A Facile Synthesis of MoS2/g-C3N4 Composite as an Anode Material with Improved Lithium Storage Capacity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Material Characterization
2.3. Characterization of Electrochemical Properties
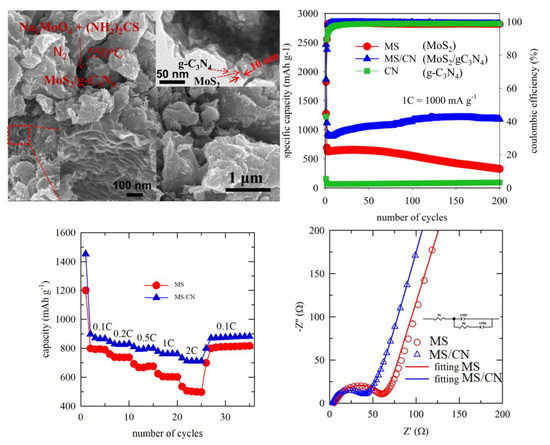
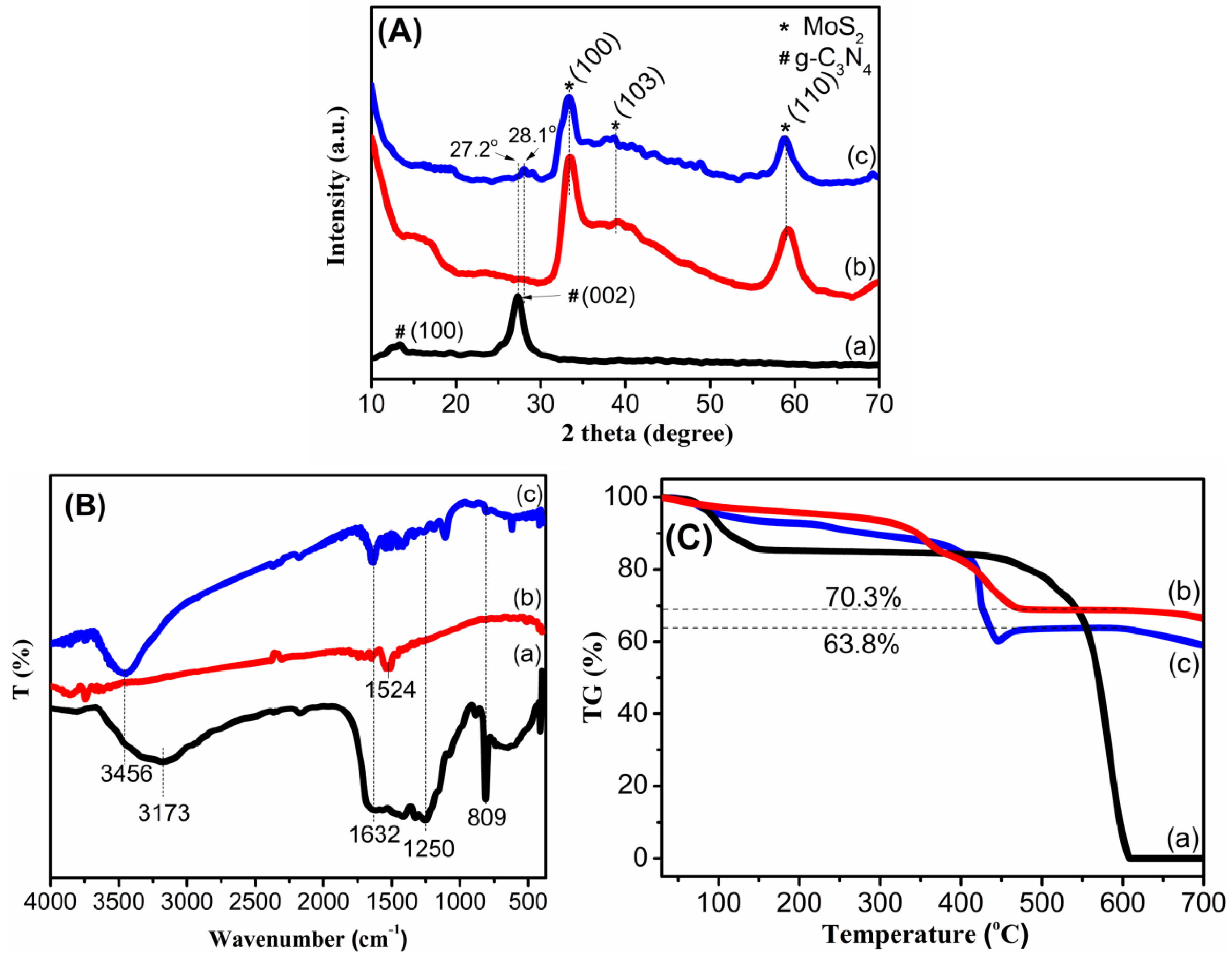
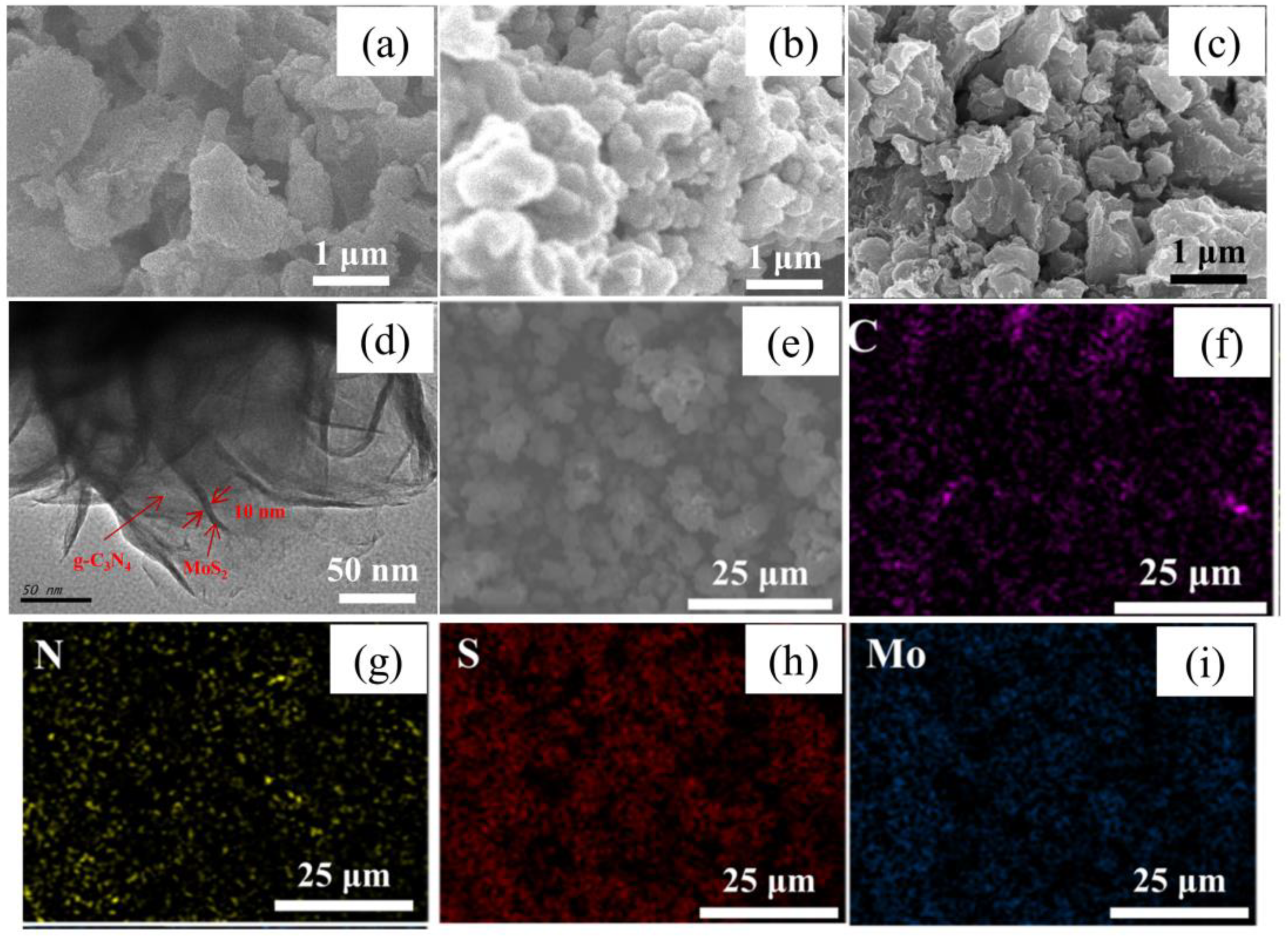
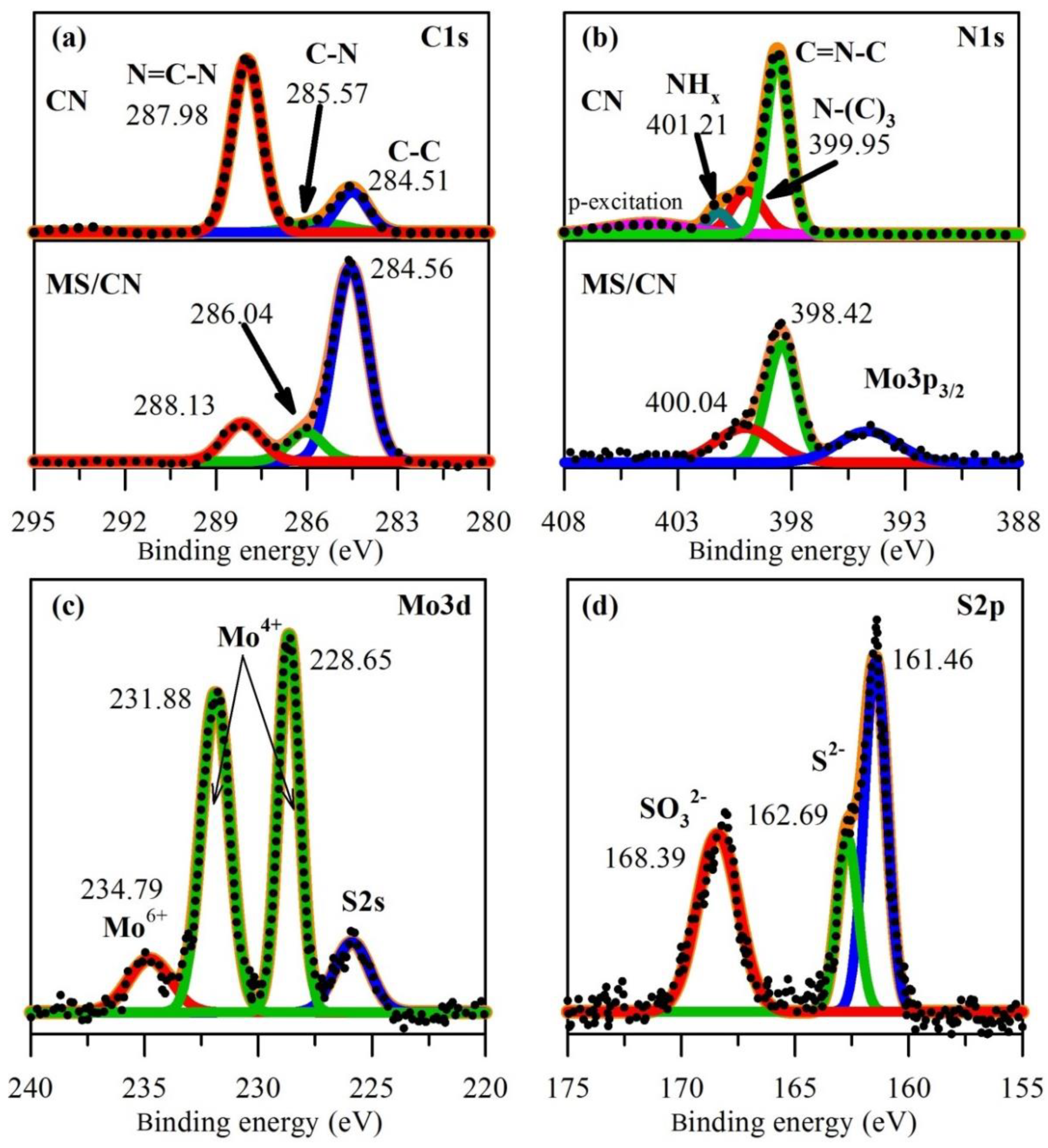
3. Results and Discussion
3.1. Characterization of the Materials
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.; Yu, L.; Lou, X.W.D. Nanostructured conversion-type anode materials for advanced lithium ion batteries. Chem 2018, 4, 972–996. [Google Scholar] [CrossRef]
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sust. Energ. Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of lithium-ion batteries: Recent advances and perspectives. J. Power Sour. 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. Recent advances in energy chemical engineering of next-generation lithium batteries. Engineering 2018, 4, 831–847. [Google Scholar] [CrossRef]
- Sun, H.; Wang, J.G.; Zhang, X.; Li, C.; Liu, F.; Zhu, W.; Shao, M. Nanoconfined construction of MoS2@C/MoS2 core-sheath nanowires for superior rate and durable Li-ion energy storage. ACS Sustain. Chem. Eng. 2019, 7, 5346–5354. [Google Scholar] [CrossRef]
- Obrovac, M.N.; Chevrier, V.L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 2014, 114, 11444–11502. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Zhang, X.F.; Cui, Y. High capacity li-ion battery anodes using Ge nanowires. Nano Lett. 2008, 8, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Tian, Z.; Fan, R.; Shao, L.; Zhang, D.; Cao, G.; Bai, Y. Research progress on silicon/carbon composite anode materials for lithium-ion battery. J. Energy Chem. 2018, 27, 1067–1090. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhi, H.; Yu, X. Recent progress in phosphorus based anode materials for lithium/sodium ion batteries. Energy Storage Mater. 2019, 16, 290–322. [Google Scholar] [CrossRef]
