CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy
Abstract
:1. Introduction
1.1. CO Biological Scope
1.2. CO Therapeutic Ways
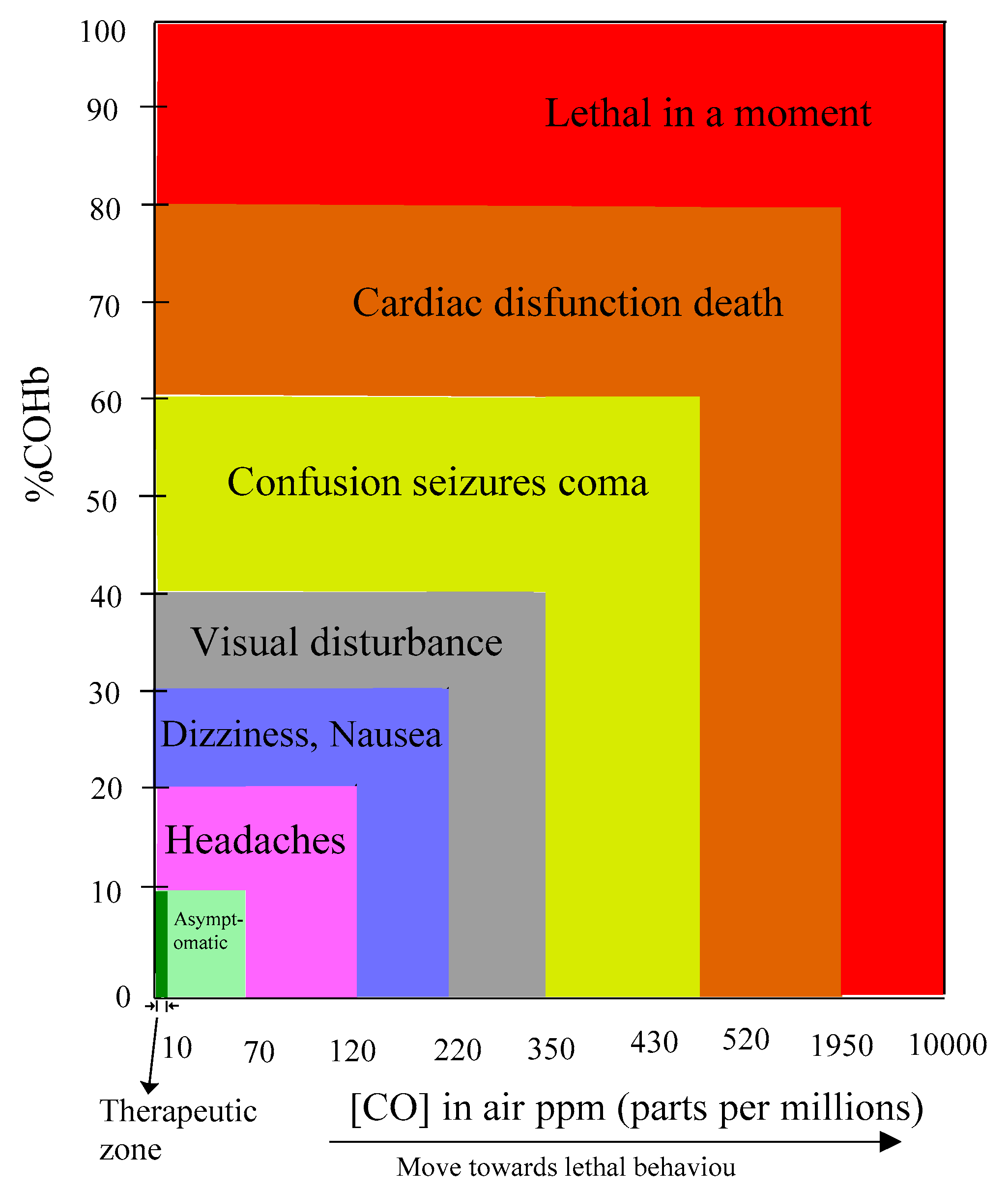
1.3. Why Exogenous Endeavor is Required?
1.4. Clinical Translations
1.5. Challenges and Demanding Features of CORMs and CORMats
- Availability, solubility and stability of reagents under ambient conditions.
- Feasibility to release the captured CO from in situ CORM.
- Controllability to release CO kinetics up to a desired level.
- Prone to toxicity which arises due to the transition metal foundation of metal-ligand fragments.
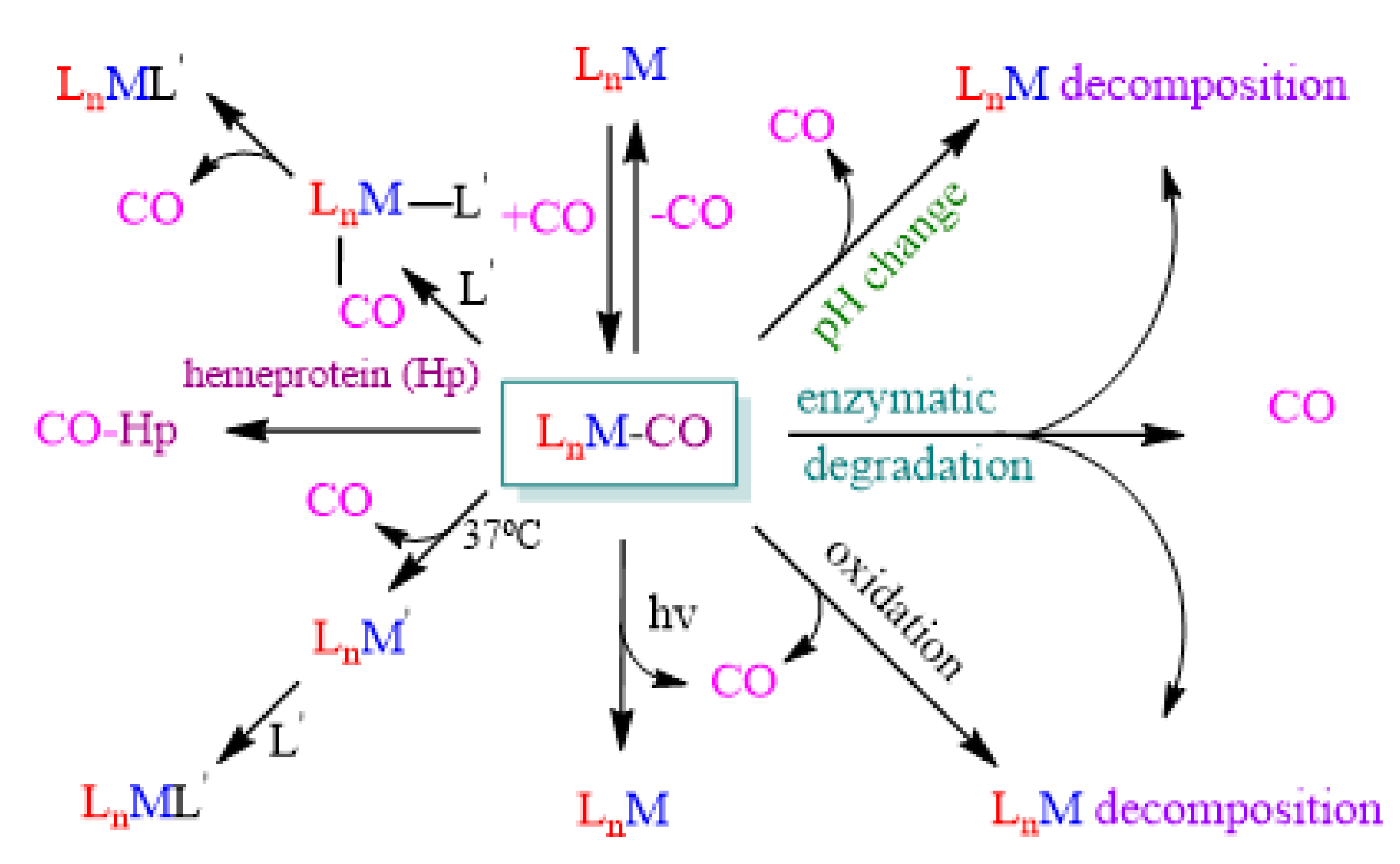
1.6. Triggers
1.7. CO Identifier
1.8. The Development Phases of CORMs Motifs
1.8.1. Metal Carbonyl Complexes (MCCs)
1.8.2. Proposed Strategies for CORMs Development
- Structural variance of unique chemistry;
- Expected divergence with different oxidation states;
- Covalently bound with the metal center;
- Assisting alterations for the attached carbonyl ligands;
- The dynamics of co-ligands binding;
- Tendency of the outer coordination sphere.
