Effects of Impurity Doping on the Luminescence Performance of Mn4+-Doped Aluminates with the Magnetoplumbite-Type Structure for Plant Cultivation
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Preparation
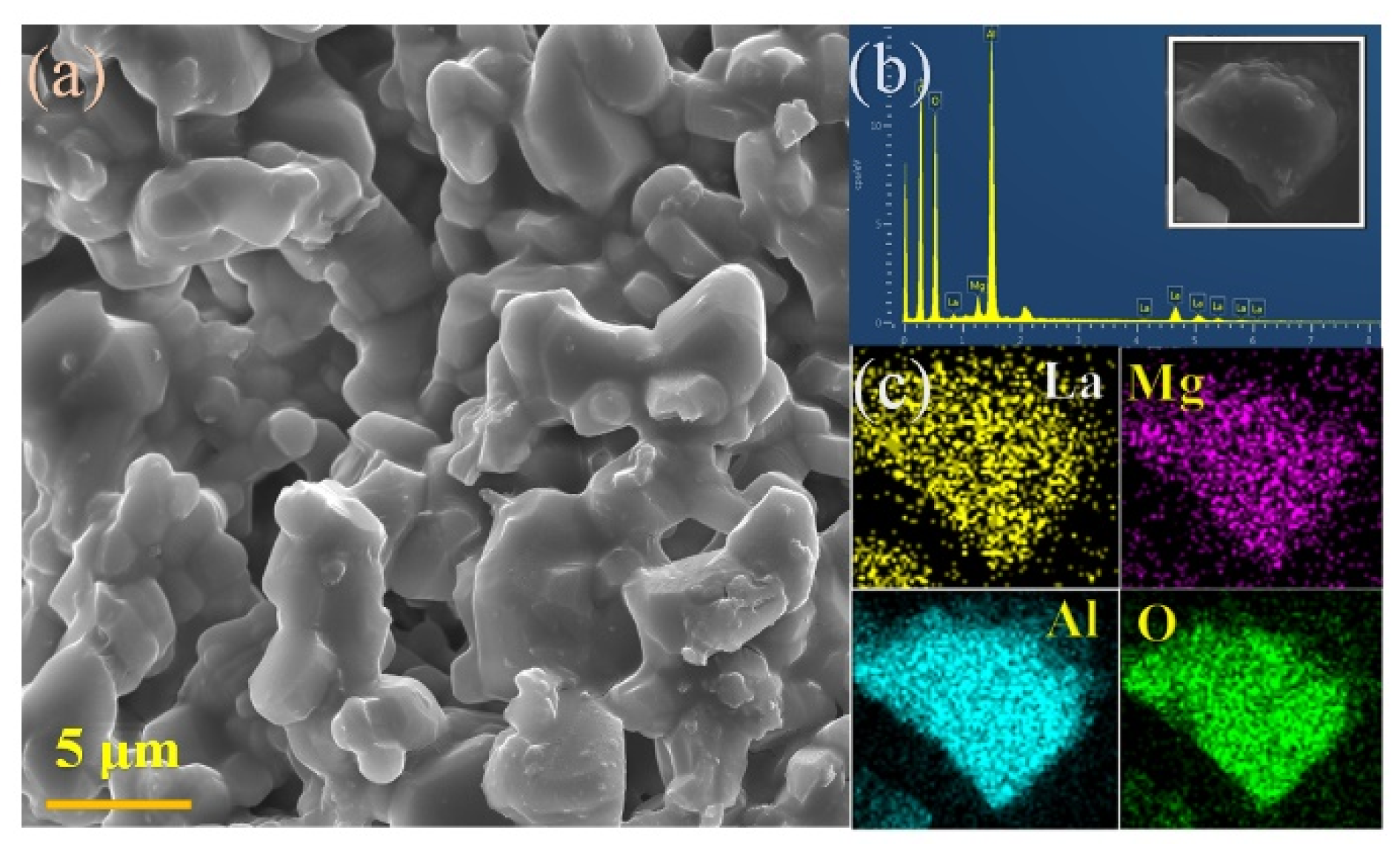
2.2. Sample Characterization
3. Results and Discussion
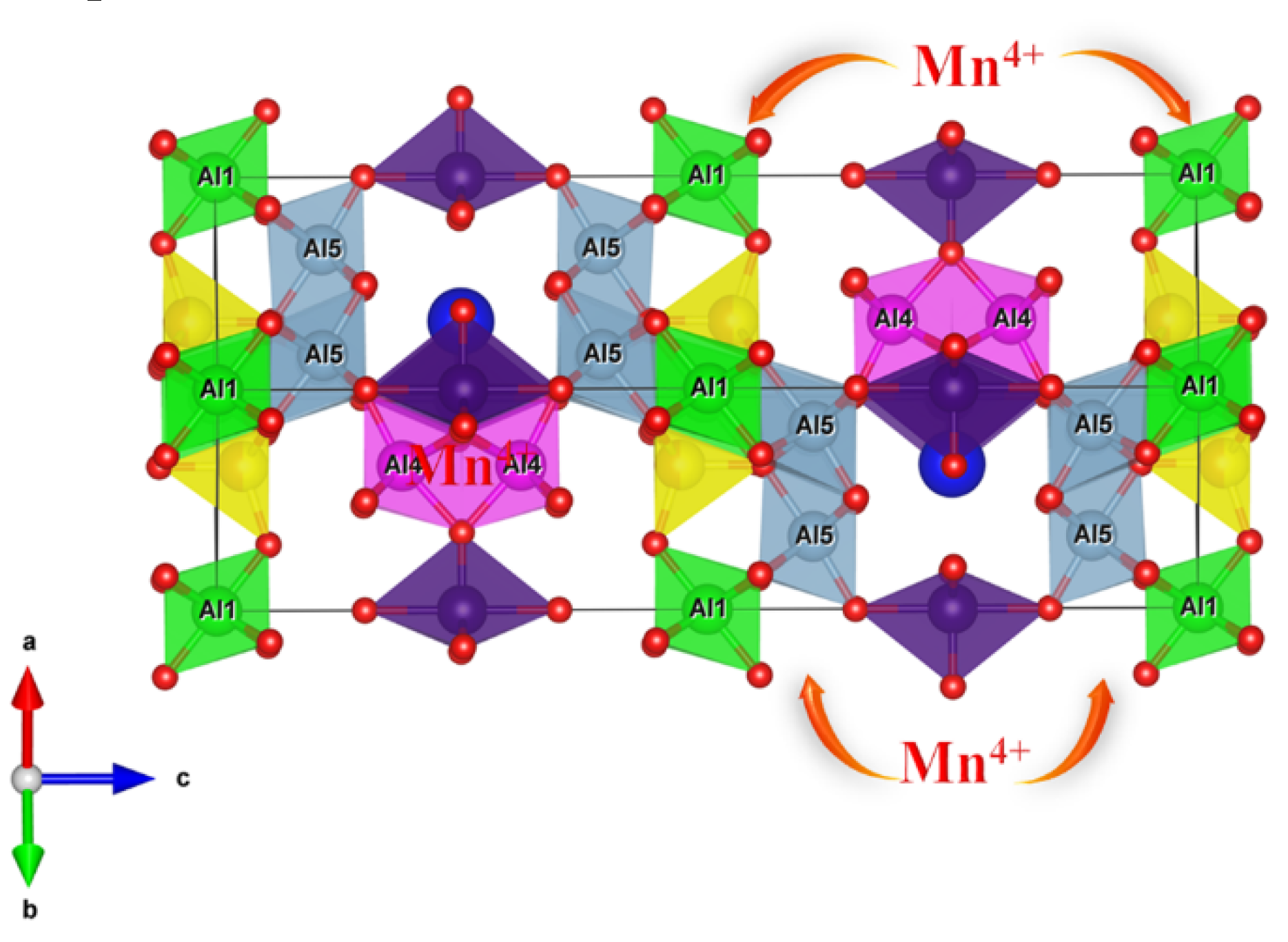
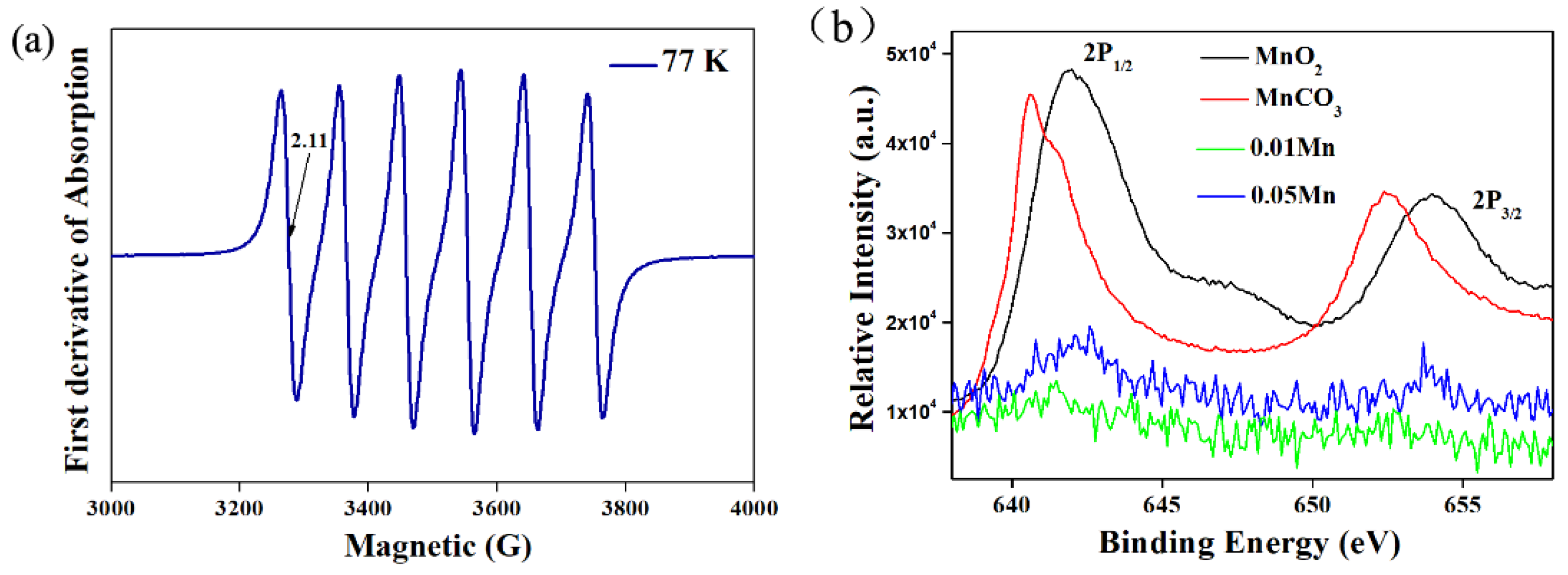
3.1. Phase Purity and Crystal Structure Analysis
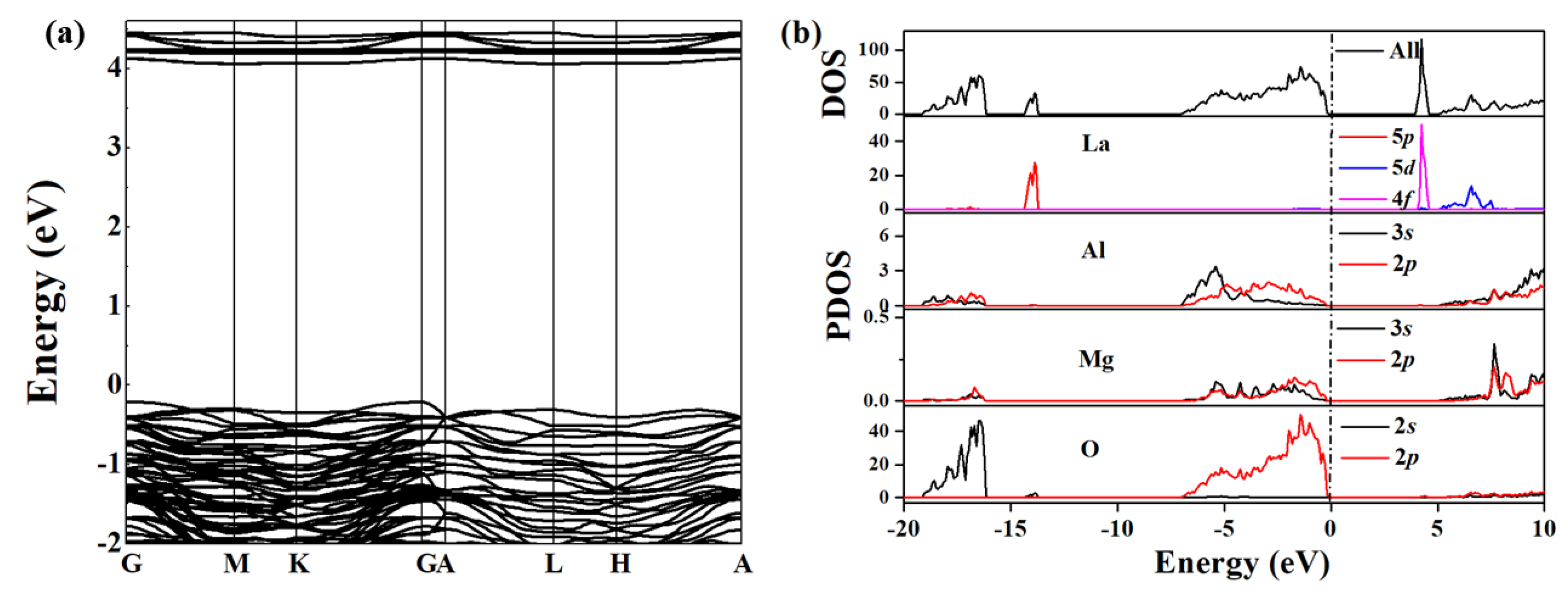
3.2. Electronic Structure Calculations of LMA
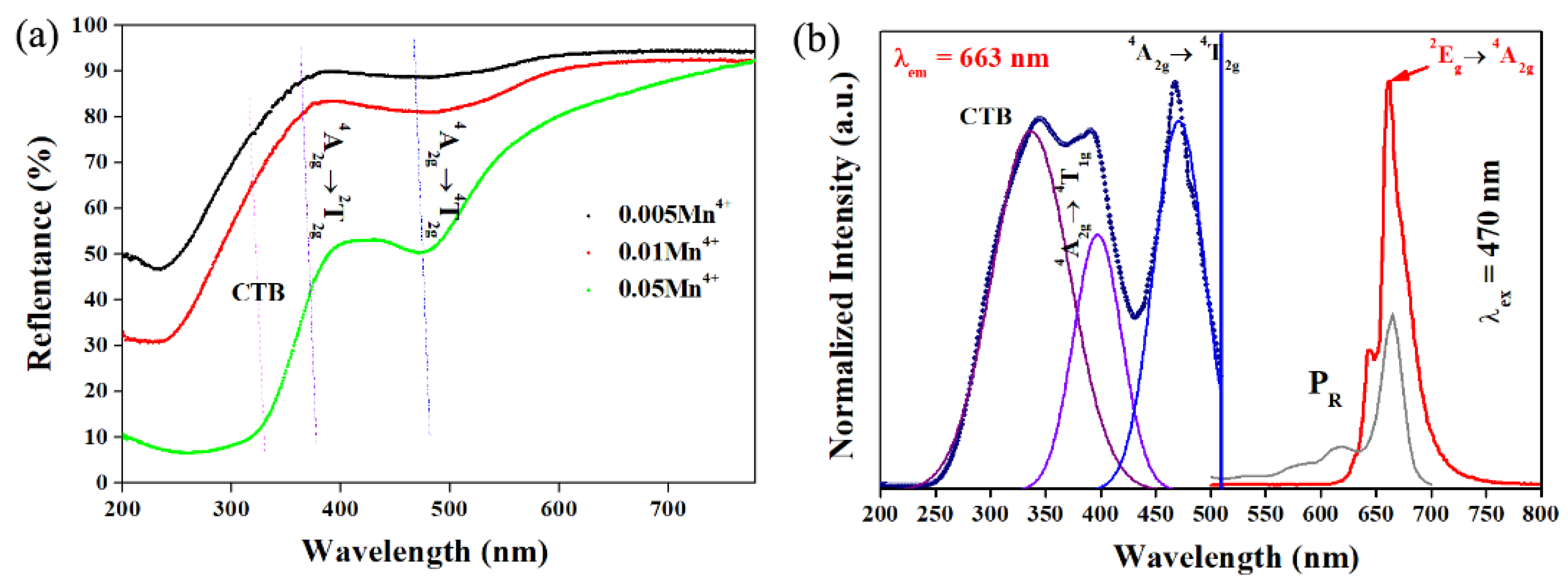
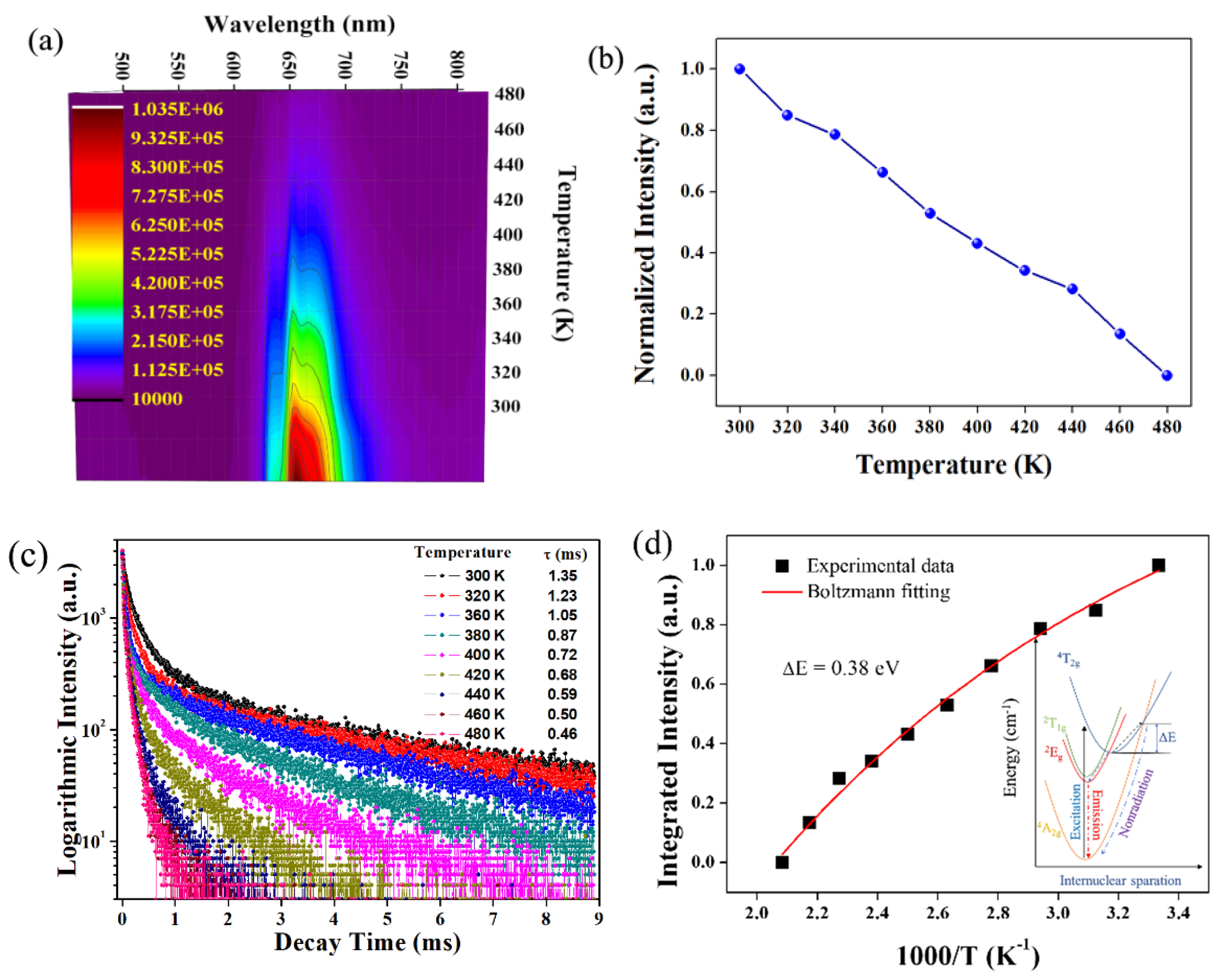
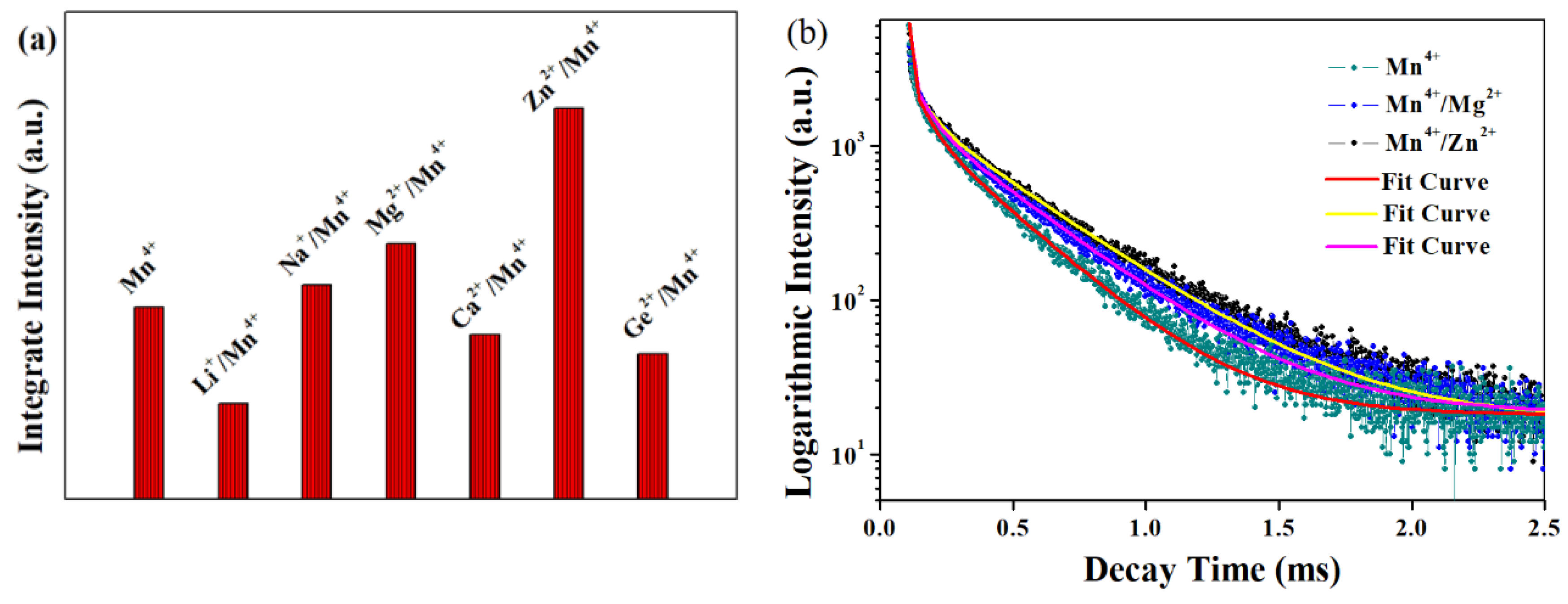
3.3. Components Luminescent Properties of LMA/Mn4+
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Seki, K.; Uematsu, K.; Toda, K.; Sato, M. Novel deep red emitting phosphors Ca14Zn6M10:Mn4+ (M =Al3+ and Ga3+). Chem. Lett. 2014, 43, 1213–1215. [Google Scholar] [CrossRef]
- Zhou, Z.; Xia, M.; Zhong, Y.; Gai, S.J.; Huang, S.X.; Tian, Y.; Lu, X.Y.; Zhou, N. Dy3+@Mn4+ co-doped Ca14Ga10-mAlmZn6O35 fa-red emitting phosphors with high brightness and improved luminescence and energy transfer properties for plant growth LED lights. J. Mater. Chem. C 2017, 5, 8201–8210. [Google Scholar] [CrossRef]
