The Influence of Inter-Cooling and Electromagnetic Stirring above Liquidus on the Formation of Primary Al3Zr and Grain Refinement in an Al-0.2%Zr Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
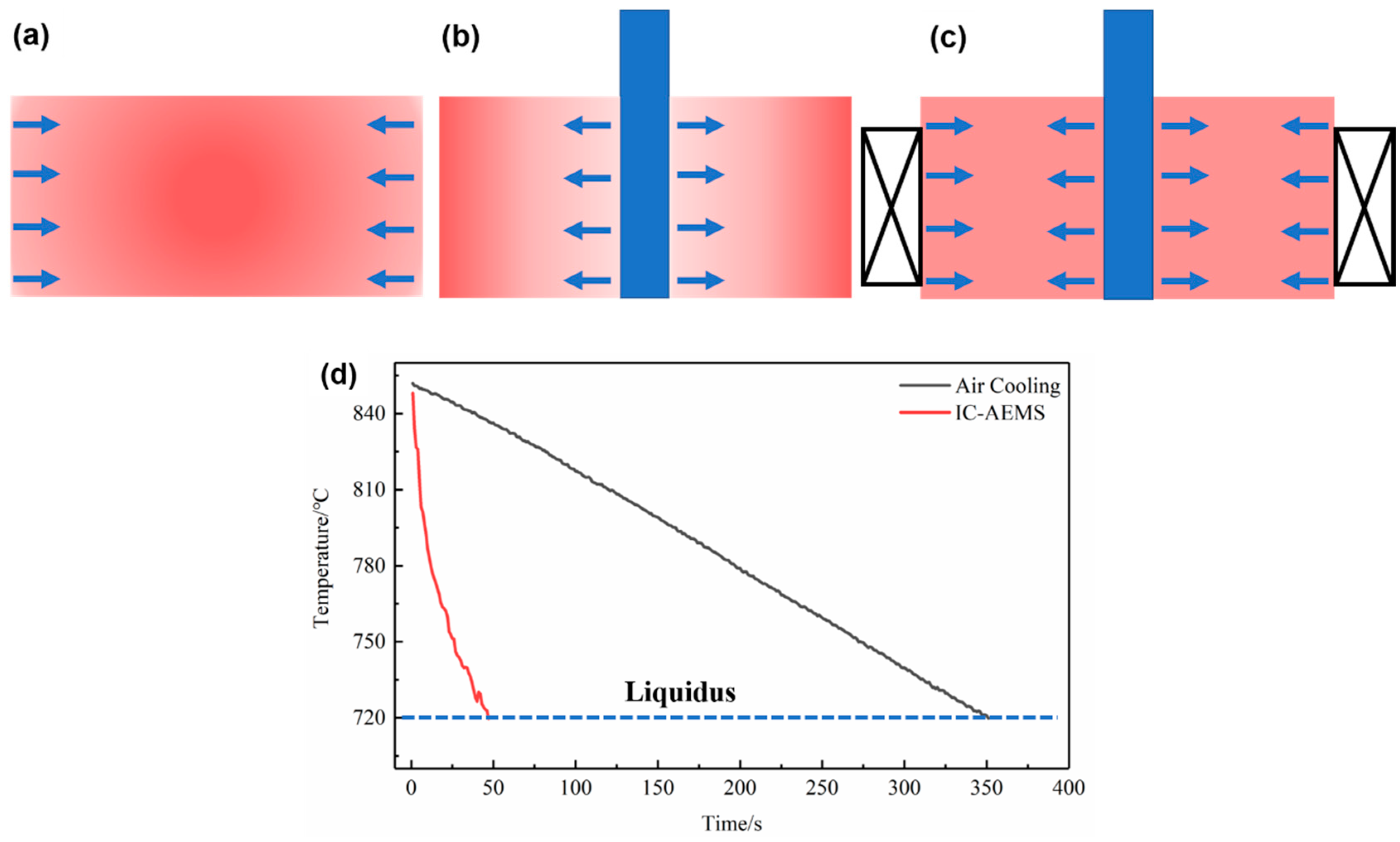
2.2. Melt Treatment Procedure
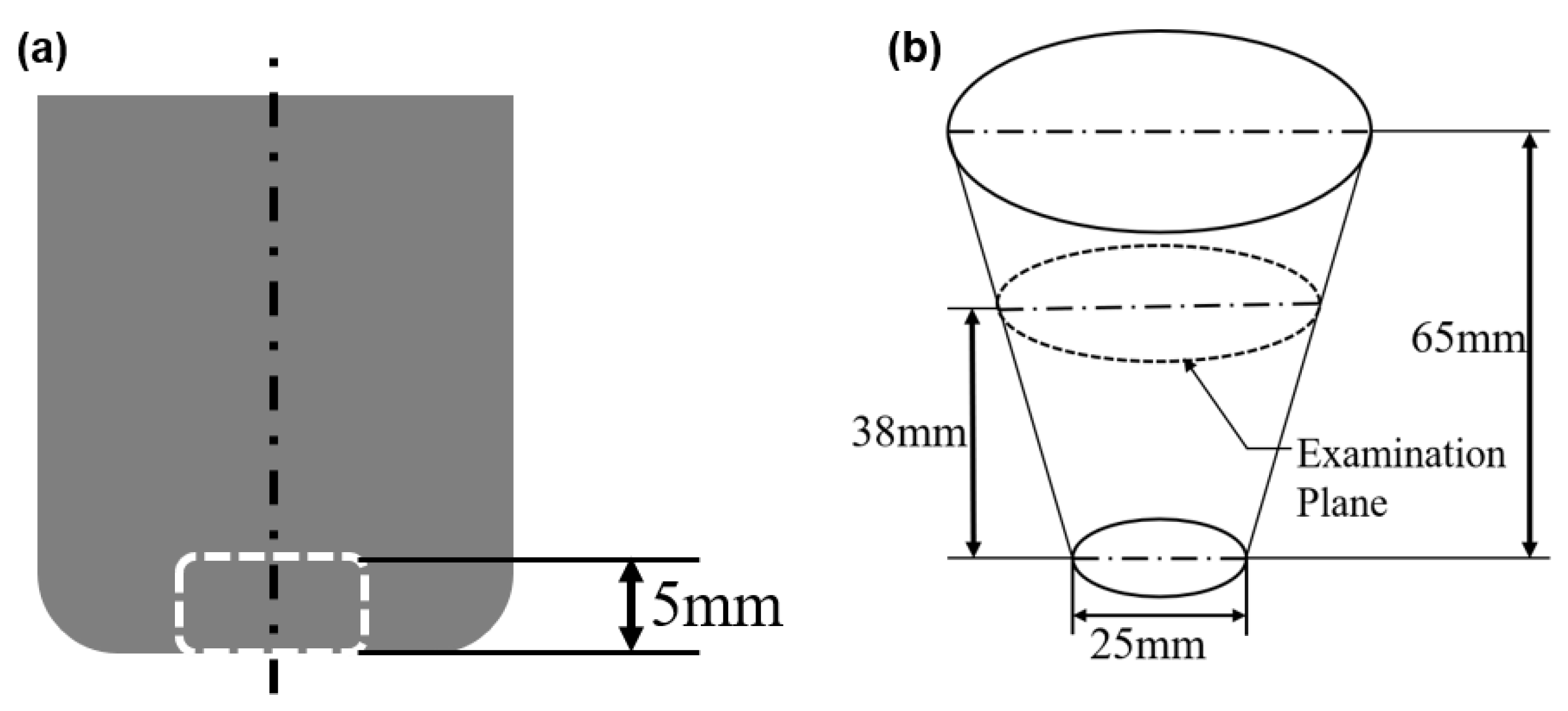
2.3. Sample Assessment
3. Results
3.1. The Effect of IC-AEMS on Grain Refinement in Al-0.2%Zr Alloys
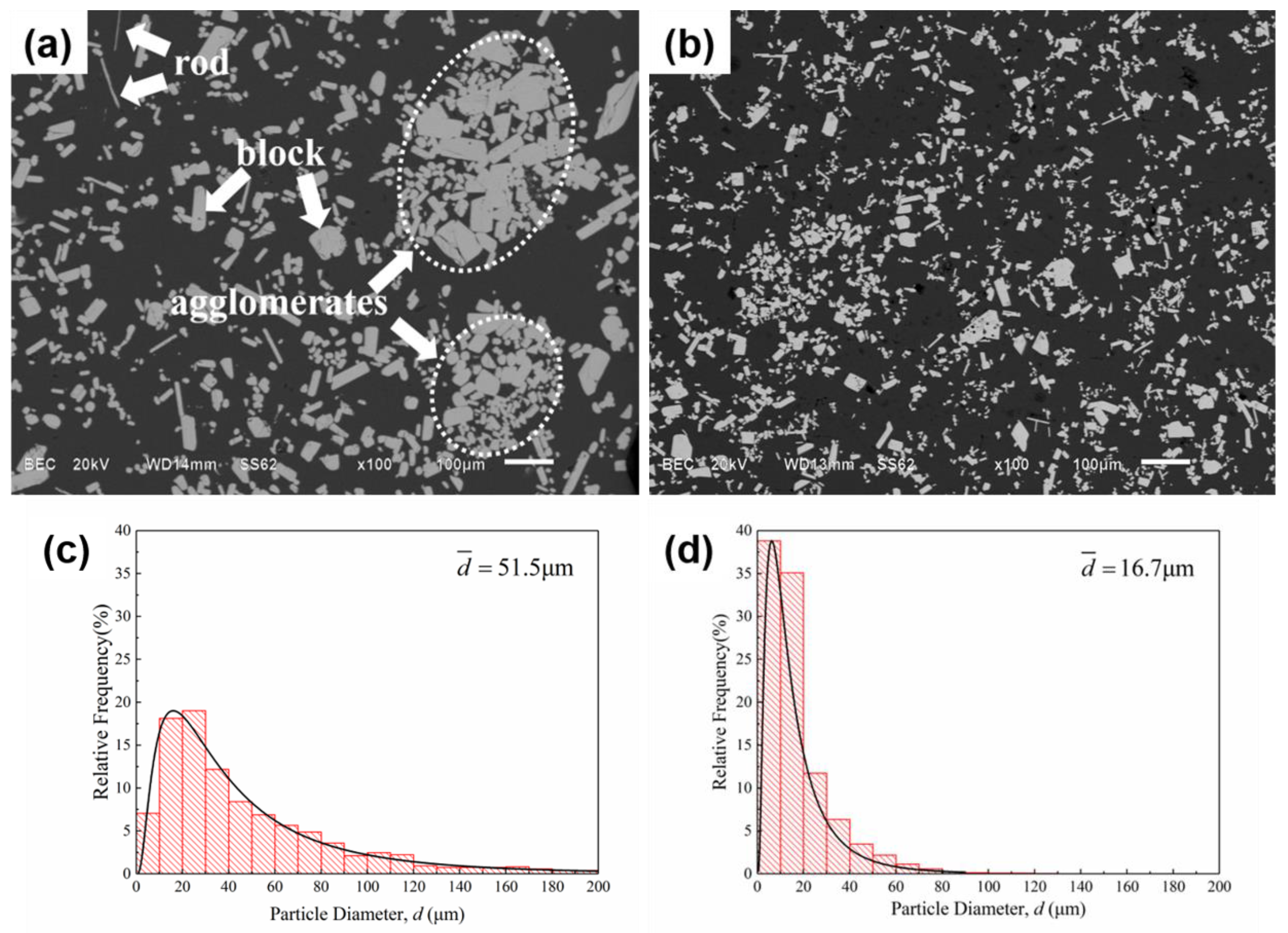
3.2. The Distribution of Primary Al3Zr Particles
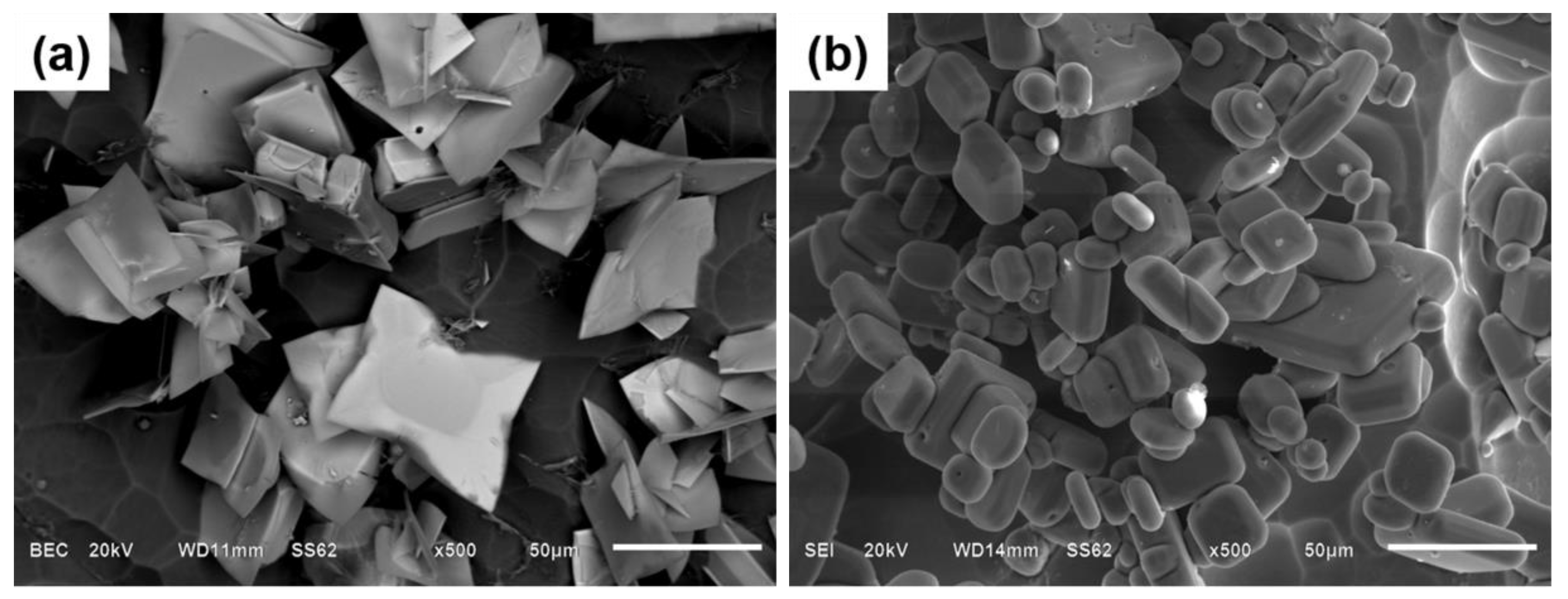
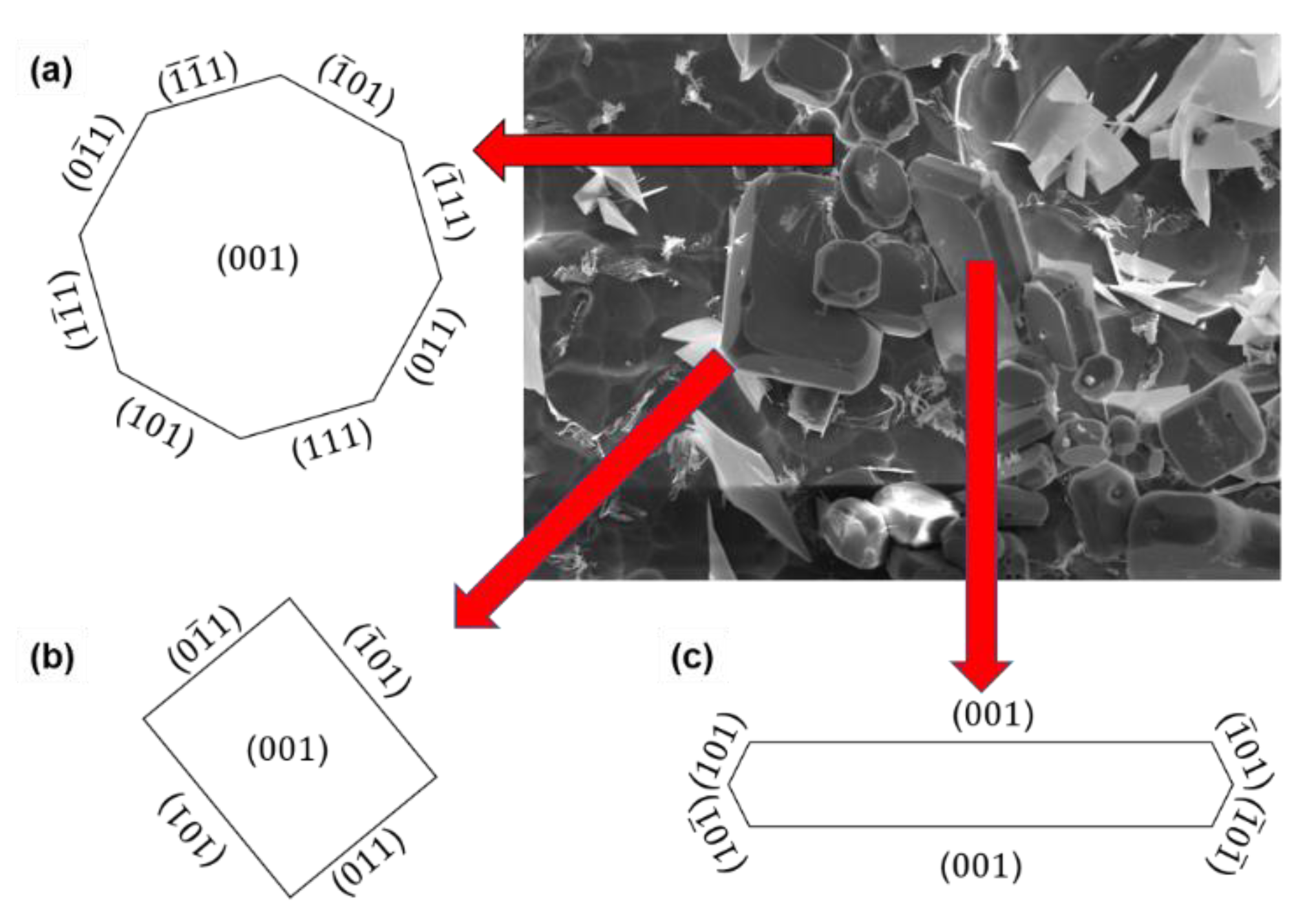
3.3. The Morphology of Primary Al3Zr Particles
4. Discussion
5. Conclusions
- (1)
- The Al3Zr particles have only a minor potency when the alloy is poured at 720 °C; the average grain size is reduced from 1383 μm to 797 μm after addition of the Al-10%Zr master alloy. However, there is significant refinement due to IC-AEMS, with an average grain size that is reduced from 797 μm to 354 μm.
- (2)
- IC-AEMS above the liquidus impacts grain refinement due to a reduction in the size of Al3Zr particles and their increased density. There is also a more uniform distribution of fine particles in the matrix. The mean particle diameter decreased from 51.5 μm to 16.7 μm, and the morphology of particles transformed from a plate/blocky shape with four fast-growing crystallographic directions to small block-like erythrocyte.