1.9. CORM’s Therapeutic Scope
1.10. Solubility
2. Research on New CO Transport Materials
2.1. Micellization
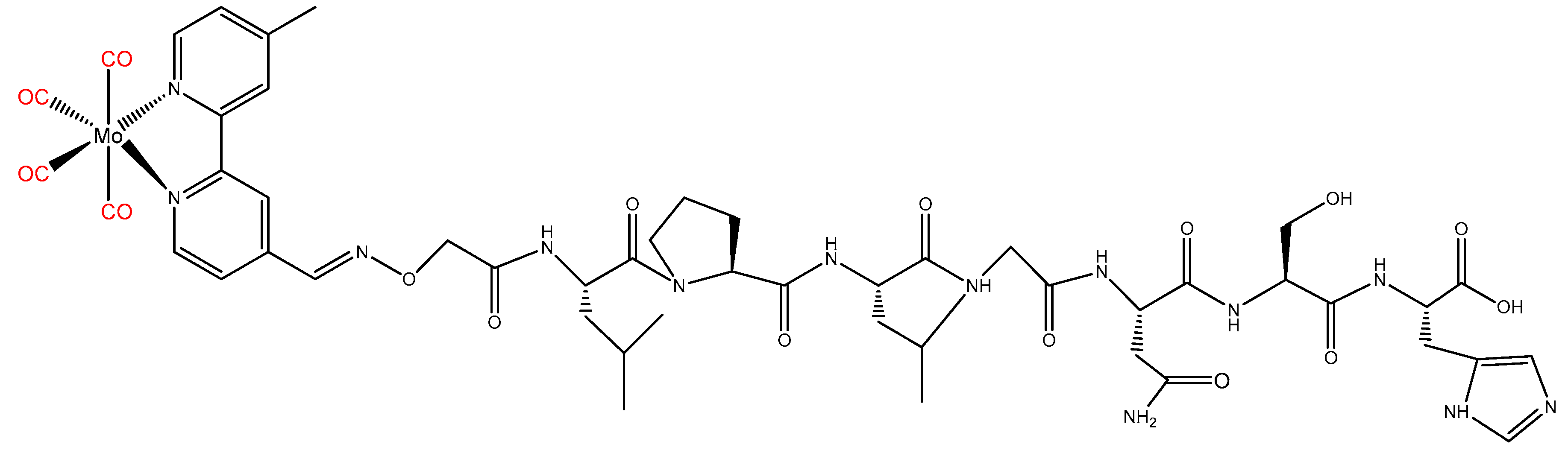
2.2. Peptide
2.3. Proteins
2.4. Vitamins
2.5. Polymers
2.6. Metal Organic Frameworks (MOFs-CORMats)
2.7. Porous Structure Materials
2.8. Nanaoparticles
2.9. Nanosheets
2.10. Metallodendrimers
2.11. Nanodiamond (ND)
3. CO-Releasing Kinetic Profile
4. CORMs/CORMats Cytotoxicity and Tissue Accumulation
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pitto-Barry, A.; Lupan, A.; Ellingford, C.; Attia, A.A.A.; Barry, N.P.E. New Class of Hybrid Materials for Detection, Capture, and "On-Demand" Release of Carbon Monoxide. ACS Appl. Mater. Interfaces 2018, 10, 13693–13701. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Otterbein, L.E. The therapeutic potential of carbon monoxide. Nat. Rev. Drug Discov. 2010, 9, 728–743. [Google Scholar] [CrossRef] [PubMed]
- Haldane, J.B.S. Carbon monoxide as a tissue poison. Biochem. J. 1927, 21, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.G.; Haldane, J.S.; Haldane, J.B.S. The laws of combination of haemoglobin with carbon monoxide and oxygen. J. Physiol. 1912, 44, 275–304. [Google Scholar] [CrossRef] [Green Version]
- Tenhunen, R.; Marver, H.S.; Schmid, R. Microsomal heme oxygenase. Characterization of the enzyme. J. Biol. Chem. 1969, 244, 6388–6394. [Google Scholar]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748–755. [Google Scholar] [CrossRef]
- Kocer, G.; Nasircilar Ulker, S.; Senturk, U.K. The contribution of carbon monoxide to vascular tonus. Microcirculation 2018, 25, e12495. [Google Scholar] [CrossRef]
- Peng, J.; Hu, T.; Li, J.; Du, J.; Zhu, K.; Cheng, B.; Li, K. Shepherd’s Purse Polyphenols Exert Its Anti-Inflammatory and Antioxidative Effects Associated with Suppressing MAPK and NF-kappaB Pathways and Heme Oxygenase-1 Activation. Oxidative Med. Cell. Longev. 2019, 2019, 7202695. [Google Scholar] [CrossRef]
- Dercho, R.A.; Nakatsu, K.; Wong, R.J.; Stevenson, D.K.; Vreman, H.J. Determination of in vivo carbon monoxide production in laboratory animals via exhaled air. J. Pharmacol. Toxicol. Methods 2006, 54, 288–295. [Google Scholar] [CrossRef]
- Heinemann, S.H.; Hoshi, T.; Westerhausen, M.; Schiller, A. Carbon monoxide—Physiology, detection and controlled release. Chem. Commun. 2014, 50, 3644–3660. [Google Scholar] [CrossRef]
- Yang, S.; Chen, M.; Zhou, L.; Zhang, G.; Gao, Z.; Zhang, W. Photo-activated CO-releasing molecules (PhotoCORMs) of robust sawhorse scaffolds [μ2-OOCR1, η1-NH2CHR2(C=O] OCH3, Ru(I)2CO4]. Dalton Trans. 2016, 45, 3727–3733. [Google Scholar] [CrossRef]
- Stupfel, M.; Bouley, G. Physiological and biochemical effects on rats and mice exposed to small concentrations of carbon monoxide for long periods. Ann. N. Y. Acad. Sci. 1970, 174, 342–368. [Google Scholar] [CrossRef]
- Ling, K.; Men, F.; Wang, W.C.; Zhou, Y.Q.; Zhang, H.W.; Ye, D.W. Carbon Monoxide and Its Controlled Release: Therapeutic Application, Detection, and Development of Carbon Monoxide Releasing Molecules (CORMs). J. Med. Chem. 2018, 61, 2611–2635. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E. Carbon monoxide: Innovative anti-inflammatory properties of an age-old gas molecule. Antioxid. Redox Signal. 2002, 4, 309–319. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]
- Zamani, M.; Aleyasin, A.; Fakhrzadeh, H.; Kiavar, M.; Raoufzadeh, S.; Larijani, B.; Mahmoodi, E. Heme Oxigenase 2 Gene Polymorphisms as Genetic Risk Factor in Atherosclerosis in Iranian Patients. Iran. Red Crescent Med. J. 2010, 12, 559–563. [Google Scholar]
- Joshi, H.P.; Kim, S.B.; Kim, S.; Kumar, H.; Jo, M.J.; Choi, H.; Kim, J.; Kyung, J.W.; Sohn, S.; Kim, K.T.; et al. Nanocarrier-mediated Delivery of CORM-2 Enhances Anti-allodynic and Anti-hyperalgesic Effects of CORM-2. Mol. Neurobiol. 2019, 56. [Google Scholar] [CrossRef]
- Motterlini, R.; Haas, B.; Foresti, R. Emerging concepts on the anti-inflammatory actions of carbon monoxide-releasing molecules (CO-RMs). Med. Gas Res. 2012, 2, 28. [Google Scholar] [CrossRef]
- Al-Huseini, L.M.; Aw Yeang, H.X.; Hamdam, J.M.; Sethu, S.; Alhumeed, N.; Wong, W.; Sathish, J.G. Heme oxygenase-1 regulates dendritic cell function through modulation of p38 MAPK-CREB/ATF1 signaling. J. Biol. Chem. 2014, 289, 16442–16451. [Google Scholar] [CrossRef]
- Stagni, E.; Privitera, M.G.; Bucolo, C.; Leggio, G.M.; Motterlini, R.; Drago, F. A water-soluble carbon monoxide-releasing molecule (CORM-3) lowers intraocular pressure in rabbits. Br. J. Ophthalmol. 2009, 93, 254–257. [Google Scholar] [CrossRef]
- Allanson, M.; Reeve, V.E. Ultraviolet A (320-400 nm) modulation of ultraviolet B (290-320 nm)-induced immune suppression is mediated by carbon monoxide. J. Investig. Dermatol. 2005, 124, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, S.; Forni, M.; Albertini, M.; Bacci, M.L.; Zannoni, A.; Gentilini, F.; Lavitrano, M.; Bach, F.H.; Otterbein, L.E.; Clement, M.G. Carbon monoxide pretreatment prevents respiratory derangement and ameliorates hyperacute endotoxic shock in pigs. FASEB J. 2005, 19, 2045–2047. [Google Scholar] [CrossRef]
- Bagul, A.; Hosgood, S.A.; Kaushik, M.; Nicholson, M.L. Carbon monoxide protects against ischemia-reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation 2008, 85, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Balla, J.; Otterbein, L.; Smith, R.N.; Brouard, S.; Lin, Y.; Csizmadia, E.; Sevigny, J.; Robson, S.C.; Vercellotti, G.; et al. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J. Immunol. 2001, 166, 4185–4194. [Google Scholar] [CrossRef]
- Chen, B.; Guo, L.; Fan, C.; Bolisetty, S.; Joseph, R.; Wright, M.M.; Agarwal, A.; George, J.F. Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am. J. Pathol. 2009, 175, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.E.; Naughton, P.; Shurey, S.; Green, C.J.; Johnson, T.R.; Mann, B.E.; Foresti, R.; Motterlini, R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ. Res. 2003, 93, e2–e8. [Google Scholar] [CrossRef]
- Yoshida, J.; Ozaki, K.S.; Nalesnik, M.A.; Ueki, S.; Castillo-Rama, M.; Faleo, G.; Ezzelarab, M.; Nakao, A.; Ekser, B.; Echeverri, G.J.; et al. Ex vivo application of carbon monoxide in UW solution prevents transplant-induced renal ischemia/reperfusion injury in pigs. Am. J. Transplant. 2010, 10, 763–772. [Google Scholar] [CrossRef]
- Sandouka, A.; Fuller, B.J.; Mann, B.E.; Green, C.J.; Foresti, R.; Motterlini, R. Treatment with CO-RMs during cold storage improves renal function at reperfusion. Kidney Int. 2006, 69, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Nakao, A.; Faleo, G.; Shimizu, H.; Nakahira, K.; Kohmoto, J.; Sugimoto, R.; Choi, A.M.; McCurry, K.R.; Takahashi, T.; Murase, N. Ex vivo carbon monoxide prevents cytochrome P450 degradation and ischemia/reperfusion injury of kidney grafts. Kidney Int. 2008, 74, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizarro, M.D.; Rodriguez, J.V.; Mamprin, M.E.; Fuller, B.J.; Mann, B.E.; Motterlini, R.; Guibert, E.E. Protective effects of a carbon monoxide-releasing molecule (CORM-3) during hepatic cold preservation. Cryobiology 2009, 58, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kaizu, T.; Ikeda, A.; Nakao, A.; Tsung, A.; Toyokawa, H.; Ueki, S.; Geller, D.A.; Murase, N. Protection of transplant-induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway downregulation. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G236–G244. [Google Scholar] [CrossRef] [Green Version]