- Chen, J.Y.; Guo, C.F.; Yang, Z.; Li, T.; Zhao, J. Li2SrSiO4:Ce3+, Pr3+ phosphor with blue, red, and near-infrared emissions used for plant growth LED. J. Am. Ceram. Soc. 2016, 99, 218–225. [Google Scholar] [CrossRef]
- Xiang, J.M.; Chen, J.Y.; Zhang, N.M.; Yao, H.B.; Guo, C.F. Far red and near infrared double-wavelength emitting phosphor Gd2ZnTiO6:Mn4+, Yb3+ for plant cultivation LEDs. Dyes Pigments 2018, 154, 257–262. [Google Scholar] [CrossRef]
- Cao, R.P.; Ye, Y.J.; Peng, Q.Y.; Zheng, G.T.; Ao, H.; Fu, J.W.; Go, Y.M.; Guo, B. Synthesis and luminescence characteristics of novel red-emitting Ba2TiGe2O8:Mn4+ phosphor. Dyes Pigments 2017, 146, 14–19. [Google Scholar] [CrossRef]
- Deng, J.K.; Zhang, H.R.; Zhang, X.J.; Zheng, Y.J.; Yuan, J.Q.; Liu, H.Z.; Liu, Y.L.; Lei, B.F.; Qiu, J.B. Ultrastable red-emitting phosphor-in-glass for superior high-power artificial plant growth LEDs. J. Mater. Chem. C 2018, 6, 1738–1745. [Google Scholar] [CrossRef]
- Huang, X.Y.; Liang, J.; Li, B.; Sun, L.L.; Lin, J. High-efficiency and thermally stable far-red-emitting NaLgMgWO6:Mn4+ phosphors for indoor plant growth light-emitting diodes. Opt. Lett. 2018, 43, 3305–3308. [Google Scholar] [CrossRef]