- (3)
- The impact of IC-AEMS on grain refinement is attributed to the improved Al3Zr precipitates, which act as heterogeneous nuclei in the melt. The use of IC-AEMS further distributes heat and improves the composition above the liquidus. The refinement can be jointly promoted by two hypotheses of pre-nucleation and explosive nucleation.
Author Contributions
Funding
Conflicts of Interest
References
- Easton, M.A.; Qian, M.A.; StJohn, D.H. Grain Refinement in Alloys: Novel Approaches. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Haghayeghi, R.; Kapranos, P. An investigation on physical and chemical refinement of aerospace aluminium alloys. Mater. Lett. 2013, 95, 121–124. [Google Scholar] [CrossRef]
- Guan, R.-G.; Tie, D. A Review on Grain Refinement of Aluminum Alloys: Progresses, Challenges and Prospects. Acta Met. Sin. (Engl. Lett.) 2017, 30, 409–432. [Google Scholar] [CrossRef]
- Rao, A.A.; Murty, B.S.; Chakraborty, M. Role of zirconium and impurities in grain refinement of aluminium lNith AI-Ti-B. Mater. Sci. Technol. 1997, 13, 769–777. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Xia, Q.; Liu, Y. Grain Refinement of the Al-Cu-Mg-Ag Alloy with Er and Sc Additions. Met. Mater. Trans. A 2007, 38, 2853–2858. [Google Scholar] [CrossRef]