- Kohmoto, J.; Nakao, A.; Sugimoto, R.; Wang, Y.; Zhan, J.; Ueda, H.; McCurry, K.R. Carbon monoxide-saturated preservation solution protects lung grafts from ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2008, 136, 1067–1075. [Google Scholar] [CrossRef]
- Minamoto, K.; Harada, H.; Lama, V.N.; Fedarau, M.A.; Pinsky, D.J. Reciprocal regulation of airway rejection by the inducible gas-forming enzymes heme oxygenase and nitric oxide synthase. J. Exp. Med. 2005, 202, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.S.; Gao, W.; Mazzola, S.; Thomas, M.N.; Csizmadia, E.; Otterbein, L.E.; Bach, F.H.; Wang, H. Heme oxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007, 21, 3450–3457. [Google Scholar] [CrossRef] [PubMed]
- Nakao, A.; Toyokawa, H.; Tsung, A.; Nalesnik, M.A.; Stolz, D.B.; Kohmoto, J.; Ikeda, A.; Tomiyama, K.; Harada, T.; Takahashi, T.; et al. Ex vivo application of carbon monoxide in University of Wisconsin solution to prevent intestinal cold ischemia/reperfusion injury. Am. J. Transplant. 2006, 6, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Bojakowski, K.; Gaciong, Z.; Grochowiecki, T.; Szmidt, J. Carbon monoxide may reduce ischemia reperfusion injury: A case report of complicated kidney transplantation from a carbon monoxide poisoned donor. Transplant. Proc. 2007, 39, 2928–2929. [Google Scholar] [CrossRef]
- Motterlini, R.; Mann, B.E.; Foresti, R. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin. Investig. Drugs 2005, 14, 1305–1318. [Google Scholar] [CrossRef]
- Ryter, S.W.; Choi, A.M. Cytoprotective and anti-inflammatory actions of carbon monoxide in organ injury and sepsis models. Novartis Found. Symp. 2007, 280, 165–175. [Google Scholar]
- Nobre, L.S.; Jeremias, H.; Romao, C.C.; Saraiva, L.M. Examining the antimicrobial activity and toxicity to animal cells of different types of CO-releasing molecules. Dalton Trans. 2016, 45, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Gonzales, A.; Foresti, R.; Clark, J.E.; Green, C.J.; Winslow, R.M. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ. Res. 1998, 83, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Taille, C.; El-Benna, J.; Lanone, S.; Boczkowski, J.; Motterlini, R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J. Biol. Chem. 2005, 280, 25350–25360. [Google Scholar] [CrossRef]
- Siow, R.C.; Sato, H.; Mann, G.E. Heme oxygenase-carbon monoxide signalling pathway in atherosclerosis: Anti-atherogenic actions of bilirubin and carbon monoxide? Cardiovasc. Res. 1999, 41, 385–394. [Google Scholar] [CrossRef]
- Crespy, D.; Landfester, K.; Schubert, U.S.; Schiller, A. Potential photoactivated metallopharmaceuticals: From active molecules to supported drugs. Chem. Commun. 2010, 46, 6651–6662. [Google Scholar] [CrossRef]
- Otterbein, L.E. The evolution of carbon monoxide into medicine. Respir. Care 2009, 54, 925–932. [Google Scholar] [CrossRef]
- Omaye, S.T. Metabolic modulation of carbon monoxide toxicity. Toxicology 2002, 180, 139–150. [Google Scholar] [CrossRef]
- Palao, E.; Slanina, T.; Muchová, L.; Šolomek, T.; Vítek, L.; Klán, P. Transition-Metal-Free CO-Releasing BODIPY Derivatives Activatable by Visible to NIR Light as Promising Bioactive Molecules. J. Am. Chem. Soc. 2016, 138, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Pierri, A.E.; Huang, P.J.; Garcia, J.V.; Stanfill, J.G.; Chui, M.; Wu, G.; Zheng, N.; Ford, P.C. A photoCORM nanocarrier for CO release using NIR light. Chem. Commun. 2015, 51, 2072–2075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Kiesewetter, D.O.; Qu, Y.; Fu, X.; Fan, J.; Huang, P.; Liu, Y.; Zhu, G.; Liu, Y.; Qian, Z.; et al. NIR-Responsive On-Demand Release of CO from Metal Carbonyl-Caged Graphene Oxide Nanomedicine. Adv. Mater. 2015, 27, 6741–6746. [Google Scholar] [CrossRef]
- Mede, R.; Hoffmann, P.; Klein, M.; Goerls, H.; Schmitt, M.; Neugebauer, U.; Gessner, G.; Heinemann, S.H.; Popp, J.; Westerhausen, M. A Water-Soluble Mn(CO)3-Based and Non-Toxic PhotoCORM for Administration of Carbon Monoxide Inside of Cells. Z. Anorg. Allg. Chem. 2017, 643, 2057–2062. [Google Scholar] [CrossRef]
- Carmona, F.J.; Rojas, S.; Sanchez, P.; Jeremias, H.; Marques, A.R.; Romao, C.C.; Choquesillo-Lazarte, D.; Navarro, J.A.; Maldonado, C.R.; Barea, E. Cation Exchange Strategy for the Encapsulation of a Photoactive CO-Releasing Organometallic Molecule into Anionic Porous Frameworks. Inorg. Chem. 2016, 55, 6525–6531. [Google Scholar] [CrossRef]
- Foresti, R.; Bani-Hani, M.G.; Motterlini, R. Use of carbon monoxide as a therapeutic agent: Promises and challenges. Intensive Care Med. 2008, 34, 649–658. [Google Scholar] [CrossRef]
- Kueh, J.T.B.; Stanley, N.J.; Hewitt, R.J.; Woods, L.M.; Larsen, L.; Harrison, J.C.; Rennison, D.; Brimble, M.A.; Sammut, I.A.; Larsen, D.S. Norborn-2-en-7-ones as physiologically-triggered carbon monoxide-releasing prodrugs. Chem. Sci. 2017, 8, 5454–5459. [Google Scholar] [CrossRef]
- Romão, C.C.; Blättler, W.A.; Seixas, J.D.; Bernardes, G.J.L. Developing drug molecules for therapy with carbon monoxide. Chem. Soc. Rev. 2012, 41, 3571–3583. [Google Scholar] [CrossRef] [PubMed]
- Stamellou, E.; Storz, D.; Botov, S.; Ntasis, E.; Wedel, J.; Sollazzo, S.; Krämer, B.K.; van Son, W.; Seelen, M.; Schmalz, H.G.; et al. Different design of enzyme-triggered CO-releasing molecules (ET-CORMs) reveals quantitative differences in biological activities in terms of toxicity and inflammation. Redox Biol. 2014, 2, 739–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, P.; Wang, C.; Shi, Z.; Johns, V.K.; Ma, L.; Oyer, J.; Copik, A.; Igarashi, R.; Liao, Y. Visible-light activatable organic CO-releasing molecules (PhotoCORMs) that simultaneously generate fluorophores. Org. Biomol. Chem. 2013, 11, 6671–6674. [Google Scholar] [CrossRef] [PubMed]
- Schatzschneider, U. Photoactivated Biological Activity of Transition-Metal Complexes. Eur. J. Inorg. Chem. 2010, 2010, 1451–1467. [Google Scholar] [CrossRef]
- Seixas, J.D.; Mukhopadhyay, A.; Santos-Silva, T.; Otterbein, L.E.; Gallo, D.J.; Rodrigues, S.S.; Guerreiro, B.H.; Gonçalves, A.M.L.; Penacho, N.; Marques, A.R.; et al. Characterization of a versatile organometallic pro-drug (CORM) for experimental CO based therapeutics. Dalton Trans. 2013, 42, 5985–5998. [Google Scholar] [CrossRef]
- Queiroga, C.S.F.; Vercelli, A.; Vieira, H.L.A. Carbon monoxide and the CNS: Challenges and achievements. Br. J. Pharmacol. 2015, 172, 1533–1545. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, U.; van der Vlies, A.J.; Simeoni, E.; Wandrey, C.; Hubbell, J.A. Carbon monoxide-releasing micelles for immunotherapy. J. Am. Chem. Soc. 2010, 132, 18273–18280. [Google Scholar] [CrossRef]
- Park, S.S.; Kim, J.; Lee, Y. Improved electrochemical microsensor for the real-time simultaneous analysis of endogenous nitric oxide and carbon monoxide generation. Anal. Chem. 2012, 84, 1792–1796. [Google Scholar] [CrossRef]