- Cao, R.P.; Shi, Z.H.; Quan, G.J.; Chen, T.; Guo, S.L.; Hu, Z.F.; Liu, P. Preparation and luminescence properties of Li2MgZrO4:Mn4+ red phosphor for plant growth. J. Lumin. 2017, 188, 577–581. [Google Scholar] [CrossRef]
- Xiang, J.M.; Zheng, J.M.; Zhou, Z.W.; Suo, H.; Zhao, Q.X.; Zhou, X.J.; Zhang, N.M.; Molokeev, M.S.; Guo, C.F. Enhancement of red emission and site analysis in Eu2+ doped new-type structure Ba3CaK(PO4)3. Chem. Eng. J. 2019, 356, 236–244. [Google Scholar] [CrossRef]
- Liu, Z.C.; Shao, G.Z.; Chen, W.; Hu, G.C.; Shen, L.L.; Cheng, Y.Z.; Liang, X.J.; Xiang, W.D. Effect of the replacement of Zn2+ with Mg2+ in Ca14Zn6Ga10O35:Mn4+. Opt. Mater. Express 2018, 8, 2532–2541. [Google Scholar] [CrossRef]
- Wang, B.; Lin, H.; Xu, J.; Chen, H.; Wang, Y.S. CaMg2Al16O27:Mn4+-based red phosphor: a potential color converter for high powered warm w-LED. ACS Appl. Mater. Interfaces 2014, 6, 22905–22913. [Google Scholar] [CrossRef]
- Cao, Li.S.; Liu, Q.F.; Wang, L.C.; Li, J.; Song, J.; Wang, D.J. Microwave-induced small size effect of (Ba,Sr)3MgSi2O8:0.06Eu2+, 0.1Mn2+ phosphor for 660 nm-featured bio-lighting. Ceram. Int. 2013, 7, 7717–7720. [Google Scholar] [CrossRef]
- Peng, M.Y.; Yin, X.W.; Tanner, P.A.; Brik, M.G.; Li, P.F. Site occupancy preference, enhancement mechanism, and thermal resistance of Mn4+ red luminescence. Chem. Mater. 2015, 27, 2938–2945. [Google Scholar] [CrossRef]
