Manganese Removal from Liquid Nickel by Hydrogen Plasma Arc Melting
Abstract
:1. Introduction
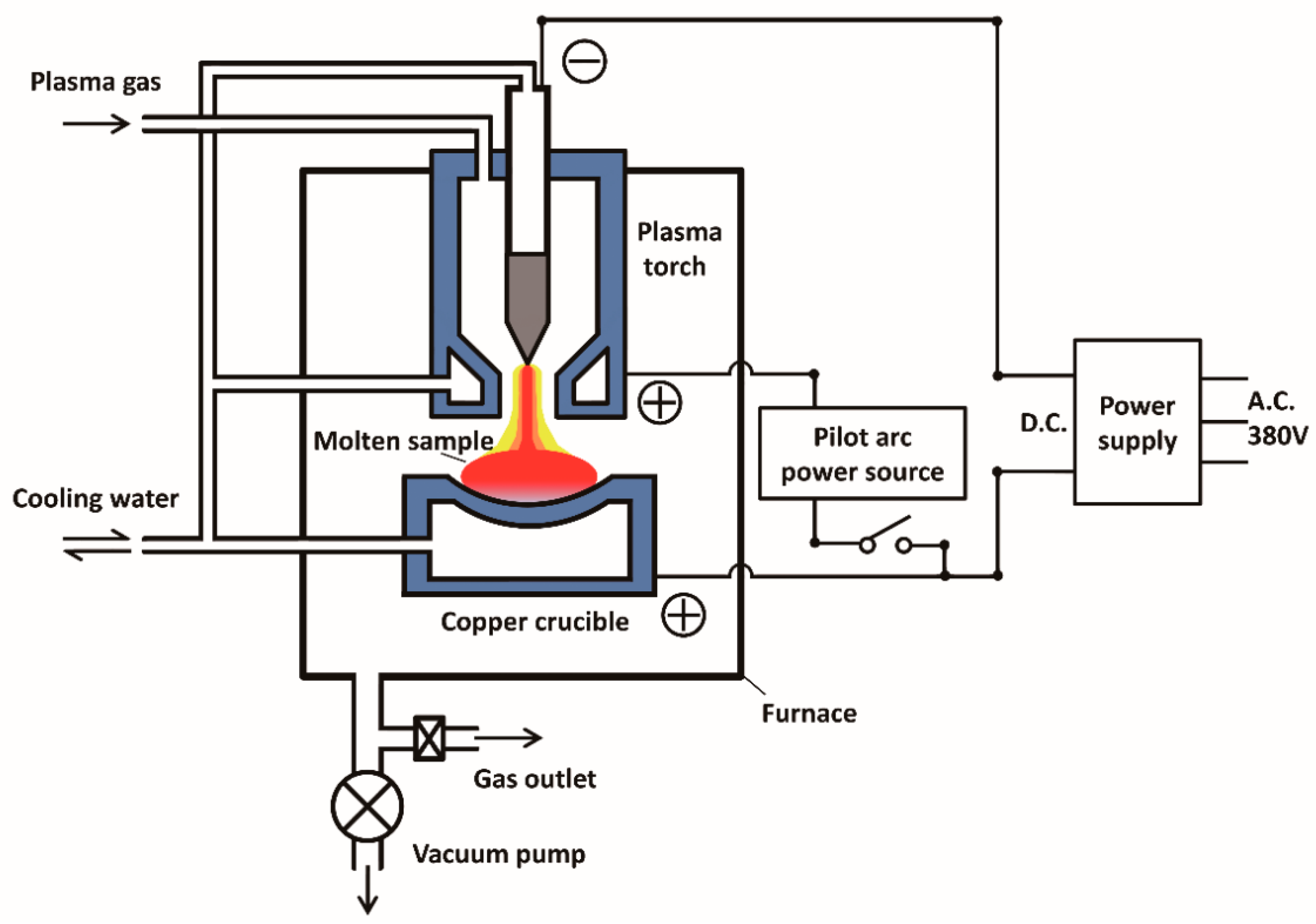
2. Experimental Sections
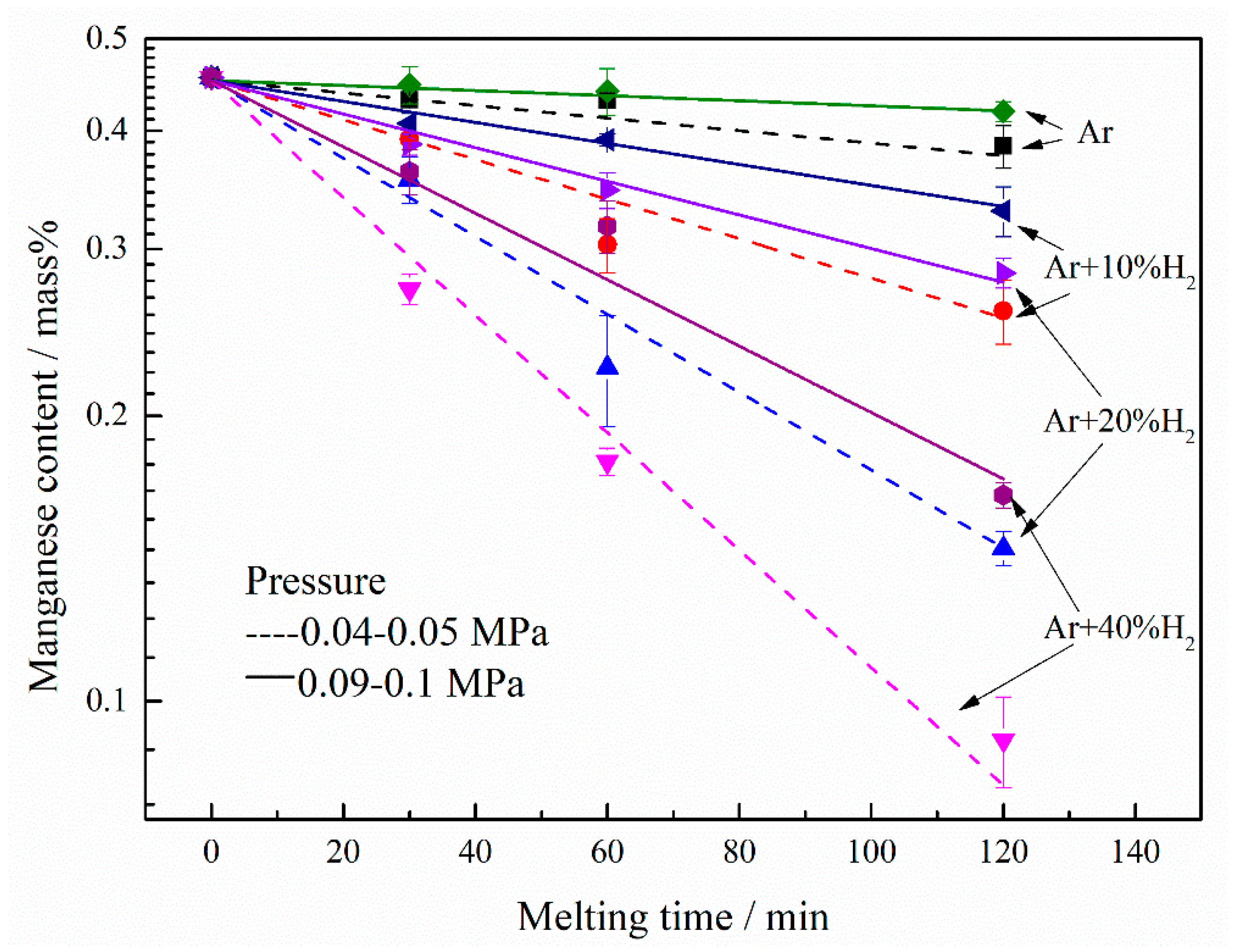
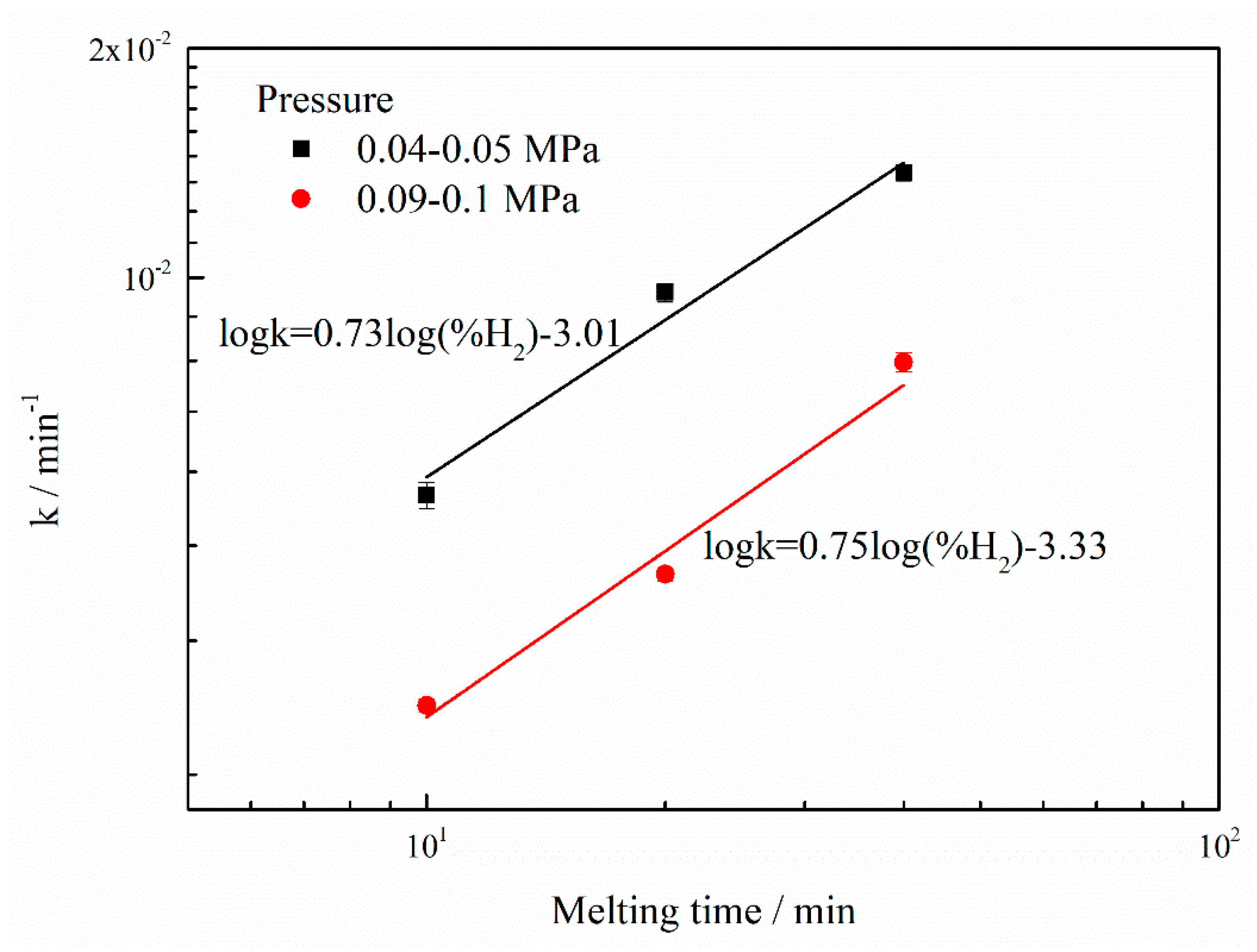
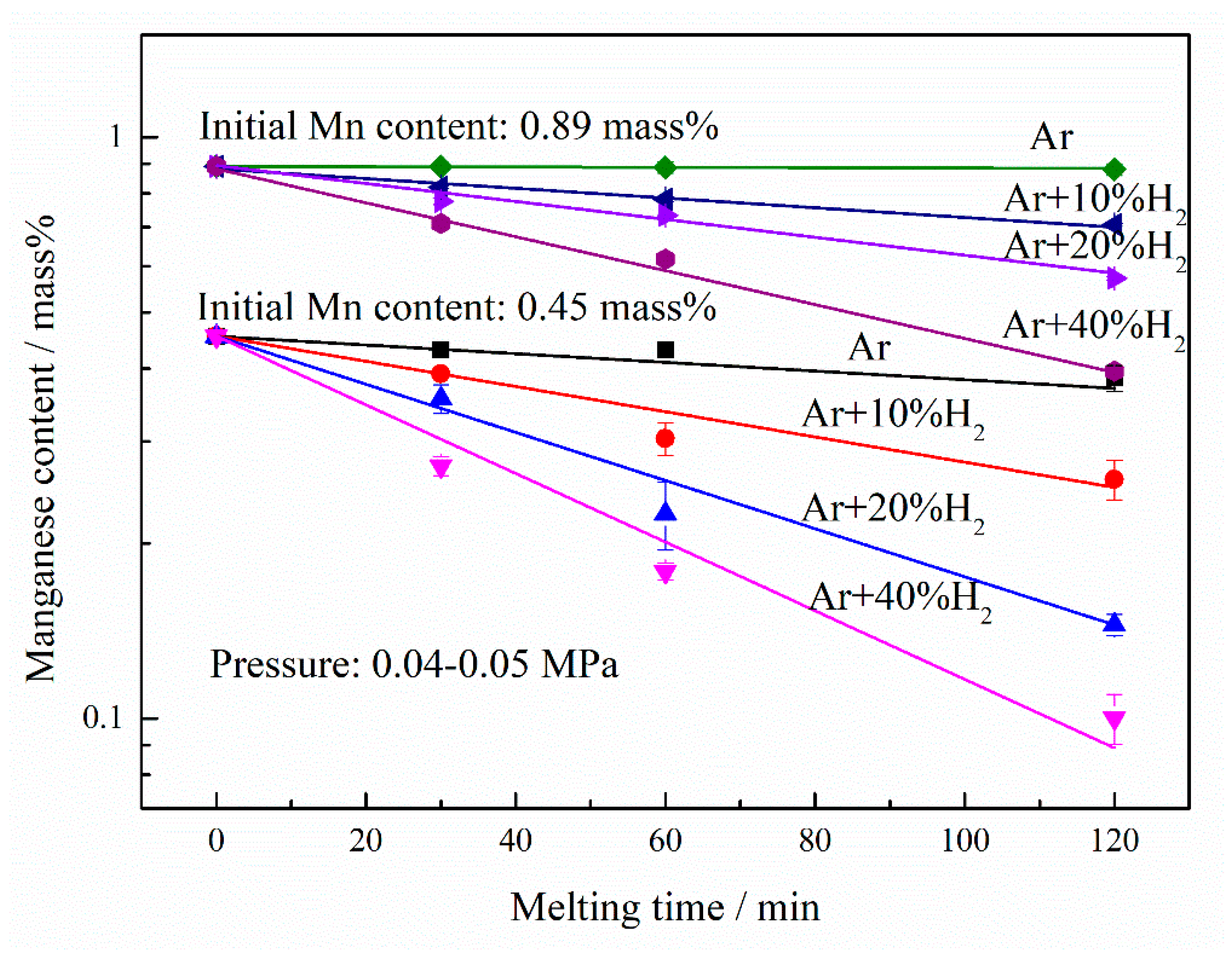
3. Results and discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Waseda, Y.; Isshiki, M. Purification Process and Characterization of Ultra High Purity Metals: Application of Basic Science to Metallurgical Processing; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Shi, L.; Sun, C.; Gao, P.; Zhou, F.; Liu, W. Mechanical properties and wear and corrosion resistance of electrodeposited Ni–Co/SiC nanocomposite coating. Appl. Surface Sci. 2006, 252, 3591–3599. [Google Scholar] [CrossRef]