- Tzeng, Y.-C.; Jian, S.-Y. Effects of the addition of trace amounts of Sc on the microstructure and mechanical properties of Al-11.6Si alloys. Mater. Sci. Eng. A 2018, 723, 22–28. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Z.; Qiu, D.; Taylor, J.A.; Easton, M.A.; Zhang, M.-X. Revisiting the role of peritectics in grain refinement of Al alloys. Acta Mater. 2013, 61, 360–370. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, D.; Liu, Z.-L.; Taylor, J.A.; Easton, M.A.; Zhang, M.-X. The grain refinement mechanism of cast aluminium by zirconium. Acta Mater. 2013, 61, 5636–5645. [Google Scholar] [CrossRef]
- Li, P.; Kandalova, E.G.; Nikitin, V.I. Grain refining performance of Al–Ti master alloys with different microstructures. Mater. Lett. 2005, 59, 723–727. [Google Scholar] [CrossRef]
- Greer, A.L. Grain refinement of alloys by inoculation of melts. Philos. Trans. R. Soc. A 2003, 361, 479. [Google Scholar] [CrossRef]
- StJohn, D.H.; Qian, M.; Easton, M.A.; Cao, P. The Interdependence Theory: The relationship between grain formation and nucleant selection. Acta Mater. 2011, 59, 4907–4921. [Google Scholar] [CrossRef]
- Quested, T.E.; Greer, A.L. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Qiu, D.; Taylor, J.A.; Zhang, M.X.; Kelly, P.M. A mechanism for the poisoning effect of silicon on the grain refinement of Al–Si alloys. Acta Mater. 2007, 55, 1447–1456. [Google Scholar] [CrossRef]