- Morimoto, Y.; Durante, W.; Lancaster, D.G.; Klattenhoff, J.; Tittel, F.K. Real-time measurements of endogenous CO production from vascular cells using an ultrasensitive laser sensor. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H483–H488. [Google Scholar] [CrossRef]
- Marks, G.S.; Vreman, H.J.; McLaughlin, B.E.; Brien, J.F.; Nakatsu, K. Measurement of endogenous carbon monoxide formation in biological systems. Antioxid. Redox Signal. 2002, 4, 271–277. [Google Scholar] [CrossRef]
- Barbe, J.M.; Canard, G.; Brandes, S.; Guilard, R. Selective chemisorption of carbon monoxide by organic-inorganic hybrid materials incorporating cobalt(III) corroles as sensing components. Chemistry 2007, 13, 2118–2129. [Google Scholar] [CrossRef]
- McLean, S.; Mann, B.E.; Poole, R.K. Sulfite species enhance carbon monoxide release from CO-releasing molecules: Implications for the deoxymyoglobin assay of activity. Anal. Biochem. 2012, 427, 36–40. [Google Scholar] [CrossRef]
- Esteban, J.; Ros-Lis, J.V.; Martinez-Manez, R.; Marcos, M.D.; Moragues, M.; Soto, J.; Sancenon, F. Sensitive and selective chromogenic sensing of carbon monoxide by using binuclear rhodium complexes. Angew. Chem. 2010, 49, 4934–4937. [Google Scholar] [CrossRef]
- Nandi, C.; Debnath, R.; Debroy, P. Intelligent Control Systems for Carbon Monoxide Detection in IoT Environments. In Guide to Ambient Intelligence in the IoT Environment: Principles, Technologies and Applications; Mahmood, Z., Ed.; Springer International Publishing: Cham, Switerzland, 2019; pp. 153–176. [Google Scholar]
- Vreman, H.J.; Stevenson, D.K. Heme oxygenase activity as measured by carbon monoxide production. Anal. Biochem. 1988, 168, 31–38. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Tan, L.; Zheng, K.; Huang, W. Lighting up carbon monoxide: Fluorescent probes for monitoring CO in living cells. Angew. Chem. 2013, 52, 1628–1630. [Google Scholar] [CrossRef]
- Michel, B.W.; Lippert, A.R.; Chang, C.J. A Reaction-Based Fluorescent Probe for Selective Imaging of Carbon Monoxide in Living Cells Using a Palladium-Mediated Carbonylation. J. Am. Chem. Soc. 2012, 134, 15668–15671. [Google Scholar] [CrossRef]
- Bauschlicher, C.W., Jr; Bagus, P.S. The metal–carbonyl bond in Ni(CO)4 and Fe(CO)5: A clear–cut analysis. J. Chem. Phys. 1984, 81, 5889–5898. [Google Scholar] [CrossRef]
- Schatzschneider, U. Novel lead structures and activation mechanisms for CO-releasing molecules (CORMs). Br. J. Pharmacol. 2015, 172, 1638–1650. [Google Scholar] [CrossRef]
- Kitazawa, M.; Wagner, J.R.; Kirby, M.L.; Anantharam, V.; Kanthasamy, A.G. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganese tricarbonyl. J. Pharmacol. Exp. Ther. 2002, 302, 26–35. [Google Scholar] [CrossRef]
- Gessner, G.; Sahoo, N.; Swain, S.M.; Hirth, G.; Schonherr, R.; Mede, R.; Westerhausen, M.; Brewitz, H.H.; Heimer, P.; Imhof, D.; et al. CO-independent modification of K(+) channels by tricarbonyldichlororuthenium(II) dimer (CORM-2). Eur. J. Pharmacol. 2017, 815, 33–41. [Google Scholar] [CrossRef]
- Mansour, A.M. Rapid green and blue light-induced CO release from bromazepam Mn(I) and Ru(II) carbonyls: Synthesis, density functional theory and biological activity evaluation. Appl. Organomet. Chem. 2017, 31, e3564. [Google Scholar] [CrossRef]
- Carmona, F.J.; Jimenez-Amezcua, I.; Rojas, S.; Romao, C.C.; Navarro, J.A.R.; Maldonado, C.R.; Barea, E. Aluminum Doped MCM-41 Nanoparticles as Platforms for the Dual Encapsulation of a CO-Releasing Molecule and Cisplatin. Inorg. Chem. 2017, 56, 10474–10480. [Google Scholar] [CrossRef]
- Wareham, L.K.; McLean, S.; Begg, R.; Rana, N.; Ali, S.; Kendall, J.J.; Sanguinetti, G.; Mann, B.E.; Poole, R.K. The Broad-Spectrum Antimicrobial Potential of [Mn(CO)4(S2CNMe(CH2CO2H))], a Water-Soluble CO-Releasing Molecule (CORM-401): Intracellular Accumulation, Transcriptomic and Statistical Analyses, and Membrane Polarization. Antioxid. Redox Signal. 2018, 28, 1286–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, A.M.; Shehab, O.R. Reactivity of visible-light induced CO releasing thiourea-based Mn(I) tricarbonyl bromide (CORM-NS1) towards lysozyme. Inorg. Chim. Acta 2018, 480, 159–165. [Google Scholar] [CrossRef]
- Aucott, B.J.; Ward, J.S.; Andrew, S.G.; Milani, J.; Whitwood, A.C.; Lynam, J.M.; Parkin, A.; Fairlamb, I.J.S. Redox-tagged carbon monoxide-releasing molecules (CORMs): Ferrocene-containing [Mn(C-N)(CO)4] complexes as a promising new CORM class. Inorg. Chem. 2017, 56, 5431–5440. [Google Scholar] [CrossRef]
- Kretschmer, R.; Gessner, G.; Goerls, H.; Heinemann, S.H.; Westerhausen, M. Dicarbonyl-bis(cysteamine)iron(II): A light induced carbon monoxide releasing molecule based on iron (CORM-S1). J. Inorg. Biochem. 2011, 105, 6–9. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, Q.; Bai, Z.; Zhao, Q.; Wang, Z.; Chen, Y.; Liu, B. Synthesis and biological activities of carbonyl cobalt CORMs with selectively inhibiting cyclooxygenase-2. J. Organomet. Chem. 2018, 874, 49–62. [Google Scholar] [CrossRef]
- Gong, Y.; Zhang, T.; Li, M.; Xi, N.; Zheng, Y.; Zhao, Q.; Chen, Y.; Liu, B. Toxicity, bio-distribution and metabolism of CO-releasing molecules based on cobalt. Free Radic. Biol. Med. 2016, 97, 362–374. [Google Scholar] [CrossRef]
- Wong, J.; MacDonald, N.; Mottillo, C.; Hiskic, I.; Butler, I.S.; Friscic, T. Synthesis of Organometallic C0-Releasing Molecules (CORMs) in the Absence of a Bulk Organic Solvent; American Chemical Society: Denver, CO, USA, 2015; INOR-785. [Google Scholar]
- Finze, M.; Bernhardt, E.; Willner, H.; Lehmann, C.W.; Aubke, F. Homoleptic, sigma-bonded octahedral superelectrophilic metal carbonyl cations of iron(II), ruthenium(II), and osmium(II). Part 2: Syntheses and characterizations of [M(CO)(6)][BF(4)](2) (M = Fe, Ru, Os). Inorg. Chem. 2005, 44, 4206–4214. [Google Scholar] [CrossRef]
- Kottelat, E.; Chabert, V.; Crochet, A.; Fromm, K.M.; Zobi, F. Towards Cardiolite-Inspired Carbon Monoxide Releasing Molecules—Reactivity of d4, d5 Rhenium and d6 Manganese Carbonyl Complexes with Isocyanide Ligands. Eur. J. Inorg. Chem. 2015, 2015, 5628–5638. [Google Scholar] [CrossRef]
- Abeyrathna, N.; Washington, K.; Bashur, C.; Liao, Y. Nonmetallic carbon monoxide releasing molecules (CORMs). Org. Biomol. Chem. 2017, 15, 8692–8699. [Google Scholar] [CrossRef]
- Friis, S.D.; Taaning, R.H.; Lindhardt, A.T.; Skrydstrup, T. Silacarboxylic acids as efficient carbon monoxide releasing molecules: Synthesis and application in palladium-catalyzed carbonylation reactions. J. Am. Chem. Soc. 2011, 133, 18114–18117. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Sawle, P.; Hammad, J.; Bains, S.; Alberto, R.; Foresti, R.; Green, C.J. CORM-A1: A new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005, 19, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Neugebauer, U.; Schmitt, M.; Popp, J. Elucidation of the CO-Release Kinetics of CORM-A1 by Means of Vibrational Spectroscopy. Chemphyschem A Eur. J. Chem. Phys. Phys. Chem. 2016, 17, 985–993. [Google Scholar] [CrossRef]
- Antony, L.A.; Slanina, T.; Sebej, P.; Solomek, T.; Klan, P. Fluorescein analogue xanthene-9-carboxylic acid: A transition-metal-free CO releasing molecule activated by green light. Org. Lett. 2013, 15, 4552–4555. [Google Scholar] [CrossRef]