- Mei, J.; Liao, T.; Sun, Z. Two-dimensional metal oxide nanosheets for rechargeable batteries. J. Energy Chem. 2018, 27, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Sun, D.; Yu, X. Metal hydrides for lithium-ion battery application: A review. J. Alloys Compd. 2018, 769, 167–185. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, Y.; Li, H.; Peng, Y. The application of nanostructured transition metal sulfides as anodes for lithium ion batteries. J. Energy Chem. 2018, 27, 1536–1554. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.T.; Li, J.C.; Xue, H.G.; Guo, S.P. Binary iron sulfides as anode materials for rechargeable batteries: Crystal structures, syntheses, and electrochemical performance. J. Power Sources 2018, 379, 41–52. [Google Scholar] [CrossRef]
- Huang, J.; Wei, Z.; Liao, J.; Ni, W.; Wang, C.; Ma, J. Molybdenum and tungsten chalcogenides for lithium/sodium-ion batteries: Beyond MoS2. J. Energy Chem. 2019, 33, 100–124. [Google Scholar] [CrossRef]
- Ma, K.; Jiang, H.; Hu, Y.; Li, C. 2D nanospace confined synthesis of pseudocapacitance-dominated MoS2-in-Ti3C2 superstructure for ultrafast and stable Li/Na-Ion batteries. Adv. Funct. Mater. 2018, 28, 1804306. [Google Scholar] [CrossRef]
- Fang, Y.; Lv, Y.; Gong, F.; Elzatahry, A.A.; Zheng, G.; Zhao, D. Synthesis of 2D-mesoporous-carbon/MoS2 heterostructures with well-defined interfaces for high-performance lithium-ion batteries. Adv. Mater. 2016, 28, 9385–9390. [Google Scholar] [CrossRef]
- Nethravathi, C.; Prabhu, J.; Lakshmipriya, S.; Rajamathi, M. Magnetic Co-doped MoS2 nanosheets for efficient catalysis of nitroarene reduction. ACS Omega 2017, 2, 5891–5897. [Google Scholar] [CrossRef]
- Qiu, W.; Xia, J.; He, S.; Xu, H.; Zhong, H.; Chen, L. Facile synthesis of hollow MoS2 microspheres/amorphous carbon composites and their lithium storage properties. Electrochim. Acta 2014, 117, 145–152. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Maitra, U.; Waghmare, U.V. Extraordinary attributes of 2-dimensional MoS2 nanosheets. Chem. Phys. Lett. 2014, 609, 172–183. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Yan, Y.; Yu, Y.; Wang, Q.; Liu, M. A high areal capacitance for lithium ions storage achieved by a hierarchical carbon/MoS2 aerogel with vertically aligned pores. ACS Appl. Energy Mater. 