- Aquilano, D.; Costa, E.; Genovese, A.; Massaro, F.R.; Rubbo, M. Heterogeneous nucleation and growth of crystalline micro-bubbles around gas cavities formed in solution. Prog. Cryst. Growth Charact. Mater. 2003, 46, 59–84. [Google Scholar] [CrossRef]
- Campanella, T.; Charbon, C.; Rappaz, M. Grain refinement induced by electromagnetic stirring: A dendrite fragmentation criterion. Metall. Mater. Trans. A 2004, 35, 3201–3210. [Google Scholar] [CrossRef]
- Ramirez, A.; Qian, M.; Davis, B.; Wilks, T.; StJohn, D.H. Potency of high-intensity ultrasonic treatment for grain refinement of magnesium alloys. Scr. Mater. 2008, 59, 19–22. [Google Scholar] [CrossRef]
- Gao, Z.; Hu, Y.; Wang, J.; Yang, J.; Li, J. Effect of electromagnetic melt treatment near liquidus on the formation of non-dendrite microstructure of superalloy. Acta. Metall. Sin-Engl. 2014, 12, 1471–1477. [Google Scholar]
- Haghayeghi, R.; Nastac, L. On microstructural refinement of an AA7449 aluminium alloy through shearing above liquidus temperature. Mater. Lett. 2011, 65, 3230–3233. [Google Scholar] [CrossRef]
- Wang, F.; Eskin, D.; Connolley, T.; Mi, J. Effect of ultrasonic melt treatment on the refinement of primary Al3Ti intermetallic in an Al–0.4Ti alloy. J. Cryst. Growth 2016, 435, 24–30. [Google Scholar] [CrossRef]
- Guan, T.Y.; Zhang, Z.F.; Bai, Y.L.; Wang, P. Effect of A-EMS above Liquidus Temperature on Structure Refinement of Al-11%Zn-3%Mg-1%Cu-0.13%Zr Alloy. Mater. Sci. Forum 2017, 898, 24–28. [Google Scholar] [CrossRef]