- Mondal, R.; Okhrimenko, A.N.; Shah, B.K.; Neckers, D.C. Photodecarbonylation of alpha-diketones: A mechanistic study of reactions leading to acenes. J. Phys.Chem. B 2008, 112, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.N.A.; Reuzel-Selke, A.; Jurisch, A.; Atrott, K.; Pascher, A.; Pratschke, J.; Buelow, R.; Neuhaus, P.; Volk, H.D.; Tullius, S.G. Induction of carbon monoxide in the donor reduces graft immunogenicity and chronic graft deterioration. Transplant. Proc. 2005, 37, 379–381. [Google Scholar] [CrossRef]
- Chauveau, C.; Bouchet, D.; Roussel, J.C.; Mathieu, P.; Braudeau, C.; Renaudin, K.; Tesson, L.; Soulillou, J.P.; Iyer, S.; Buelow, R.; et al. Gene Transfer of Heme Oxygenase-1 and Carbon Monoxide Delivery Inhibit Chronic Rejection. Am. J. Transplant. 2002, 2, 581–592. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.N.; Richards, J.M.; Esquer, H.J.; Benninghoff, A.D.; Arif, A.M.; Berreau, L.M. A Structurally-Tunable 3-Hydroxyflavone Motif for Visible Light-Induced Carbon Monoxide-Releasing Molecules (CORMs). ChemistryOpen 2015, 4, 590–594. [Google Scholar] [CrossRef]
- Petrovski, Ž.; Norton de Matos, M.R.P.; Braga, S.S.; Pereira, C.C.L.; Matos, M.L.; Gonçalves, I.S.; Pillinger, M.; Alves, P.M.; Romão, C.C. Synthesis, characterization and antitumor activity of 1,2-disubstituted ferrocenes and cyclodextrin inclusion complexes. J. Organomet. Chem. 2008, 693, 675–684. [Google Scholar] [CrossRef]
- Motterlini, R.; Clark, J.E.; Foresti, R.; Sarathchandra, P.; Mann, B.E.; Green, C.J. Carbon monoxide-releasing molecules: Characterization of biochemical and vascular activities. Circ. Res. 2002, 90, E17–E24. [Google Scholar] [CrossRef]
- Steiger, C.; Luhmann, T.; Meinel, L. Oral drug delivery of therapeutic gases—Carbon monoxide release for gastrointestinal diseases. J. Control. Release 2014, 189, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ferrandiz, M.L.; Maicas, N.; Garcia-Arnandis, I.; Terencio, M.C.; Motterlini, R.; Devesa, I.; Joosten, L.A.; van den Berg, W.B.; Alcaraz, M.J. Treatment with a CO-releasing molecule (CORM-3) reduces joint inflammation and erosion in murine collagen-induced arthritis. Ann. Rheum. Dis. 2008, 67, 1211–1217. [Google Scholar] [CrossRef]
- Nobre, L.S.; Seixas, J.D.; Romao, C.C.; Saraiva, L.M. Antimicrobial action of carbon monoxide-releasing compounds. Antimicrob. Agents Chemother. 2007, 51, 4303–4307. [Google Scholar] [CrossRef] [PubMed]
- Bikiel, D.E.; Gonzalez Solveyra, E.; Di Salvo, F.; Milagre, H.M.; Eberlin, M.N.; Correa, R.S.; Ellena, J.; Estrin, D.A.; Doctorovich, F. Tetrachlorocarbonyliridates: Water-soluble carbon monoxide releasing molecules rate-modulated by the sixth ligand. Inorg. Chem. 2011, 50, 2334–2345. [Google Scholar] [CrossRef] [PubMed]
- Braud, L.; Pini, M.; Wilson, J.L.; Czibik, G.; Sawaki, D.; Derumeaux, G.; Foresti, R.; Motterlini, R. A carbon monoxide-releasing molecule (CORM-401) induces a metabolic switch in adipocytes and improves insulin-sensitivity on high fat diet-induced obesity in mice. Arch. Cardiovasc. Dis. Suppl. 2018, 10, 188. [Google Scholar] [CrossRef]
- Wareham, L.K.; Poole, R.K.; Tinajero-Trejo, M. CO-releasing Metal Carbonyl Compounds as Antimicrobial Agents in the Post-antibiotic Era. J. Biol. Chem. 2015, 290, 18999–19007. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Atkin, A.J.; Thatcher, R.J.; Whitwood, A.C.; Fairlamb, I.J.; Lynam, J.M. Diversity and design of metal-based carbon monoxide-releasing molecules (CO-RMs) in aqueous systems: Revealing the essential trends. Dalton Trans. 2009, 4351–4358. [Google Scholar] [CrossRef] [PubMed]
- Seixas, J.D.; Santos, M.F.; Mukhopadhyay, A.; Coelho, A.C.; Reis, P.M.; Veiros, L.F.; Marques, A.R.; Penacho, N.; Goncalves, A.M.; Romao, M.J.; et al. A contribution to the rational design of Ru(CO)3Cl2L complexes for in vivo delivery of CO. Dalton Trans. 2015, 44, 5058–5075. [Google Scholar] [CrossRef]
- Suliman, H.B.; Zobi, F.; Piantadosi, C.A. Heme Oxygenase-1/Carbon Monoxide System and Embryonic Stem Cell Differentiation and Maturation into Cardiomyocytes. Antioxid. Redox Signal. 2016, 24, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Musameh, M.D.; Green, C.J.; Mann, B.E.; Fuller, B.J.; Motterlini, R. Improved myocardial function after cold storage with preservation solution supplemented with a carbon monoxide-releasing molecule (CORM-3). J. Heart Lung Transplant. 2007, 26, 1192–1198. [Google Scholar] [CrossRef]
- Ulbrich, F.; Hagmann, C.; Buerkle, H.; Romao, C.C.; Schallner, N.; Goebel, U.; Biermann, J. The Carbon monoxide releasing molecule ALF-186 mediates anti-inflammatory and neuroprotective effects via the soluble guanylate cyclase ß1 in rats’ retinal ganglion cells after ischemia and reperfusion injury. J. Neuroinflamm. 2017, 14, 130. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.Q.; Whitwood, A.C.; Fairlamb, I.J.; Lynam, J.M. Group 6 carbon monoxide-releasing metal complexes with biologically-compatible leaving groups. Inorg. Chem. 2010, 49, 8941–8952. [Google Scholar] [CrossRef]
- Long, L.; Jiang, X.; Wang, X.; Xiao, Z.; Liu, X. Water-soluble diiron hexacarbonyl complex as a CO-RM: Controllable CO-releasing, releasing mechanism and biocompatibility. Dalton Trans. 2013, 42, 15663–15669. [Google Scholar] [CrossRef]
- Zobi, F.; Degonda, A.; Schaub, M.C.; Bogdanova, A.Y. CO releasing properties and cytoprotective effect of cis-trans-[Re(II)(CO)2Br2L2]n complexes. Inorg. Chem. 2010, 49, 7313–7322. [Google Scholar] [CrossRef]
- Schatzschneider, U. PhotoCORMs: Light-triggered release of carbon monoxide from the coordination sphere of transition metal complexes for biological applications. Inorg. Chim. Acta 2011, 374, 19–23. [Google Scholar] [CrossRef]
- Bohlender, C.; Gläser, S.; Klein, M.; Weisser, J.; Thein, S.; Neugebauer, U.; Popp, J.; Wyrwa, R.; Schiller, A. Light-triggered CO release from nanoporous non-wovens. J. Mater. Chem. B 2014, 2, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Lomont, J.P.; Nguyen, S.C.; Harris, C.B. Exploring the utility of tandem thermal-photochemical CO delivery with CORM-2. Organometallics 2014, 33, 6179–6185. [Google Scholar] [CrossRef]
- Bannenberg, G.L.; Vieira, H.L. Therapeutic applications of the gaseous mediators carbon monoxide and hydrogen sulfide. Expert Opin. Ther. Pat. 2009, 19, 663–682. [Google Scholar] [CrossRef]
- Motterlini, R.; Mann, B.E.; Johnson, T.R.; Clark, J.E.; Foresti, R.; Green, C.J. Bioactivity and pharmacological actions of carbon monoxide-releasing molecules. Curr. Pharm. Des. 2003, 9, 2525–2539. [Google Scholar] [CrossRef]
- Fiumana, E.; Parfenova, H.; Jaggar, J.H.; Leffler, C.W. Carbon monoxide mediates vasodilator effects of glutamate in isolated pressurized cerebral arterioles of newborn pigs. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1073–H1079. [Google Scholar] [CrossRef] [Green Version]
- Cepinskas, G.; Katada, K.; Bihari, A.; Potter, R.F. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G184–G191. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, V.G.; Chawla, N.; Mangla, D.; Gomes, S.B.; Arkebauer, M.R.; Wasko, K.A.; Sadacharam, K.; Vosseller, K. Carbon monoxide-releasing molecule-2 enhances coagulation in rabbit plasma and decreases bleeding time in clopidogrel/aspirin-treated rabbits. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2011, 22, 756–759. [Google Scholar] [CrossRef]