2018, 1, 4814–4823. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, B.; Wang, X.; Yang, J.; Zhang, X.X.; Zhou, Y.; Chen, J. MoS2 nanosheets vertically grown on the carbonized corn stalks as lithium-ion battery anode. ACS Appl. Mater. Interfaces 2018, 10, 22067–22073. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, J.; Wen, Z.; Cui, S.; Yuan, C.; Chen, J. N-doped graphene/porous g-C3N4 nanosheets supported layered-MoS2 hybrid as robust anode materials for lithium-ion batteries. Nano Energy 2014, 8, 157–164. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Park, A.R.; Rauf, A.; Hong, S.H.; Choi, Y.; Park, J.; Yoo, P.J. Highly interdigitated and porous architected ternary composite of SnS2, g-C3N4, and reduced graphene oxide (rGO) as high performance lithium ion battery anodes. RSC Adv. 2017, 7, 3125–3135. [Google Scholar] [CrossRef]
- Tran, H.H.; Nguyen, P.H.; Cao, V.H.; Nguyen, L.T.; Tran, V.M.; Le, M.L.P.; Kim, S.J.; Vo, V. SnO2 nanosheets/graphite oxide/g-C3N4 composite as enhanced performance anode material for lithium ion batteries. Chem. Phys. Lett. 2019, 715, 284–292. [Google Scholar] [CrossRef]
- Vo, V.; Thi, X.D.N.; Jin, Y.S.; Thi, G.L.; Trung, N.T.; Quang, D.T.; Kim, S.J. SnO2 nanosheets/g-C3N4 composite with improved lithium storage capabilities. Chem. Phys. Lett. 2017, 674, 42–47. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Hau Ng, Y.; Yong, S.T.; Chai, S.P. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Wu, M.; Wang, Q.; Sun, Q.; Jena, P. Functionalized graphitic carbon nitride for efficient energy storage. J. Phys. Chem. C 2013, 117, 6055–6059. [Google Scholar] [CrossRef]
- Senthil, S.; Kishore, S.C.; Sasidharan, M. Ultrathin MoS2 sheets supported on N-rich carbon nitride nanospheres with enhanced lithium storage properties. Appl. Surf. Sci. 2017, 410, 215–224. [Google Scholar]
- Peng, W.; Li, X. Synthesis of MoS2/g-C3N4 as a solar light-responsive photocatalyst for organic degradation. Catal. Commun. 2014, 49, 63–67. [Google Scholar] [CrossRef]
