layer Graphene in Different Aqueous Electrolytes. J. Electrochem. Soc. 2016, 163, A2768–A2775. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, S.; Dang, G.; Min, F.; Li, H.; Zhang, Q.; Xie, J. Solvothermal Synthesized γ-Fe2O3/graphite Composite for Supercapacitor. Int. J. Electrochem. Sci. 2017, 12, 6292–6303. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Q.; Brett, D.J.L.; He, G.; Wang, R.; Key, J.; Ji, S. Double-shelled tremella-like NiO@Co3O4 @MnO2 as a high-performance cathode material for alkaline supercapacitors. J. Power Sources 2017, 343, 76–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, H.; Wu, X.; Wang, L.; Zhang, A.; Xia, T.; Dong, H.; Li, X.; Zhang, L. Progress of electrochemical capacitor electrode materials: A review. Int. J. Hydrog. Energy 2009, 34, 4889–4899. [Google Scholar] [CrossRef]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Gupta, V.; Kawaguchi, T.; Miura, N. Synthesis and electrochemical behavior of nanostructured cauliflower-shape Co–Ni/Co–Ni oxides composites. Mater. Res. Bull. 2009, 44, 202–206. [Google Scholar] [CrossRef]
- Han, D.; Jing, X.; Wang, J.; Yang, P.; Song, D.; Liu, J. Porous lanthanum doped NiO microspheres for supercapacitor application. J. Electroanal. Chem. 2012, 682, 37–44. [Google Scholar] [CrossRef]
- Su, X.; Zhang, J.; Mu, H.; Zhao, J.; Wang, Z.; Zhao, Z.; Han, C.; Ye, Z. Effects of etching temperature and ball milling on the preparation and capacitance of Ti3C2 MXene. J. Alloys Compd. 2018, 752, 32–39. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, Y.; Tan, Y.; Yang, S.; Feng, X.; Müllen, K. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Li, Z.; Hu, T.; Zhu, S.; Zhang, C.; Wang, X. High-Capacitance Mechanism for Ti3C2Tx MXene by in Situ Electrochemical Raman Spectroscopy Investigation. ACS Nano 2016, 10, 11344–11350. [Google Scholar] [CrossRef] [PubMed]
- AL-Osta, A.; Jadhav, V.V.; Zate, M.K.; Mane, R.S.; Hui, K.N.; Han, S. Electrochemical supercapacitors of anodized-brass-templated NiO nanostrutured electrodes. Scr. Mater. 2015, 99, 29–32. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Wang, S.; Chen, C.; Chien, T.; Lee, P.; Lin, C. Supercapacitive properties of spray pyrolyzed iron-added manganese oxide powders deposited by electrophoretic deposition technique. Thin Solid Films 2008, 517, 1234–1238. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, W.; Zhu, J.; Zhao, W.; Ma, J.; Mhaisalkar, S.; Maria, T.L.; Yang, Y.; Zhang, H.; Hng, H.H.; et al. Synthesis of porous NiO nanocrystals with controllable surface area and their application as supercapacitor electrodes. Nano Res. 2010, 3, 643–652. [Google Scholar] [CrossRef]
- Zheng, Y.; Ding, H.; Zhang, M. Preparation and electrochemical properties of nickel oxide as a supercapacitor electrode material. Mater. Res. Bull. 2009, 44, 403–407. [Google Scholar] [CrossRef]




| Materials | Solvent | Absorption Capacity | Reference | |
|---|---|---|---|---|
| Ms/MM (g/g) | Ms/VM (g/cm3) | |||
| porous 3D Ti3C2Tx | deionized water | 22 | 72.6 | This work |
| machine oils | 14.6 | 48.2 | ||
| g-C3N4/GO-wrapped sponge | n-hexane | 49.8 | - | [22] |
| UFA | crude oils | 290 | - | [17] |
| graphene sponge | oils | 129 | - | [23] |
| graphene-based sponge | soybean oil | - | 1.12 | [20] |
| chloroform | - | 1.86 | ||
| Materials | Specific Capacitance | Capacitance Retention (%) | Electrolyte | Refs. |
|---|---|---|---|---|
| porous 3D Ti3C2Tx | 266 F/cm3, 67 F/g 2 mV/s | 100% over 2500 cycles | 1 M Na2SO4 | This work |
| porous 3D Ti3C2Tx-20 wt. % NiO | 291 F/cm3, 72 F/g 2 mv/s | - | 1 M Na2SO4 | |
| porous 3D Ti3C2Tx-33 wt. % NiO | 336 F/cm3, 85 F/g 2 mv/s | - | 1 M Na2SO4 | |
| porous 3D Ti3C2Tx-50 wt. % NiO | 341 F/cm3, 77 F/g 2 mv/s | 114% over 2500 cycles | 1 M Na2SO4 | |
| porous 3D Ti3C2Tx-67 wt. % NiO | 283 F/cm3, 56 F/g 2 mv/s | - | 1 M Na2SO4 | |
| Graphene oxide and resol aerogel | 99 F/g a, 100 mA/g | 97% over 10,000 cycles | 6 M KOH | [21] |
| rGO aerogel/NF | 366 F/g, 2 A/g | 60% over 1000 cycles | 6 M KOH | [19] |
| 3D GA-based mesoporous carbon | 226 F/g, 1 mv/s | 142% over 5000 cycles | 1 M H2SO4 | [36] |
| porous carbon nanofibers | 202 F/g, 1 A/g | 97% over 3000 cycles | 6 M KOH | [18] |
| porous MXene | 410 F/cm3, 5 mv/s | 103% over5000 cycles | 1 M NaCl | [25] |
| 3D MXene-rGO aerogel | 34.6 mF/cm2 a, 1 mv/s | 91% over 15,000 cycles | - | [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Ying, G.; Liu, L.; Ma, F.; Su, L.; Zhang, C.; Wu, D.; Wang, X.; Zhou, Y. Three-Dimensional Porous Ti3C2Tx-NiO Composite Electrodes with Enhanced Electrochemical Performance for Supercapacitors. Materials 2019, 12, 188. https://doi.org/10.3390/ma12010188
Zhang K, Ying G, Liu L, Ma F, Su L, Zhang C, Wu D, Wang X, Zhou Y. Three-Dimensional Porous Ti3C2Tx-NiO Composite Electrodes with Enhanced Electrochemical Performance for Supercapacitors. Materials. 2019; 12(1):188. https://doi.org/10.3390/ma12010188
Chicago/Turabian StyleZhang, Kaicheng, Guobing Ying, Lu Liu, Fengchen Ma, Lin Su, Chen Zhang, Donghai Wu, Xiang Wang, and Ying Zhou. 2019. "Three-Dimensional Porous Ti3C2Tx-NiO Composite Electrodes with Enhanced Electrochemical Performance for Supercapacitors" Materials 12, no. 1: 188. https://doi.org/10.3390/ma12010188
APA StyleZhang, K., Ying, G., Liu, L., Ma, F., Su, L., Zhang, C., Wu, D., Wang, X., & Zhou, Y. (2019). Three-Dimensional Porous Ti3C2Tx-NiO Composite Electrodes with Enhanced Electrochemical Performance for Supercapacitors. Materials, 12(1), 188. https://doi.org/10.3390/ma12010188