- Santos-Silva, T.; Mukhopadhyay, A.; Seixas, J.D.; Bernardes, G.J.; Romao, C.C.; Romao, M.J. Towards improved therapeutic CORMs: Understanding the reactivity of CORM-3 with proteins. Curr. Med. Chem. 2011, 18, 3361–3366. [Google Scholar] [CrossRef]
- Maines, M.D. Heme oxygenase: Function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J. 1988, 2, 2557–2568. [Google Scholar] [CrossRef]
- Freitas, A.; Alves-Filho, J.C.; Secco, D.D.; Neto, A.F.; Ferreira, S.H.; Barja-Fidalgo, C.; Cunha, F.Q. Heme oxygenase/carbon monoxide-biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br. J. Pharmacol. 2006, 149, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Chaves-Ferreira, M.; Albuquerque, I.S.; Matak-Vinkovic, D.; Coelho, A.C.; Carvalho, S.M.; Saraiva, L.M.; Romao, C.C.; Bernardes, G.J. Spontaneous CO release from Ru(II)(CO)2-protein complexes in aqueous solution, cells, and mice. Angew. Chem. 2015, 54, 1172–1175. [Google Scholar] [CrossRef]
- Chora, A.A.; Fontoura, P.; Cunha, A.; Pais, T.F.; Cardoso, S.; Ho, P.P.; Lee, L.Y.; Sobel, R.A.; Steinman, L.; Soares, M.P. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J. Clin. Investig. 2007, 117, 438–447. [Google Scholar] [CrossRef]
- Vitek, L.; Gbelcova, H.; Muchova, L.; Vanova, K.; Zelenka, J.; Konickova, R.; Suk, J.; Zadinova, M.; Knejzlik, Z.; Ahmad, S.; et al. Antiproliferative effects of carbon monoxide on pancreatic cancer. Dig. Liver Dis. 2014, 46, 369–375. [Google Scholar] [CrossRef]
- Chlopicki, S.; Lomnicka, M.; Fedorowicz, A.; Grochal, E.; Kramkowski, K.; Mogielnicki, A.; Buczko, W.; Motterlini, R. Inhibition of platelet aggregation by carbon monoxide-releasing molecules (CO-RMs): Comparison with NO donors. Naunyn Schmiedeberg’s Arch. Pharmacol. 2012, 385, 641–650. [Google Scholar] [CrossRef]
- Chung, S.W.; Liu, X.; Macias, A.A.; Baron, R.M.; Perrella, M.A. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Investig. 2008, 118, 239–247. [Google Scholar] [CrossRef]
- Desmard, M.; Davidge, K.S.; Bouvet, O.; Morin, D.; Roux, D.; Foresti, R.; Ricard, J.D.; Denamur, E.; Poole, R.K.; Montravers, P.; et al. A carbon monoxide-releasing molecule (CORM-3) exerts bactericidal activity against Pseudomonas aeruginosa and improves survival in an animal model of bacteraemia. FASEB J. 2009, 23, 1023–1031. [Google Scholar] [CrossRef]
- Desmard, M.; Foresti, R.; Morin, D.; Dagouassat, M.; Berdeaux, A.; Denamur, E.; Crook, S.H.; Mann, B.E.; Scapens, D.; Montravers, P.; et al. Differential antibacterial activity against Pseudomonas aeruginosa by carbon monoxide-releasing molecules. Antioxid. Redox Signal. 2012, 16, 153–163. [Google Scholar] [CrossRef]
- Murray, T.S.; Okegbe, C.; Gao, Y.; Kazmierczak, B.I.; Motterlini, R.; Dietrich, L.E.; Bruscia, E.M. The carbon monoxide releasing molecule CORM-2 attenuates Pseudomonas aeruginosa biofilm formation. PLoS ONE 2012, 7, e35499. [Google Scholar] [CrossRef]
- Wegiel, B.; Larsen, R.; Gallo, D.; Chin, B.Y.; Harris, C.; Mannam, P.; Kaczmarek, E.; Lee, P.J.; Zuckerbraun, B.S.; Flavell, R.; et al. Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation. J. Clin. Investig. 2014, 124, 4926–4940. [Google Scholar] [CrossRef]
- Otterbein, L.E.; May, A.; Chin, B.Y. Carbon monoxide increases macrophage bacterial clearance through Toll-like receptor (TLR)4 expression. Cell. Mol. Biol. 2005, 51, 433–440. [Google Scholar]
- Tavares, A.F.; Teixeira, M.; Romao, C.C.; Seixas, J.D.; Nobre, L.S.; Saraiva, L.M. Reactive oxygen species mediate bactericidal killing elicited by carbon monoxide-releasing molecules. J. Biol. Chem. 2011, 286, 26708–26717. [Google Scholar] [CrossRef]
- Hu, C.M.; Lin, H.H.; Chiang, M.T.; Chang, P.F.; Chau, L.Y. Systemic expression of heme oxygenase-1 ameliorates type 1 diabetes in NOD mice. Diabetes 2007, 56, 1240–1247. [Google Scholar] [CrossRef]
- Wegiel, B.; Gallo, D.J.; Raman, K.G.; Karlsson, J.M.; Ozanich, B.; Chin, B.Y.; Tzeng, E.; Ahmad, S.; Ahmed, A.; Baty, C.J.; et al. Nitric oxide-dependent bone marrow progenitor mobilization by carbon monoxide enhances endothelial repair after vascular injury. Circulation 2010, 121, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Boyer, C. Macromolecular and Inorganic Nanomaterials Scaffolds for Carbon Monoxide Delivery: Recent Developments and Future Trends. ACS Biomater. Sci. Eng. 2015, 1, 895–913. [Google Scholar] [CrossRef]
- Kautz, A.C.; Kunz, P.C.; Janiak, C. CO-releasing molecule (CORM) conjugate systems. Dalton Trans. 2016, 45, 18045–18063. [Google Scholar] [CrossRef] [Green Version]
- Schallner, N.; Otterbein, L.E. Friend or foe? Carbon monoxide and the mitochondria. Front. Physiol. 2015, 6, 17. [Google Scholar] [CrossRef]
- Diring, S.; Carné-Sánchez, A.; Zhang, J.; Ikemura, S.; Kim, C.; Inaba, H.; Kitagawa, S.; Furukawa, S. Light responsive metal–organic frameworks as controllable CO-releasing cell culture substrates. Chem. Sci. 2017, 8, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Noei, H.; Mienert, B.; Niesel, J.; Bill, E.; Muhler, M.; Fischer, R.A.; Wang, Y.; Schatzschneider, U.; Metzler-Nolte, N. Iron metal-organic frameworks MIL-88B and NH2-MIL-88B for the loading and delivery of the gasotransmitter carbon monoxide. Chemistry 2013, 19, 6785–6790. [Google Scholar] [CrossRef]
- Pai, S.; Radacki, K.; Schatzschneider, U. Sonogashira, CuAAC, and Oxime Ligations for the Synthesis of MnI Tricarbonyl PhotoCORM Peptide Conjugates. Eur. J. Inorg. Chem. 2014, 2014, 2886–2895. [Google Scholar] [CrossRef]
- Pfeiffer, H.; Rojas, A.; Niesel, J.; Schatzschneider, U. Sonogashira and Click reactions for the N-terminal and side-chain functionalization of peptides with [Mn(CO)3(tpm)]+-based CO releasing molecules (tpm = tris(pyrazolyl)methane). Dalton Trans. 2009, 4292–4298. [Google Scholar] [CrossRef]
- Jackson, C.S.; Schmitt, S.; Dou, Q.P.; Kodanko, J.J. Synthesis, characterization, and reactivity of the stable iron carbonyl complex [Fe(CO)(N4Py)](ClO4)2: Photoactivated carbon monoxide release, growth inhibitory activity, and peptide ligation. Inorg. Chem. 2011, 50, 5336–5338. [Google Scholar] [CrossRef]
- Matson, J.B.; Webber, M.J.; Tamboli, V.K.; Weber, B.; Stupp, S.I. A peptide-based material for therapeutic carbon monoxide delivery. Soft Matter 2012, 8, 6689–6692. [Google Scholar] [CrossRef]
- Bischof, C.; Joshi, T.; Dimri, A.; Spiccia, L.; Schatzschneider, U. Synthesis, spectroscopic properties, and photoinduced CO-release studies of functionalized ruthenium(II) polypyridyl complexes: Versatile building blocks for development of CORM-peptide nucleic acid bioconjugates. Inorg. Chem. 2013, 52, 9297–9308. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, H.; Sowik, T.; Schatzschneider, U. Bioorthogonal oxime ligation of a Mo(CO)4(N–N) CO-releasing molecule (CORM) to a TGF β-binding peptide. J. Organomet. Chem. 2013, 734, 17–24. [Google Scholar] [CrossRef]