- Liu, Y.D.; Ren, L.; Qi, X.; Yang, L.W.; Hao, G.L.; Li, J.; Wei, X.L.; Zhong, J.X. Preparation, characterization and photoelectrochemical property of ultrathin MoS2 nanosheets via hydrothermal intercalation and exfoliation route. J. Alloy. Compd. 2013, 57, 37–42. [Google Scholar] [CrossRef]
- Li, J.; Liu, E.; Ma, Y.; Hu, X.; Wan, J.; Sun, L.; Fan, J. Synthesis of MoS2/g-C3N4 nanosheets as 2D heterojunction photocatalysts with enhanced visible light activity. Appl. Surf. Sci. 2016, 364, 694–702. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Zscheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Lu, X.; Jin, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y. Controllable synthesis of graphitic C3N4/ultrathin MoS2 nanosheets hybrid nanostructures with enhanced photocatalytic performance. Dalton Trans. 2016, 45, 15406–15414. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liang, L.; Wang, F.; Liu, M.; Sun, J. Enhanced photocatalytic activity of degrading rhodamine B over MoS2/g-C3N4 photocatalyst under visible light. Energy Environ. Focus 2015, 4, 74–81. [Google Scholar] [CrossRef]
- Chen, J.; Mao, Z.; Zhang, L.; Wang, D.; Xu, R.; Bie, L.; Fahlman, B.D. Nitrogen-deficient graphitic carbon nitride with enhanced performance for lithium ion battery anodes. ACS Nano 2017, 11, 12650–12657. [Google Scholar] [CrossRef]
- Zou, H.; Yan, X.; Ren, J.; Wu, X.; Dai, Y.; Sha, D.; Pan, J.; Liu, J. Photocatalytic activity enhancement of modified g-C3N4 by ionothermal copolymerization. J. Mater. 2015, 1, 340–347. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Zhang, J.; Zhang, H.; Tian, W.; Li, X.; Tade, M.O.; Sun, H.; Wang, S. Flower-like MoS2 on graphitic carbon nitride for enhanced photocatalytic and electrochemical hydrogen evolutions. Appl. Catal. B 2018, 239, 334–344. [Google Scholar] [CrossRef]
- Zang, Y.; Li, L.; Li, X.; Lin, R.; Li, G. Synergistic collaboration of g-C3N4/SnO2 composites for enhanced visible-light photocatalytic activity. Chem. Eng. J. 2014, 246, 277–286. [Google Scholar] [CrossRef]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Liu, H.; Chen, D.; Wang, Z.; Jing, H.; Zhang, R. Microwave-assisted molten-salt rapid synthesis of isotype triazine-/heptazine based g-C3N4 heterojunctions with highly enhanced photocatalytic hydrogen evolution performance. App. Catal. B Environ. 2017, 203, 300–313. [Google Scholar] [CrossRef]
