Metal-Free Organic Chromophores Featuring an Ethynyl-Thienothiophene Linker with an n-Hexyl Chain for Translucent Dye-Sensitized Solar Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis
2.2. Characterization
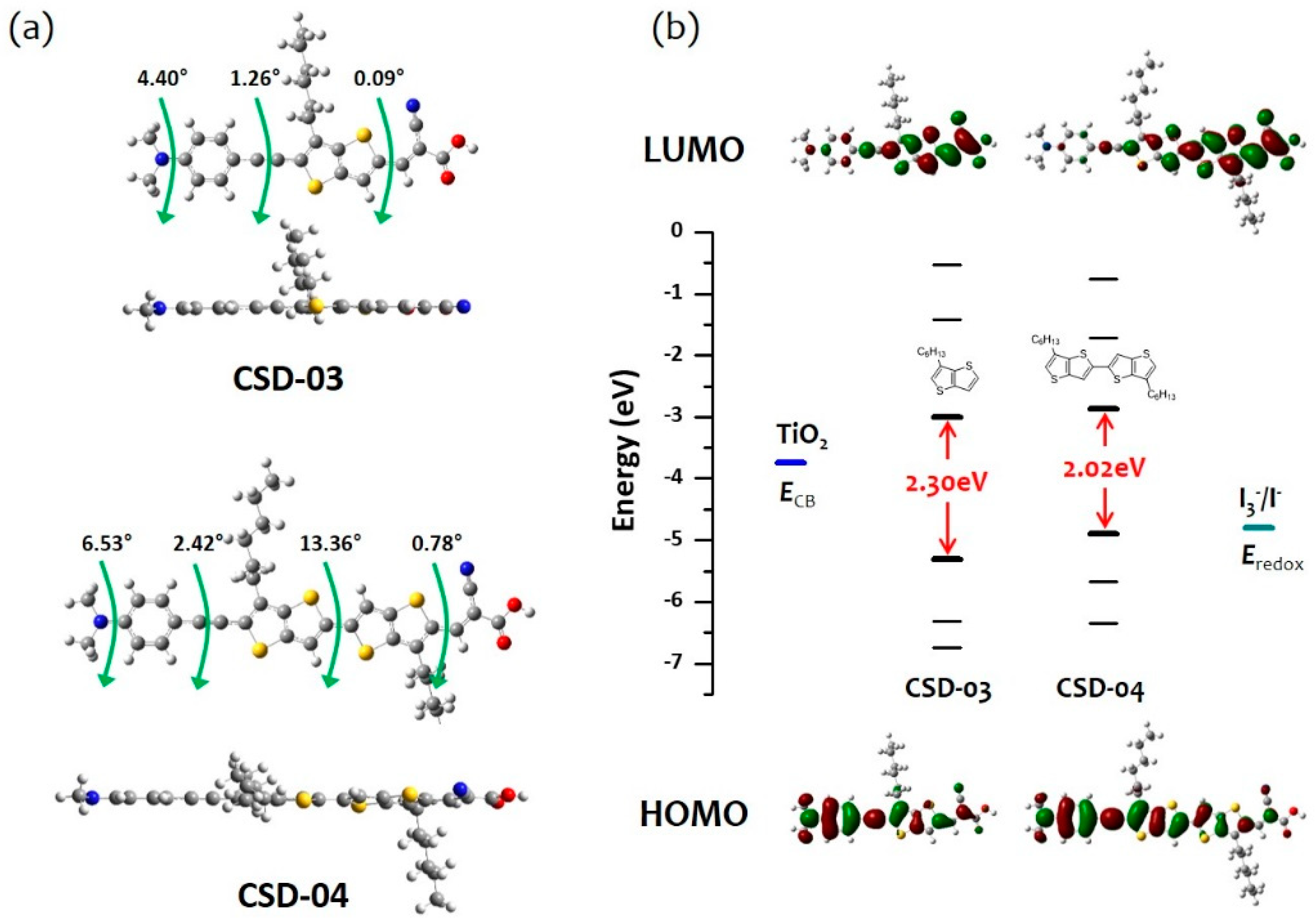
2.3. Theoretical Calculations
2.4. Dye-Sensitized Solar Cells
3. Materials and Methods
3.1. General
3.2. Syntheses
3.3. Computational Details
3.4. DSSC Fabrication and Photovoltaic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Petterson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Brown, T.M.; Fabregat-Santiago, F.; Bisquert, J. A perspective on the production of dye-sensitized solar modules. Energ. Environ. Sci. 2014, 7, 3952–3981. [Google Scholar] [CrossRef]
- Freitag, M.; Teuscher, J.; Saygil, Y.; Zhang, X.; Giordano, F.; Liska, P.; Hua, J.; Zakeeruddin, S.M.; Moser, J.-E.; Grätzel, M.; et al. Dye-sensitized solar cells for efficient power generation under ambient lightning. Nat. Photonics 2017, 11, 372–378. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Angelis, F.D.; Fantacci, S.; Selloni, A.; Viscardi, G.; Liska, P.; Ito, S.; Takeru, B.; Grätzel, M. Combined experimental and DFT-TDDFT computational study of photoelectrochemical cell ruthenium sensitizers. J. Am. Chem. Soc. 2005, 127, 16835–16847. [Google Scholar] [CrossRef]
- Robertson, N. Optimizing dyes for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2006, 45, 2338–2345. [Google Scholar] [CrossRef]
- Kanaparthi, R.K.; Kandhadi, J.; Giribabu, L. Metal-free organic dyes for dye-sensitized solar cells: Recent advances. Tetrahedron 2012, 68, 8383–8393. [Google Scholar] [CrossRef]
- Liang, M.; Chen, J. Arylamine organic dyes for dye-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 3453–3488. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A. Triphenylamine based dyes for dye sensitized solar cells: A review. Sol. Energy 2016, 123, 127–144. [Google Scholar] [CrossRef]
- Zhang, G.; Bai, Y.; Shi, D.; Wegner, S.; Zakeeruddin, S.M.; Grätzel, M.; Wang, P. Employ a bisthienothiophene linker to construct an organic chromophore for efficient and stable dye-sensitized solar cells. Energy Environ. Sci. 2009, 2, 92–95. [Google Scholar] [CrossRef]
- Al-Eid, M.; Lim, S.H.; Park, K.-W.; Fitzpatrick, B.; Han, C.-H.; Kwak, K.; Hong, J.; Cooke, G. Facile synthesis of metal-free organic dyes featuring a thienylethynyl spacer for dye sensitized solar cells. Dyes Pigm. 2014, 104, 197–203. [Google Scholar] [CrossRef]
- Park, K.-W.; Ahn, S.; Baek, M.H.; Lim, D.-S.; Wiles, A.A.; Kim, M.G.; Hong, J. Coplanar D-π-A organic sensitizers featuring a thienylethynyl spacer for efficient dye-sensitized solar cells. Mater. Express 2017, 7, 43–50. [Google Scholar] [CrossRef]