- Hu, T.; Lin, H.; Gao, Y.; Xu, J.; Wang, J.M.; Xiang, X.Q.; Wang, Y.S. Host sensitization of Mn4+ in self-activated Na2WO2F4:Mn4+. J. Am. Ceram. Soc. 2018, 101, 3437–3442. [Google Scholar] [CrossRef]
- Li, H.; Hu, T.; Huang, Q.M.; Cheng, Y.; Wang, B.; Xu, J.; Wang, J.M.; Wang, Y.S. Non-rare-earth K2XF7:Mn4+ (X = Ta, Nb): A highly-efficient narrow-band red phosphor enabling the application in wide-color-gamut LCD. Laser Photonics Rev. 2017, 11, 1700148. [Google Scholar] [CrossRef]
- Wang, B.; Lin, H.; Huang, F.; Xu, J.; Chen, H.; Lin, Z.B.; Wang, Y.S. Non-rare-earth BaMgAl10-2xO17:xMn4+,xMg2+: a narrow-band red phosphor for use as a high-power warm w-LED. Chem. Mater. 2016, 28, 3515–3524. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Y.W.; Zhang, X.J.; Zou, R.; Pan, F.J.; Wang, J.; Wu, M.M. HF-free hydrothermal route for synthesis of highly efficient narrow-band red emitting phosphor K2Si1-xF6:xMn4+ for warm white light emitting diodes. Chem. Mater. 2016, 28, 1495–1502. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Zheng, J.M.; Shi, R.; Zhang, N.M.; Chen, J.Y.; Zhang, R.Y.; Suo, Y.; Goldys, E.M.; Cuo, C.F. Ab initio site occupancy and far red emission of Mn4+ in cubic-phase La(MgTi)1/2O3 for plant cultivation. ACS Appl. Mater. Interfaces 2017, 9, 6177–6185. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rodriguez, R.; Valiente, R.; Rodriguez, F.; Speghini, A.; Bettinlli, M. Temperature dependence and temporal dynamics of Mn2+ upconversion luminescence sensitized by Yb3+ in codoped LaMgAl11O19. Phys. Rev. B 2010, 82, 075117. [Google Scholar] [CrossRef]
- Brandle, C.D.; Berkstresser, G.W.; Shmulovich, J.; Valentino, A.J. Czochralski growth and evaluation of LaMgAl11O19 based phoshpors. J. Cryst. Growth. 1987, 85, 234–239. [Google Scholar] [CrossRef]
- Wang, B.Y.; Zu, J.Z.; Chen, H.; Zhang, Z.H. Luminescence Properties of LaMgAl11O19:Mn2+ and LaMgAl11O19: RE, Mn2+(RE=Eu2+, Gd3+) under UV/VUV Excitation. Rare Metal Mater. Eng. 2009, 38, 389–392. [Google Scholar] [CrossRef]
- Singh, V.; Chakradhar, R.P.S.; Rao, J.L.; Dhoble, S.J.; Kim, S.H. Electron paramagnetic resonance and photoluminescence studies of LaMgAl11O19:Mn2+ green phosphors. J. Elecron. Mater. 2014, 43, 4041. [Google Scholar] [CrossRef]
- Jovanić, B.R.; Viana, B. High-pressure and optical properties of lanthanum magnesium hexa-aluminate doped with Mn2+ (LMA:Mn2+) laser material. Opt. Commun. 2009, 282, 1798–1800. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Payne, M.C.; Teter, M.P.; Allan, D.