- Li, R.; Yang, L.; Xiong, T.; Wu, Y.; Cao, L.; Yuan, D.; Zhou, W. Nitrogen doped MoS2 nanosheets synthesized via a low-temperature process as electrocatalysts with enhanced activity for hydrogen evolution reaction. J. Power Sour. 2017, 356, 133–139. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, G.; Hou, Y.; Wang, X. Layering MoS2 on soft hollow g-C3N4 nanostructures for photocatalytic hydrogen evolution. Appl. Catal. A 2016, 521, 2–8. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, J.; Yang, Z.; Yu, J.; Wang, A.; Sang, Y.; Zhou, W.; Liu, H. One-step synthesis of CdS nanoparticles/MoS2 nanosheets heterostructure on porous molybdenum sheet for enhanced photocatalytic H2 evolution. Appl. Catal. B 2017, 210, 290–296. [Google Scholar] [CrossRef]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar] [CrossRef]
- Xu, X.; Fan, Z.; Yu, X.; Ding, S.; Yu, D.; Lou, X.W.D. A nanosheets-on-channel architecture constructed from MoS2 and CMK-3 for high-capacity and long-cycle-life lithium storage. Adv. Energy Mater. 2014, 4, 1400902. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Li, N.; Liu, Z.; Gao, Q.; Li, X.; Wang, R.; Yan, X.; Li, Y. In situ synthesis of concentric C@MoS2 core–shell nanospheres as anode for lithium ion battery. J. Mater. Sci. 2017, 52, 13183–13191. [Google Scholar] [CrossRef]
- Debela, T.T.; Lim, Y.R.; Seo, H.W.; Kwon, I.S.; Kwak, I.H.; Park, J.; Cho, W.I.; Kang, H.S. Two-dimensional WS2@Nitrogen-doped graphite for high performance lithium ion batteries: experiments and molecular dynamics simulations. ACS Appl. Mater. Interfaces 2018, 10, 37928–37936. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.B.; Kim, H.S.; Yan, Y.; Ko, J.S.; Robbennolt, S.; Dunn, B.; Tolbert, S.H. Mesoporous MoS2 as a transition metal dichalcogenide exhibiting pseudocapacitive Li and Na-ion charge storage. Adv. Energy Mater. 2016, 6, 1501937. [Google Scholar] [CrossRef]