- Bai, Y.-L.; Xu, J.; Zhang, Z.-F.; Shi, L.-K. Annulus electromagnetic stirring for preparing semisolid A357 aluminum alloy slurry. Trans. Nonferr. Met. Soc. China 2009, 19, 1104–1109. [Google Scholar] [CrossRef]
- ASTM. Standard Test Procedure for Aluminium Alloy Grain Refiners: TP-1; The Aluminium Association: Washington, DC, USA, 1987. [Google Scholar]
- Dyson, F.J. Electron Spin Resonance Absorption in Metals. II. Theory of Electron Diffusion and the Skin Effect. Phys. Rev. 1955, 98, 349–359. [Google Scholar] [CrossRef]
- Spencer, D.; Mehrabian, R.; Flemings, M.C. Rheological behavior of Sn-15 pct Pb in the crystallization range. Metall. Trans. 1972, 3, 1925–1932. [Google Scholar] [CrossRef]
- Nadella, R.; Eskin, D.G.; Du, Q.; Katgerman, L. Macrosegregation in direct-chill casting of aluminium alloys. Prog. Mater. Sci. 2008, 53, 421–480. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Gao, F.; Peng, G.S.; Alba-Baena, N. Effect of potent TiB2 addition levels and impurities on the grain refinement of Al. J. Alloys Compd. 2016, 689, 401–407. [Google Scholar] [CrossRef]
- Zhang, M.X.; Kelly, P.M.; Easton, M.A.; Taylor, J.A. Crystallographic study of grain refinement in aluminum alloys using the edge-to-edge matching model. Acta Mater. 2005, 53, 1427–1438. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, F.; Wang, J.; Wang, Q.; Wang, W.; Cui, J. Effects of cooling rate on solidification structure of Al-5Zr master alloys. Chin. J. Nonferr. Met. 2017, 8–14. [Google Scholar]