- Lü, X.; Bao, X.; Huang, Y.; Qu, Y.; Lu, H.; Lu, Z. Mechanisms of cytotoxicity of nickel ions based on gene expression profiles. Biomaterials 2009, 30, 141–148. [Google Scholar] [CrossRef] [PubMed]
- French, B.J. Nickel-Base Alloys Having a Low Coefficient of Thermal Expansion. Google Patents 11 November 1975. [Google Scholar]
- Birol, Y. Ni-based superalloy as a potential tool material for thixoforming of steels. Ironmak. Steelmak. 2009, 36, 555–560. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Nickel-and cobalt-based catalysts for hydrogen generation by hydrolysis of borohydride. J. Alloys Compd. 2006, 415, 288–293. [Google Scholar] [CrossRef]
- Ishii, F.; Ban-Ya, S. Deoxidation Equilibrium of Silicon in Liquid Nickel-Chromium, Nickel-Molybdenum and Nickel-Tungsten Alloys. ISIJ Int. 1992, 32, 1097–1101. [Google Scholar] [CrossRef]
- Ban-Ya, S.; Ishii, F.; Ohtaki, D. Deoxidation Equilibrium of Hafnium in Liquid Iron, Nickel and Iron-Nickel Alloys. ISIJ Int. 1994, 34, 484–490. [Google Scholar] [CrossRef]
- Ishii, F.; Ban-Ya, S. Equilibrium between Yttrium and Oxygen in Liquid Iron and Nickel. ISIJ Int. 1995, 35, 280–285. [Google Scholar] [CrossRef]
- Ishii, F.; Ban-Ya, S.; Hino, M. Thermodynamics of the Deoxidation Equilibrium of Aluminum in Liquid Nickel and Nickel-Iron Alloys. ISIJ Int. 1996, 36, 25–31. [Google Scholar] [CrossRef]
- Yonemoto, L.M.; Miki, T.; Hino, M. Magnesium Deoxidation Equilibrium of Molten Fe–Ni Alloy Expressed by Quadratic Formalism and Redlich–Kister Type Polynomial. ISIJ Int. 2008, 48, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Dashevskiy, V.Y.; Aleksandrov, A.A.; Kanevskiy, A.G.; Leont’ev, L.I. Deoxidation Equilibrium of Zirconium in the Iron-Nickel Melts. ISIJ Int. 2013, 53, 1120–1124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Shang, Z.; Chen, M.; He, J.; Lv, B.; Wang, X.; Xiong, X. High-Purity Nickel Prepared by Electron Beam Melting: Purification Mechanism. Metall. Mater. Trans. B 2014, 45, 164–174. [Google Scholar] [CrossRef]
- Oh, J.M.; Roh, K.M.; Lim, J.W. Brief review of removal effect of hydrogen-plasma arc melting on refining of pure titanium and titanium alloys. Int. J. Hydrog. Energy 2016, 41, 23033–23041. [Google Scholar] [CrossRef]
- Su, Y.; Liu, X.; Luo, L.; Zhao, L.; Guo, J.; Fu, H. Deoxidation of Ti–Al Intermetallics via Hydrogen Treatment. Int. J. Hydrog. Energy 2010, 35, 9214–9217. [Google Scholar] [CrossRef]
- Elanski, D.; Lim, J.W.; Mimura, K.; Isshiki, M. Impurity removal from Zr, Nb and Ta metals by hydrogen plasma arc melting and thermodynamic estimation of hydride formation. J. Alloys Compd. 2006, 413, 251–258. [Google Scholar] [CrossRef]
- Mimura, K.; Kornukai, T.; Isshiki, M. Purification of chromium by hydrogen plasma-arc zone melting. Mater. Sci. Eng. A 2005, 403, 11–16. [Google Scholar] [CrossRef]
- Mimura, K.; Matsumoto, K.; Isshiki, M. Purification of Hafnium by Hydrogen Plasma Arc Melting. Mater. Trans. 2011, 52, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Li, L.; Yang, C.; Tian, W.; Li, X. Removal of gaseous impurities from terbium by hydrogen plasma arc melting. Int. J. Hydrog. Energy 2015, 40, 7943–7948. [Google Scholar] [CrossRef]