- Pei, J.; Geng, H.; Ang, E.H.; Zhang, L.; Cao, X.; Zheng, J.; Gu, H. Controlled synthesis of hollow C@TiO2@MoS2 hierarchical nanospheres for high-performance lithium-ion batteries. Nanoscale 2018, 10, 17327–17334. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Tian, W.; Shen, J.; Qiao, X.; Sun, T.; Wu, H.; Zhao, J.; Liu, X.; Zhang, Y. Facile fabrication of a jarosite ultrathin KFe3(SO4)2(OH)6@rGO nanosheet hybrid composite with pseudocapacitive contribution as a robust anode for lithium-ion batteries. Inorg. Chem. Front. 2019, 6, 192–198. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, H.; Zhou, R.; Liu, X.; Wei, B. Onion-like nanospheres organized by carbon encapsulated few-layer MoS2 nanosheets with enhanced lithium storage performance. J. Power Sour. 2019, 413, 327–333. [Google Scholar] [CrossRef]
- Wang, C.; Sawicki, M.; Emani, S.; Liu, C.; Shaw, L.L. Na3MnCO3PO4—A high capacity, multi-electron transfer redox cathode material for sodium ion batteries. Electrochim. Acta 2015, 161, 322–328. [Google Scholar] [CrossRef]
- Wang, C.; Sawicki, M.; Kaduk, J.A.; Shaw, L.L. Roles of processing, structural defects and ionic conductivity in the electrochemical performance of Na3MnCO3PO4 cathode material. J. Electrochem. Soc. 2015, 162, A1601–A1609. [Google Scholar] [CrossRef]
- Liu, K.; Man, J.; Cui, J.; Zhang, H.; Li, T.; Yang, J.; Wen, Z.; Sun, J. Li4Ti5O12/g-C3N4 composite with an improved lithium storage capability. Mater. Lett. 2019, 234, 117–120. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Z.; Yang, Y.-E.; Yin, J.; Kong, W.; Li, S.; Sun, J.; Ji, S. Ultra-long life Si@rGO/g-C3N4 with a multiply synergetic effect as an anode material for lithium-ion batteries. J. Mater. Chem. A 2018, 6, 7557–7565. [Google Scholar] [CrossRef]
- Veith, G.M.; Baggetto, L.; Adamczyk, L.A.; Guo, B.; Brown, S.S.; Sun, X.G.; Albert, A.A.; Humble, J.R.; Barnes, C.E.; Bojdys, M.J.; et al. Electrochemical and solid-state lithiation of graphitic C3N4. Chem. Mater. 2013, 25, 503–508. [Google Scholar] [CrossRef]