- Yin, H.; Fang, J.; Liao, L.; Nakamura, H.; Maeda, H. Styrene-maleic acid copolymer-encapsulated CORM2, a water-soluble carbon monoxide (CO) donor with a constant CO-releasing property, exhibits therapeutic potential for inflammatory bowel disease. J. Control. Release 2014, 187, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Tabe, H.; Fujita, K.; Abe, S.; Tsujimoto, M.; Kuchimaru, T.; Kizaka-Kondoh, S.; Takano, M.; Kitagawa, S.; Ueno, T. Preparation of a Cross-Linked Porous Protein Crystal Containing Ru Carbonyl Complexes as a CO-Releasing Extracellular Scaffold. Inorg. Chem. 2015, 54, 215–220. [Google Scholar] [CrossRef]
- Tabe, H.; Shimoi, T.; Fujita, K.; Abe, S.; Ijiri, H.; Tsujimoto, M.; Kuchimaru, T.; Kizaka-Kondo, S.; Mori, H.; Kitagawa, S.; et al. Design of a CO-releasing Extracellular Scaffold Using in Vivo Protein Crystals. Chem. Lett. 2015, 44, 342–344. [Google Scholar] [CrossRef]
- Albuquerque, I.S.; Jeremias, H.F.; Chaves-Ferreira, M.; Matak-Vinkovic, D.; Boutureira, O.; Romão, C.C.; Bernardes, G.J.L. An artificial CO-releasing metalloprotein built by histidine-selective metallation. Chem. Commun. 2015, 51, 3993–3996. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Tanaka, Y.; Sho, T.; Ozeki, S.; Abe, S.; Hikage, T.; Kuchimaru, T.; Kizaka-Kondoh, S.; Ueno, T. Intracellular CO release from composite of ferritin and ruthenium carbonyl complexes. J. Am. Chem. Soc. 2014, 136, 16902–16908. [Google Scholar] [CrossRef]
- Zobi, F.; Blacque, O.; Jacobs, R.A.; Schaub, M.C.; Bogdanova, A.Y. 17 e−rhenium dicarbonyl CO-releasing molecules on a cobalamin scaffold for biological application. Dalton Trans. 2012, 41, 370–378. [Google Scholar] [CrossRef]
- Wilson, J.L.; Fayad Kobeissi, S.; Oudir, S.; Haas, B.; Michel, B.; Dubois Rande, J.L.; Ollivier, A.; Martens, T.; Rivard, M.; Motterlini, R.; et al. Design and synthesis of new hybrid molecules that activate the transcription factor Nrf2 and simultaneously release carbon monoxide. Chemistry 2014, 20, 14698–14704. [Google Scholar] [CrossRef]
- Pena, A.C.; Penacho, N.; Mancio-Silva, L.; Neres, R.; Seixas, J.D.; Fernandes, A.C.; Romao, C.C.; Mota, M.M.; Bernardes, G.J.; Pamplona, A. A novel carbon monoxide-releasing molecule fully protects mice from severe malaria. Antimicrob. Agents Chemother. 2012, 56, 1281–1290. [Google Scholar] [CrossRef]
- Marques, A.R.; Kromer, L.; Gallo, D.J.; Penacho, N.; Rodrigues, S.S.; Seixas, J.D.; Bernardes, G.J.L.; Reis, P.M.; Otterbein, S.L.; Ruggieri, R.A.; et al. Generation of Carbon Monoxide Releasing Molecules (CO-RMs) as Drug Candidates for the Treatment of Acute Liver Injury: Targeting of CO-RMs to the Liver. Organometallics 2012, 31, 5810–5822. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef]
- Brückmann, N.E.; Wahl, M.; Reiß, G.J.; Kohns, M.; Wätjen, W.; Kunz, P.C. Polymer Conjugates of Photoinducible CO-Releasing Molecules. Eur. J. Inorg. Chem. 2011, 2011, 4571–4577. [Google Scholar] [CrossRef]
- Kunz, P.C.; Brückmann, N.E.; Spingler, B. Towards Polymer Diagnostic Agents―Copolymers of N-(2-Hydroxypropyl)methacrylamide and Bis(2-pyridylmethyl)-4-vinylbenzylamine: Synthesis, Characterisation and Re(CO)3-Labelling. Eur. J. Inorg. Chem. 2007, 2007, 394–399. [Google Scholar] [CrossRef]
- Dördelmann, G.; Pfeiffer, H.; Birkner, A.; Schatzschneider, U. Silicium Dioxide Nanoparticles As Carriers for Photoactivatable CO-Releasing Molecules (PhotoCORMs). Inorg. Chem. 2011, 50, 4362–4367. [Google Scholar] [CrossRef]
- Gonzales, M.A.; Han, H.; Moyes, A.; Radinos, A.; Hobbs, A.J.; Coombs, N.; Oliver, S.R.J.; Mascharak, P.K. Light-triggered carbon monoxide delivery with Al-MCM-41-based nanoparticles bearing a designed manganese carbonyl complex. J. Mater. Chem. B 2014, 2, 2107–2113. [Google Scholar] [CrossRef]
- Dordelmann, G.; Meinhardt, T.; Sowik, T.; Krueger, A.; Schatzschneider, U. CuAAC click functionalization of azide-modified nanodiamond with a photoactivatable CO-releasing molecule (PhotoCORM) based on [Mn(CO)3(tpm)]+. Chem. Commun. 2012, 48, 11528–11530. [Google Scholar] [CrossRef]
- Kunz, P.C.; Meyer, H.; Barthel, J.; Sollazzo, S.; Schmidt, A.M.; Janiak, C. Metal carbonyls supported on iron oxide nanoparticles to trigger the CO-gasotransmitter release by magnetic heating. Chem. Commun. 2013, 49, 4896–4898. [Google Scholar] [CrossRef]
- Govender, P.; Pai, S.; Schatzschneider, U.; Smith, G.S. Next generation PhotoCORMs: Polynuclear tricarbonylmanganese(I)-functionalized polypyridyl metallodendrimers. Inorg. Chem. 2013, 52, 5470–5478. [Google Scholar] [CrossRef]
- Cadranel, A.; Pieslinger, G.E.; Tongying, P.; Kuno, M.K.; Baraldo, L.M.; Hodak, J.H. Spectroscopic signatures of ligand field states in {Ru(II)(imine)} complexes. Dalton Trans. 2016, 45, 5464–5475. [Google Scholar] [CrossRef]
- Gonzales, M.A. Iron, Manganese and Ruthenium Metal Carbonyls as Photoactive Carbon Monoxide Releasing Molecules (photoCORMS): Ligand Design Strategies, Syntheses and Structure Characterizations. Ph.D. Thesis, University of California, Santa Cruz, CA, USA, 2013. [Google Scholar]
- Khramov, D.M.; Lynch, V.M.; Bielawski, C.W. N-Heterocyclic Carbene−Transition Metal Complexes: Spectroscopic and Crystallographic Analyses of π-Back-bonding Interactions. Organometallics 2007, 26, 6042–6049. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, L.; Liu, Z. Drug delivery with upconversion nanoparticles for multi-functional targeted cancer cell imaging and therapy. Biomaterials 2011, 32, 1110–1120. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, Y.; Lin, C.; Hu, D.; Wang, F.; Chen, X.; Chen, Z.; Zheng, N. Multifunctional core-shell upconverting nanoparticles for imaging and photodynamic therapy of liver cancer cells. Chem. Asian J. 2012, 7, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.W.; Seo, Y.-K.; Hwang, Y.K.; Chang, J.-S.; Leclerc, H.; Wuttke, S.; Bazin, P.; Vimont, A.; Daturi, M.; Bloch, E.; et al. Controlled Reducibility of a Metal–Organic Framework with Coordinatively Unsaturated Sites for Preferential Gas Sorption. Angew. Chem. Intl. Ed. 2010, 49, 5949–5952. [Google Scholar] [CrossRef]
- van der Vlies, A.J.; Inubushi, R.; Uyama, H.; Hasegawa, U. Polymeric Framboidal Nanoparticles Loaded with a Carbon Monoxide Donor via Phenylboronic Acid-Catechol Complexation. Bioconjugate Chem. 2016, 27, 1500–1508. [Google Scholar] [CrossRef]
- Muhammad, N.; Mao, Q.; Xia, H. CAR T-cells for cancer therapy. Biotechnol. Genet. Eng. Rev. 2017, 33, 190–226. [Google Scholar] [CrossRef]
- Johnson, T.R.; Mann, B.E.; Teasdale, I.P.; Adams, H.; Foresti, R.; Green, C.J.; Motterlini, R. Metal carbonyls as pharmaceuticals? [Ru(CO)3Cl(glycinate)], a CO-releasing molecule with an extensive aqueous solution chemistry. Dalton Trans. 2007, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.P.; Choi, C.H.; Hao, L.; Calabrese, C.M.; Auyeung, E.; Zhang, C.; Goor, O.J.; Mirkin, C.A. The Sequence-Specific Cellular Uptake of Spherical Nucleic Acid Nanoparticle Conjugates. Small (Weinh. Bergstr. Ger. ) 2015, 11, 4173–4182. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.; Adnan, N.N.; Oliver, S.; Boyer, C. The Interaction of CORM-2 with Block Copolymers Containing Poly(4-vinylpyridine): Macromolecular Scaffolds for Carbon Monoxide Delivery in Biological Systems. Macromol. Rapid Comm. 2016, 37, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gallego, S.; Bernardes, G.J. Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo. Angew. Chem. 2014, 53, 9712–9721. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.L.; Scragg, J.L.; Peers, C. Modulation of hTREK-1 by carbon monoxide. Neuroreport 2008, 19, 345–348. [Google Scholar] [CrossRef]