- Elanski, D.; Lim, J.W.; Mimura, K.; Isshiki, M. Impurity removal from Fe, Cr, Ti, and V metals by hydrogen plasma arc melting and thermodynamic estimation of hydride and sulfide formation. J. Alloys Compd. 2006, 421, 209–216. [Google Scholar] [CrossRef]
- Jung-Min, O.; Back-Kyu, L.; Chang-Youl, S.; Jae-Won, L. Removal of metallic impurities from Ti binary alloy scraps using hydrogen plasma arc melting. J. Alloys Compd. 2013, 574, 1–5. [Google Scholar]
- Li, G.; Li, L.; Fu, K.; Wang, C.; Zheng, J.; Xu, L.; Tian, W.; Li, X. Hydrogen in-situ refining method for preparing high purity gadolinium. J. Alloys Compd. 2015, 648, 29–33. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Fromm, E. Reduction of Metal Evaporation Losses by Inert Gas Atmospheres. Metall. Trans. A 1978, 9, 1835–1838. [Google Scholar] [CrossRef]
- Mimura, K.; Lee, S.; Isshiki, M. Removal of alloying elements from zirconium alloys by hydrogen plasma-arc melting. J. Alloys Compd. 1995, 221, 267–273. [Google Scholar] [CrossRef]
- Dembovský, V. Plasma Metallurgy: The Principles; Butterworth-Heinemann Limited: Amsterdam, The Netherlands, 1985. [Google Scholar]

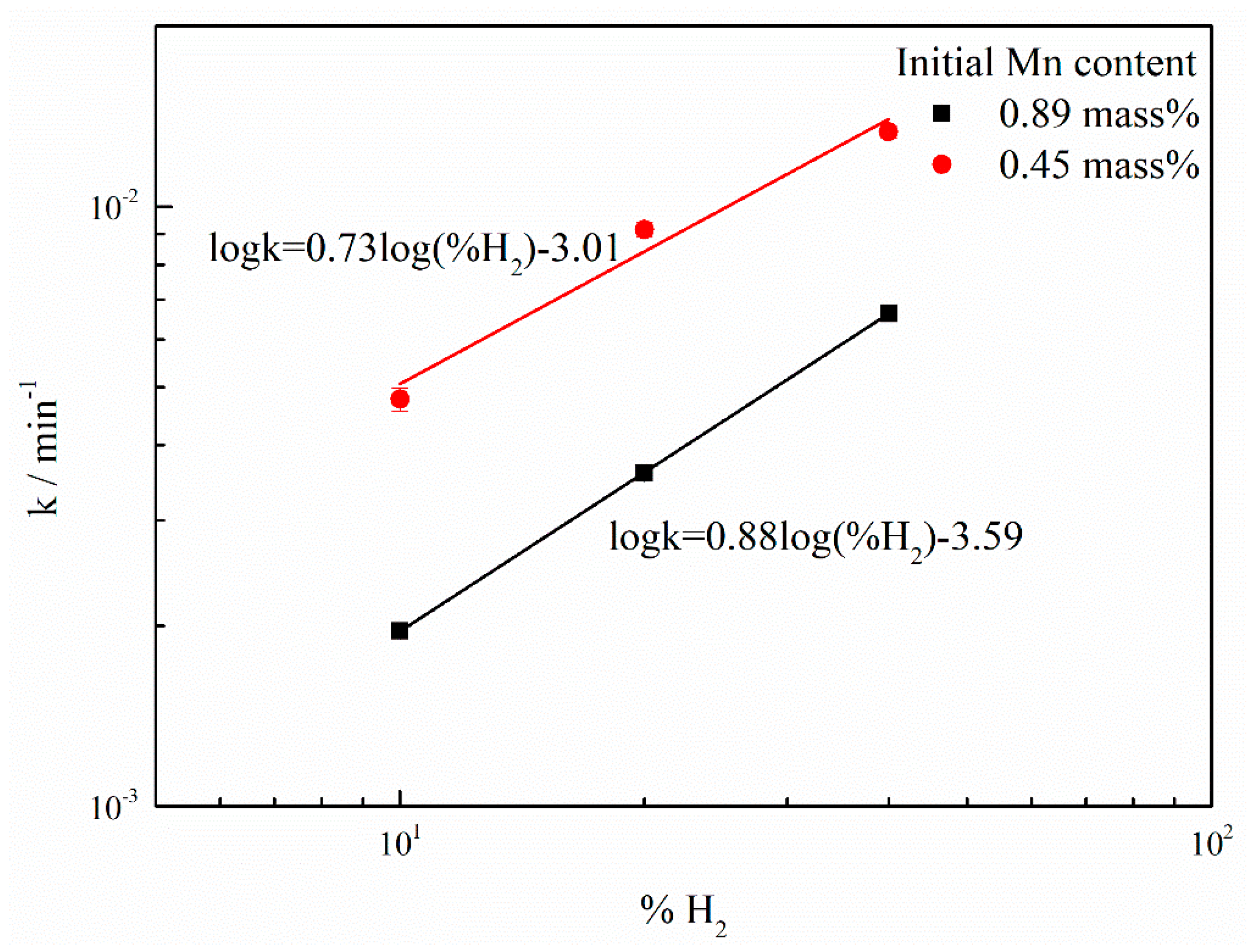
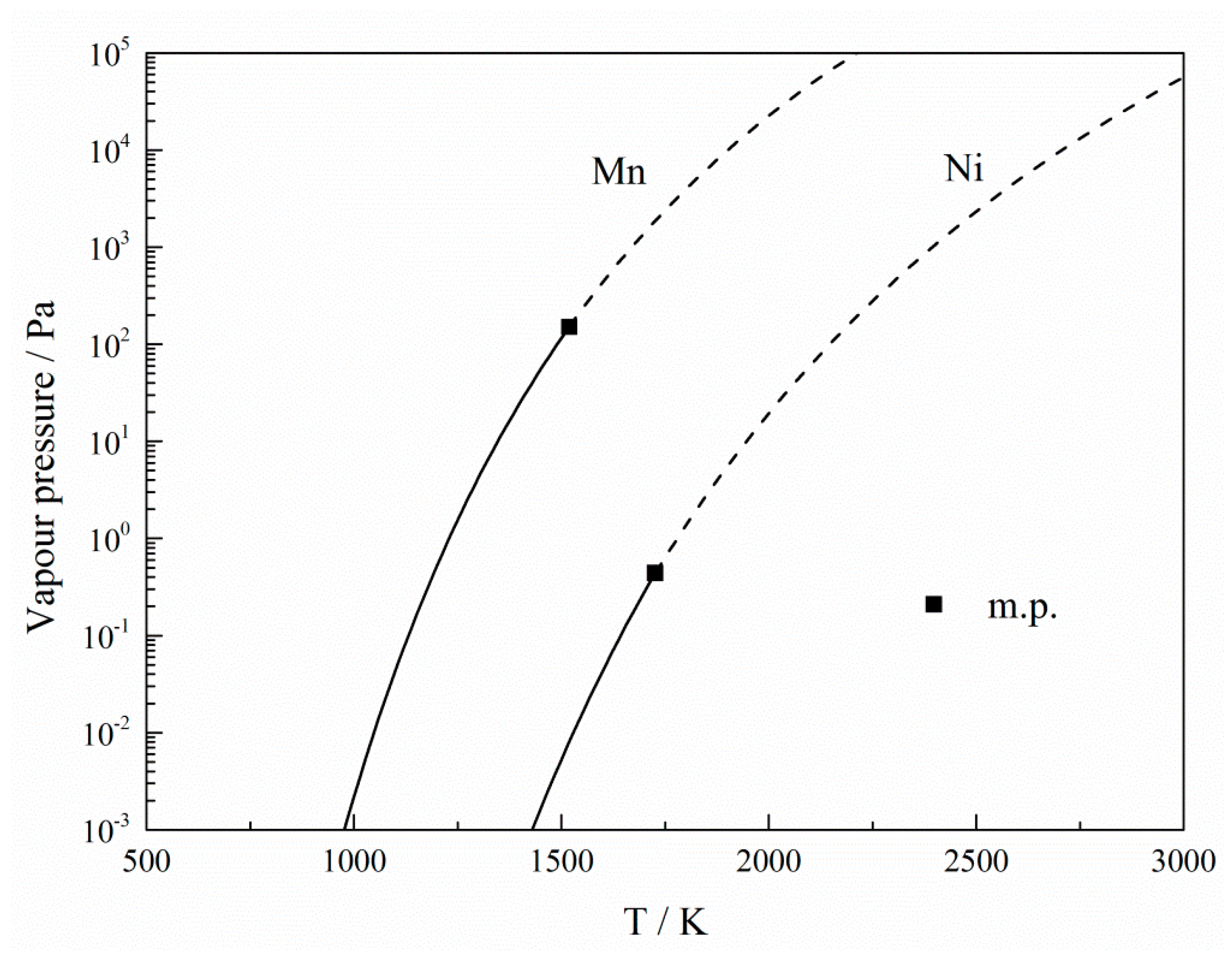
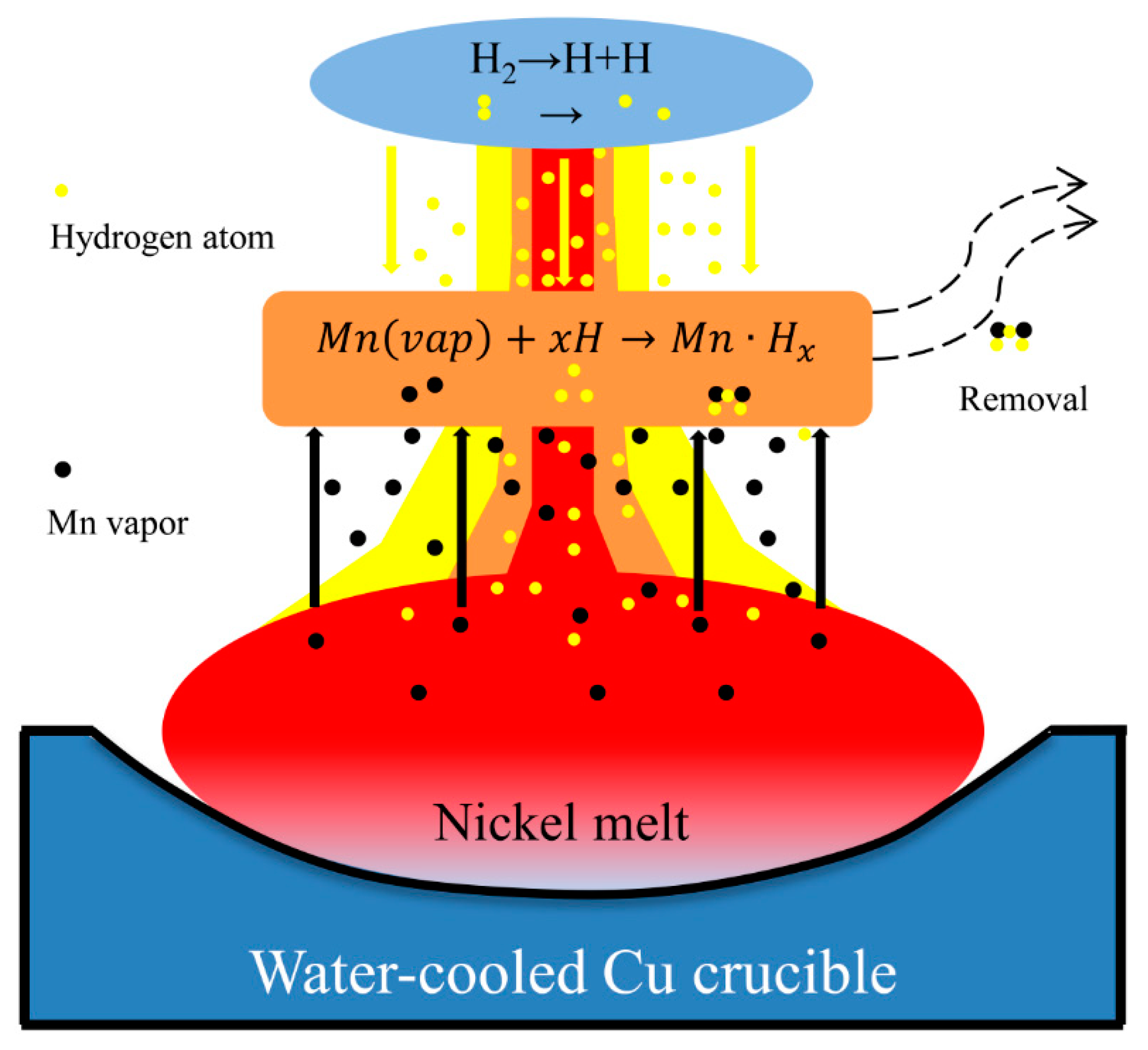
| Plasma gas | under 0.09–0.1 MPa | Standard Error | under 0.04–0.05 MPa | Standard Error |
|---|---|---|---|---|
| Ar | 6.24 × 10−4 | 2.0 × 10−5 | 1.20 × 10−3 | 5.4 × 10−5 |
| Ar-10%H2 | 2.74 × 10−3 | 5.6 × 10−5 | 4.97 × 10−3 | 2.1 × 10−4 |
| Ar-20%H2 | 4.08 × 10−3 | 8.3 × 10−5 | 9.56 × 10−3 | 2.7 × 10−4 |
| Ar-40%H2 | 7.74 × 10−3 | 2.2 × 10−4 | 1.37 × 10−2 | 3.3 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Yu, J.; Hou, Y.; Zhang, Y.; Wang, J.; Li, X.; Liao, H.; Ren, Z. Manganese Removal from Liquid Nickel by Hydrogen Plasma Arc Melting. Materials 2019, 12, 33. https://doi.org/10.3390/ma12010033
Guo X, Yu J, Hou Y, Zhang Y, Wang J, Li X, Liao H, Ren Z. Manganese Removal from Liquid Nickel by Hydrogen Plasma Arc Melting. Materials. 2019; 12(1):33. https://doi.org/10.3390/ma12010033
Chicago/Turabian StyleGuo, Xiliang, Jianbo Yu, Yuan Hou, Yujia Zhang, Jiang Wang, Xia Li, Hanlin Liao, and Zhongming Ren. 2019. "Manganese Removal from Liquid Nickel by Hydrogen Plasma Arc Melting" Materials 12, no. 1: 33. https://doi.org/10.3390/ma12010033