- Li, F.; Zhu, Q.; Li, L.; Jia, Z.; Shao, B.; Cui, J. Effect of Pouring Temperature on the Primary Al3Zr Phase in Al-5Zr Master Alloy. Rare Met. Mater. Eng. 2015, 44, 2029–2033. [Google Scholar]
- Zhang, L.; Jiang, H.; Zhao, J.; He, J. Microstructure and grain refining efficiency of Al-5Ti-1B master alloys prepared by halide salt route. J. Mater. Process. Technol. 2017, 246, 205–210. [Google Scholar] [CrossRef]
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef] [Green Version]
- Erdemir, D.; Lee, A.Y.; Myerson, A.S. Nucleation of Crystals from Solution: Classical and Two-Step Models. Acc. Chem. Res. 2009, 42, 621–629. [Google Scholar] [CrossRef]
- Vives, C.; Perry, C. Effects of electromagnetic stirring during the controlled solidification of tin. Int. J. Heat Mass Transf. 1986, 29, 21–33. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Esling, C.; Jiang, H.; Zhao, Z.; Zuo, Y.; Cui, J. Crystallographic features of the primary Al3Zr phase in as-cast Al-1.36wt% Zr alloy. J. Cryst. Growth 2011, 316, 172–176. [Google Scholar] [CrossRef]



| Zr | Fe | Si | Al |
|---|---|---|---|
| 0.217 | 0.005 | 0.007 | Bal. |