- Lindstrom, H.; Sodergren, S.; Solbrand, A.; Rensmo, H.; Hjelm, J.; Hagfeldt, A.; Lindquist, S.E. Li+ ion insertion in TiO2 (Anatase). 2. Voltammetry on nanoporous films. J. Phys. Chem. B 1997, 101, 7717–7722. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Method: Fundamentals and Applications; John Wiley & Sons: New York, NY, USA, 1980. [Google Scholar]
- Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931. [Google Scholar] [CrossRef]
- Yu, F.; Liu, Z.; Zhou, R.; Tan, D.; Wang, H.; Wang, F. Pseudocapacitance contribution in boron-doped graphite sheets for anion storage enables high-performance sodium-ion capacitors. Mater. Horiz. 2018, 5, 529–535. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, Z.; Wang, S.; Hossain, M.S.A.; Yu, J.; Kumar, N.A.; Yamauchi, Y. Pseudocapacitive behavior of the Fe2O3 anode and its contribution to high reversible capacity in lithium ion batteries. Nanoscale 2018, 10, 18010–18018. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Peng, X.; Zhang, Y.; Jin, D.; Chu, P.K.; Zhang, L. Elucidating the intercalation pseudocapacitance mechanism of MoS2-carbon monolayer interoverlapped superstructure: toward high-performance sodium-ion-based hybrid supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 32745–32755. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.B.; Kim, H.-S.; Lin, T.C.; Lai, C.-H.; Dunn, B.; Tolbert, S.H. Pseudocapacitive charge storage in thick composite MoS2 nanocrystal-based electrodes. Adv. Energy Mater. 2017, 7, 1601283. [Google Scholar] [CrossRef]
- Liu, T.C.; Pell, W.G.; Conway, B.E.; Roberson, S.L. Behavior of Molybdenum Nitrides as Materials for Electrochemical Capacitors: Comparison with Ruthenium Oxide. J. Electrochem. Soc. 1998, 145, 1882–1888. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, D.-M.; Kim, S.-J.; Kim, M.-C.; Choe, H.-S.; Lee, K.H.; Sohn, J.I.; Cha, S.N.; Kim, J.M.; Park, K.-W. In-situ synthesis and characterization of Ge embedded electrospun carbon nanostructures as high performance anode material for lithium-ion batteries. ACS Appl. Mater. Interfaces 2016, 8, 7022–7029. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.K.; Johari, P.; Basu, S.; Nayak, C.; Mitra, S. An experimental and computational study to understand the lithium storage mechanism in molybdenum disulfide. Nanoscale 2014, 6, 10243–10254. [Google Scholar] [CrossRef]