- Lundvig, D.M.; Immenschuh, S.; Wagener, F.A. Heme oxygenase, inflammation, and fibrosis: The good, the bad, and the ugly? Front. Pharmacol. 2012, 3, 81. [Google Scholar] [CrossRef]
- Szeremeta, M.; Petelska, A.D.; Kotynska, J.; Niemcunowicz-Janica, A.; Figaszewski, Z.A. The effect of fatal carbon monoxide poisoning on the surface charge of blood cells. J. Membr. Biol. 2013, 246, 717–722. [Google Scholar] [CrossRef]
- Petelska, A.D.; Kotynska, J.; Figaszewski, Z.A. The effect of fatal carbon monoxide poisoning on the equilibria between cell membranes and the electrolyte solution. J. Membr. Biol. 2015, 248, 157–161. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Zhao, Q.; Chen, Y.; Liu, B.; Zhang, B.; Zheng, Q. Syntheses and evaluation of drug-like properties of CO-releasing molecules containing ruthenium and group 6 metal. Eur. J. Med. Chem. 2014, 74, 199–215. [Google Scholar] [CrossRef]
- Winburn, I.C.; Gunatunga, K.; McKernan, R.D.; Walker, R.J.; Sammut, I.A.; Harrison, J.C. Cell damage following carbon monoxide releasing molecule exposure: Implications for therapeutic applications. Basic Clin. Pharmacol. Toxicol. 2012, 111, 31–41. [Google Scholar] [CrossRef]






























| List | CORMs | Therapeutic Implications | Refs |
|---|---|---|---|
| 1 | CORM-1 | Increase coronary perfusion pressures; attenuates the L-NAME-mediated; restore unstable blood pressure and modulates vessel contractility ex-vivo in animals. | [95] |
| 2 | CORM-2 | Attenuates inflammatory response in lungs and liver; induces vasorelaxation; protects against IRI; activates K+/Ca+2 channels; possible for pulmonary hypertension. | [95,96] |
| 3 | CORM-3 | Improves the liver & kidney functions during transplantation; Vasorelaxation induction; prevents sepsis & cardiac graft rejection; helps in bacterial infections; support rheumatoid arthritis; RBF improvement in the treatment of cynomolgus for monkeys. | [23,28,71,97,98,99] |
| 4 | CORM-401 | Improves insulin-sensitivity and metabolic switch induces in adipocytes. | [100] |
| 5 | ALF492 | In severe malaria, fully protects with artesunate combination. | [101] |
| 6 | CORM-A1 | Induces the vasorelaxation; Increases RBF and reduced vascular; gives resistance in the kidney of mice; good cerebroprotective agent for epileptic seizures treatments. | [102] |
| 7 | Re-CORM-1 | Anti-oxidative characteristic and protects against IRI from the affected neonatal rat of cardiomyocytes. | [103] |
| 8 | B12-ReCORM-2 | Protects against IRI (neonatal rat cardio-myocyte); hindrance cell mortality up to 80%; support the cardiac repairing and cardiac disease (ameliorates degenerative); anti-oxidative agent; augments and direct cardiomyogenesis. | [104,105] |
| 9 | 3-hydroxyflavon CORMs | Exerting anti-oxidative activity; anti-inflammatory services and anti-cancer effects. | [46,55,89] |
| 10 | ALF 186 | Protective effects for gastric ulcers and neuro protective, while IRI-induced apoptosis of retinal ganglion cells (RGC). | [106,107,108,109] |
| 11 | CORMA-1-PLA | Prevents fibroblasts and internalized into 3T3 cells during metabolic and hypoxia depletion conditions. | [110] |
| 12 | α-DK-CORMs | Absorbs in acute myeloid leukemia (AML) KG-1 cells and releases CO In-vivo upon 470nm irradiation. | [111] |
| Sr. # | CORMats | Therapeutic Implications | Refs |
|---|---|---|---|
| 1 | Micellization | Bioactive in a murine model of inflammatory colitis; potential for curing the ROS affected inflammatory disease; In response to human monocytes and attenuates the LPS-induced inflammatory. | [55,59,145] |
| 2 | Proteins | Regulation the cytokines IL-6 and IL-10; artificial metallohydrolase performance and elevated NF-κB factor (10 folds). | [121,146,147,148,149] |
| 3 | Vitamins | Shows HO-1 expression; inducing nuclear accumulation of Nrf2; antimalarial drug artesunate and acute liver failure. | [150,151,152,153] |
| 4 | Polymers | HCT116 human colon cancer and HepG2 liver cancer cells; enhance the EPR effect and targeting the tumor sites; achieve special and selective physiological targets. | [47,154,155,156] |
| 5 | Porous structure materials | Inflammatory skin issues and topical skin cancer treatment. Surprisingly, no toxicity was found in mouse fibroblast 3T3 cells. | [50,111]. |
| 6 | Nanoparticles | Cysteine and subdues feedback to pro-inflammatory mediator’s IL-6; cardiovascular therapy and relax the rat aorta muscle rings. | [157,168] |
| 7 | Peptide | Human prostate cancer cell line (PC-3) and supported cardiomyocyte viability. | [139,140,141,142,143,144] |
| 8 | Nano-sheets | Controllable CO release (e.g. GO-MnCORMats) suitable for inflammatory diseases after LPS stimulation and responsive intracellular CO release. | [48] |
| 9 | Nano-diamond | Nano-diamond precursor compatible with photons, hopefully, could be modified for special cell targeting. | [159] |
| 10 | MOFs | Inflammatory bowel disease and expected pharmacological applications by downsizing the MOF crystals to the nanoscale. | [137,138] |
| 11 | Metallodendrimers | Potential for inflammatory disease and cancer cells. | [161] |
| Strategies | CO-Release Mechanism | Molecules/Materials |
|---|---|---|
| CO-releasing molecules (CORMs) | organometallics | CORM-1, CORM-2, CORM-3, ALF492, CORM-A1, B12-ReCORM-2, Re-CORM-1, CORMA-1-PLA and ALF186. |
| nonmetallic | Silica-carboxylates, boranocarbonates, boranocarbamates, xanthene carboxylic acid (XCA), hydroxy-flavones, 1,2-disubstituted ferrocenes, methylene chloride, meso-carboxy BODIPYs, unsaturated cyclic and diketones (DKs). | |
| CO-releasing materials (CORMats) | conjugated systems | Micellization, peptide, vitamins, proteins, polymers, metal organic framework, nanoparticles, nano-sheets, porous structure materials, metallodendrimer and nano-diamond. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizan, M.; Muhammad, N.; Niazi, K.U.K.; Hu, Y.; Wang, Y.; Wu, Y.; Sun, H.; Liu, R.; Dong, W.; Zhang, W.; et al. CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy. Materials 2019, 12, 1643. https://doi.org/10.3390/ma12101643
Faizan M, Muhammad N, Niazi KUK, Hu Y, Wang Y, Wu Y, Sun H, Liu R, Dong W, Zhang W, et al. CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy. Materials. 2019; 12(10):1643. https://doi.org/10.3390/ma12101643
Chicago/Turabian StyleFaizan, Muhammad, Niaz Muhammad, Kifayat Ullah Khan Niazi, Yongxia Hu, Yanyan Wang, Ya Wu, Huaming Sun, Ruixia Liu, Wensheng Dong, Weiqiang Zhang, and et al. 2019. "CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy" Materials 12, no. 10: 1643. https://doi.org/10.3390/ma12101643
APA StyleFaizan, M., Muhammad, N., Niazi, K. U. K., Hu, Y., Wang, Y., Wu, Y., Sun, H., Liu, R., Dong, W., Zhang, W., & Gao, Z. (2019). CO-Releasing Materials: An Emphasis on Therapeutic Implications, as Release and Subsequent Cytotoxicity Are the Part of Therapy. Materials, 12(10), 1643. https://doi.org/10.3390/ma12101643