| Alloy | Temperature of the Phase Formation during Solidification (°C) | Temperature Range of Melt Treatment (°C) | Treatment Condition | Casting Condition | |
|---|---|---|---|---|---|
| Mold | Cooling Rate, °C/s | ||||
| Al | 660(Al) | 850–720 | - | TP-1 | 3.5 |
| Al-0.2%Zr | 720(Al3Zr) | - | TP-1 | 3.5 | |
| Al-0.2%Zr | 720(Al3Zr) | IC-AEMS | TP-1 | 3.5 | |
| Al-0.2%Zr | 720(Al3Zr) | - | Graphite crucible | 0.4 | |
| Al-0.2%Zr | 720(Al3Zr) | IC-AEMS | Graphite crucible | 0.4 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, T.; Zhang, Z.; Bai, Y.; He, M.; Zheng, H.; Zhao, H.; Li, X.; Wang, P. The Influence of Inter-Cooling and Electromagnetic Stirring above Liquidus on the Formation of Primary Al3Zr and Grain Refinement in an Al-0.2%Zr Alloy. Materials 2019, 12, 22. https://doi.org/10.3390/ma12010022
Guan T, Zhang Z, Bai Y, He M, Zheng H, Zhao H, Li X, Wang P. The Influence of Inter-Cooling and Electromagnetic Stirring above Liquidus on the Formation of Primary Al3Zr and Grain Refinement in an Al-0.2%Zr Alloy. Materials. 2019; 12(1):22. https://doi.org/10.3390/ma12010022
Chicago/Turabian StyleGuan, Tianyang, Zhifeng Zhang, Yuelong Bai, Min He, Hansen Zheng, Haodong Zhao, Xiaopeng Li, and Ping Wang. 2019. "The Influence of Inter-Cooling and Electromagnetic Stirring above Liquidus on the Formation of Primary Al3Zr and Grain Refinement in an Al-0.2%Zr Alloy" Materials 12, no. 1: 22. https://doi.org/10.3390/ma12010022