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for abinitio total-energy calculations-molecular-dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Kleinman, L.; Bylander, D.M. Efficacious form for model pseudopotentials. Phys. Rev. Lett. 1982, 48, 1425–1428. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for brillouin-zone integrations. Phys. Rev. B. 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.S.; Chen, Y.Q.; Chen, Y.; Zhou, J.C.; Zeng, Q.G. Long persistent and photo-stimulated luminescence in Pr3+-doped layered perovskite phosphor for optical data storage. J. Am. Ceram. Soc. 2018, 101, 4598–4607. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.Y.; Sharafudeen, K.; Dong, G.P.; Zhou, S.F.; Ma, Z.J.; Peng, M.Y.; Qiu, J.B. A strategy for developing near infrared long-persistent phosphors: taking MAlO3:Mn4+, Ge4+ (M = La, Gd) as an example. J. Mater. Chem. C 2014, 2, 2019–2027. [Google Scholar] [CrossRef]
- Peng, M.Y.; Yin, X.W.; Tanner, P.A.; Liang, C.Q.; Li, P.F.; Zhang, Q.Y.; Qiu, J.R. Orderly-layered tetravalent manganese-doped strontium aluminate Sr4Al14O25:Mn4+: an efficient red phosphor for warm white light emitting diodes. J. Am. Ceram. Soc. 2013, 96, 2870–2876. [Google Scholar] [CrossRef]