| Sample | Rs (Internal Ohmic Resistance) (Ω) | Rct (Charge Transfer Resistance) (Ω) | Warburg Coefficient (Ω·s−1/2) | Li+ Ion Diffusion Coefficient DLi+ (cm2·s−1/2) |
|---|---|---|---|---|
| Initial MS | 4.651 | 60.017 | 311.060 | 9.05 × 10−13 |
| Initial MS/CN | 3.333 | 40.887 | 120.246 | 6.05 × 10−12 |
| 200th MS | 4.424 | 33.025 | 99.283 | 8.88 × 10−12 |
| 200th MS/CN | 4.279 | 35.017 | 26.333 | 1.26 × 10−10 |
| Sample | Initial Discharge Capacity | Cycle Number | Rate | Reversible Capacity | Ref. |
|---|---|---|---|---|---|
| MS/CN | 2467 mA·h·g−1 | 200 | 1 C | 1204 mA·h·g−1 | This work |
| VA-C/MoS2 | 678 mA·h·g−1 | 1000 | 5 A·g−1 | 613 mA·h·g−1 | [20] |
| g-C3N4/MoS2 | 2390 mA·h·g−1 | 50 | 0.1 C | 864 mA·h·g−1 | [28] |
| MoS2/CCS | 1409.5 mA·h·g−1 | 250 | 100 mA·g−1 | 1230.9 mA·h·g−1 | [21] |
| MoS2MACC | 1245.4 mA·h·g−1 | 100 | 100 mA·g−1 | 1147 mA·h·g−1 | [18] |
| C@TiO2@MoS2 | 1687 mA·h·g−1 | 100 | 200 mA·g−1 | 993.2 mA·h·g−1 | [52] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran Huu, H.; Nguyen Thi, X.D.; Nguyen Van, K.; Kim, S.J.; Vo, V. A Facile Synthesis of MoS2/g-C3N4 Composite as an Anode Material with Improved Lithium Storage Capacity. Materials 2019, 12, 1730. https://doi.org/10.3390/ma12111730
Tran Huu H, Nguyen Thi XD, Nguyen Van K, Kim SJ, Vo V. A Facile Synthesis of MoS2/g-C3N4 Composite as an Anode Material with Improved Lithium Storage Capacity. Materials. 2019; 12(11):1730. https://doi.org/10.3390/ma12111730
Chicago/Turabian StyleTran Huu, Ha, Xuan Dieu Nguyen Thi, Kim Nguyen Van, Sung Jin Kim, and Vien Vo. 2019. "A Facile Synthesis of MoS2/g-C3N4 Composite as an Anode Material with Improved Lithium Storage Capacity" Materials 12, no. 11: 1730. https://doi.org/10.3390/ma12111730
APA StyleTran Huu, H., Nguyen Thi, X. D., Nguyen Van, K., Kim, S. J., & Vo, V. (2019). A Facile Synthesis of MoS2/g-C3N4 Composite as an Anode Material with Improved Lithium Storage Capacity. Materials, 12(11), 1730. https://doi.org/10.3390/ma12111730