- Blasse, G.; Dirksen, G.J. Luminescence and crystal-structure of LiNbGeO5. J. Solid State Chem. 1986, 65, 283–286. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 210, 836–850. [Google Scholar] [CrossRef]
- Chen, H.; Lin, H.; Xu, J.; Wang, B.; Lin, Z.B.; Zhou, J.C.; Wang, Y.S. Chromaticity-tunable phosphor-in-glass for long-lifetime high-power warm w-LEDs. J. Mater. Chem. C 2015, 31, 8080–8089. [Google Scholar] [CrossRef]
- Xie, R.J.; Hirosaki, N.; Kimura, N.; Sakuma, K.; Mitomo, M. 2-phosphor-converted white light-emitting diodes using oxynitride/nitride phosphors. Appl. Phys. Lett. 2007, 90, 191101. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 2015, 32, 751–767. [Google Scholar] [CrossRef]


| Atom | x | y | z | Occ | U | Coordination Number |
|---|---|---|---|---|---|---|
| La | 0.33333 | 0.66667 | 0.75000 | 1 | 0.00911 | |
| Mg | 0.33333 | 0.66667 | 0.02720 | 0.5 | 0.00240 | 4 |
| Al1 | 0.00000 | 0.00000 | 0.00000 | 1 | 0.00190 | 6 |
| Al2 | 0.00000 | 0.00000 | 0.25000 | 1 | 0.02305 | 5 |
| Al3 | 0.33333 | 0.66667 | 0.02720 | 0.5 | 0.00240 | 4 |
| Al4 | 0.33333 | 0.66667 | 0.18950 | 1 | 0.00228 | 6 |
| Al5 | 0.16740 | 0.33480 | 0.89200 | 1 | 0.00304 | 6 |
| O1 | 0.00000 | 0.00000 | 0.15100 | 1 | 0.00278 | |
| O2 | 0.33333 | 0.66667 | 0.94200 | 1 | 0.00329 | |
| O3 | 0.18000 | 0.36000 | 0.25000 | 1 | 0.00709 | |
| O4 | 0.15200 | 0.30400 | 0.05300 | 1 | 0.00455 | |
| O5 | 0.50500 | 0.010000 | 0.15100 | 1 | 0.00380 | |
| Symmetry: | hexagonal | Space group: | P63/mmc | |||
| Lattice parameters: a = b = 5.5950 Å; c = 21.9680 Å; α = β = 90°; γ = 120°; V = 594.86 Å3 | ||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chen, Z.; Wang, B.; Liang, R.; Li, Y.; Kang, L.; Liu, P. Effects of Impurity Doping on the Luminescence Performance of Mn4+-Doped Aluminates with the Magnetoplumbite-Type Structure for Plant Cultivation. Materials 2019, 12, 86. https://doi.org/10.3390/ma12010086
Li X, Chen Z, Wang B, Liang R, Li Y, Kang L, Liu P. Effects of Impurity Doping on the Luminescence Performance of Mn4+-Doped Aluminates with the Magnetoplumbite-Type Structure for Plant Cultivation. Materials. 2019; 12(1):86. https://doi.org/10.3390/ma12010086
Chicago/Turabian StyleLi, Xiaoshuang, Zikun Chen, Bo Wang, Ruizhao Liang, Yongting Li, Lei Kang, and Pengfei Liu. 2019. "Effects of Impurity Doping on the Luminescence Performance of Mn4+-Doped Aluminates with the Magnetoplumbite-Type Structure for Plant Cultivation" Materials 12, no. 1: 86. https://doi.org/10.3390/ma12010086