- Zhao, H.; Long, J.; Zhao, B.; Tan, S. 2-ethynyl-6-methylthieno[3,2-b]thiophene as an efficient π spacer for porphyrin-based dyes. Dyes Pigm. 2015, 122, 168–176. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Mesquita, I.; Andrade, L.; Mendes, A.; Justino, L.L.G.; Burrows, H.D.; Raposo, M.M.M. Synthesis and characterization of push-pull bithiophene and thieno[3,2-b]thiophene derivatives bearing an ethyne linker as sensitizers for dye-sensitized solar cells. Org. Electron. 2017, 49, 194–205. [Google Scholar] [CrossRef]
- Horiuchi, T.; Miura, H.; Uchida, S. Highly-efficient metal-free organic dyes for dye-sensitized solar cells. Chem. Commun. 2003, 9, 3036–3037. [Google Scholar] [CrossRef]
- Wang, P.; Klein, C.; Humphry-Baker, R.; Zakeeruddin, S.M.; Grätzel, M. A high molar extinction coefficient sensitizer for stable dye-sensitized solar cells. J. Am. Chem. Soc. 2005, 127, 808–809. [Google Scholar] [CrossRef]
- Won, Y.S.; Yang, Y.S.; Kim, J.H.; Ryu, J.-H.; Kim, K.K.; Park, S.S. Organic photosensitizers based on terthiophene with alkyl chain and double acceptors for application in dye-sensitized solar cells. Energy Fuels 2010, 24, 3676–3681. [Google Scholar] [CrossRef]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R.F.; Bässler, H.; Porsch, M.; Daub, J. Efficient two layer leds on a polymer blend basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- Han, L.; Koide, N.; Chiba, Y.; Islam, A.; Komiya, R.; Fuke, N.; Fukui, A.; Yamanaka, R. Improvement of efficiency of dye-sensitized solar cells by reduction of internal resistance. Appl. Phys. Lett. 2005, 86, 213501. [Google Scholar] [CrossRef]
- Hoshikawa, T.; Yamada, M.; Kikuchi, R.; Eguchi, K. Impedance analysis of internal resistance affecting the photoelectrochemical performance of dye-sensitized solar cells. J. Electrochem. Soc. 2015, 152, E68–E73. [Google Scholar] [CrossRef]
- Gaussian, R.A.; Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; et al. Gaussian 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Cho, T.-Y.; Han, C.-W.; Jun, Y.; Yoon, S.-G. Formation of artificial pores in nano-TiO2 photo-electrode films using acetylene-black for high-efficiency dye-sensitized solar cells. Sci. Rep. 2013, 3, 1496. [Google Scholar] [CrossRef]
- Ito, S.; Nazeeruddin, M.K.; Liska, P.; Comte, P.; Charvet, R.; Péchy, P.; Jirousek, M.; Kay, A.; Zakeeruddin, S.M.; Grätzel, M. Photovoltaic characterization of dye-sensitized solar cells: Effect of device masking on conversion efficiency. Prog. Photovolt: Res. Appl. 2006, 14, 589–601. [Google Scholar] [CrossRef]

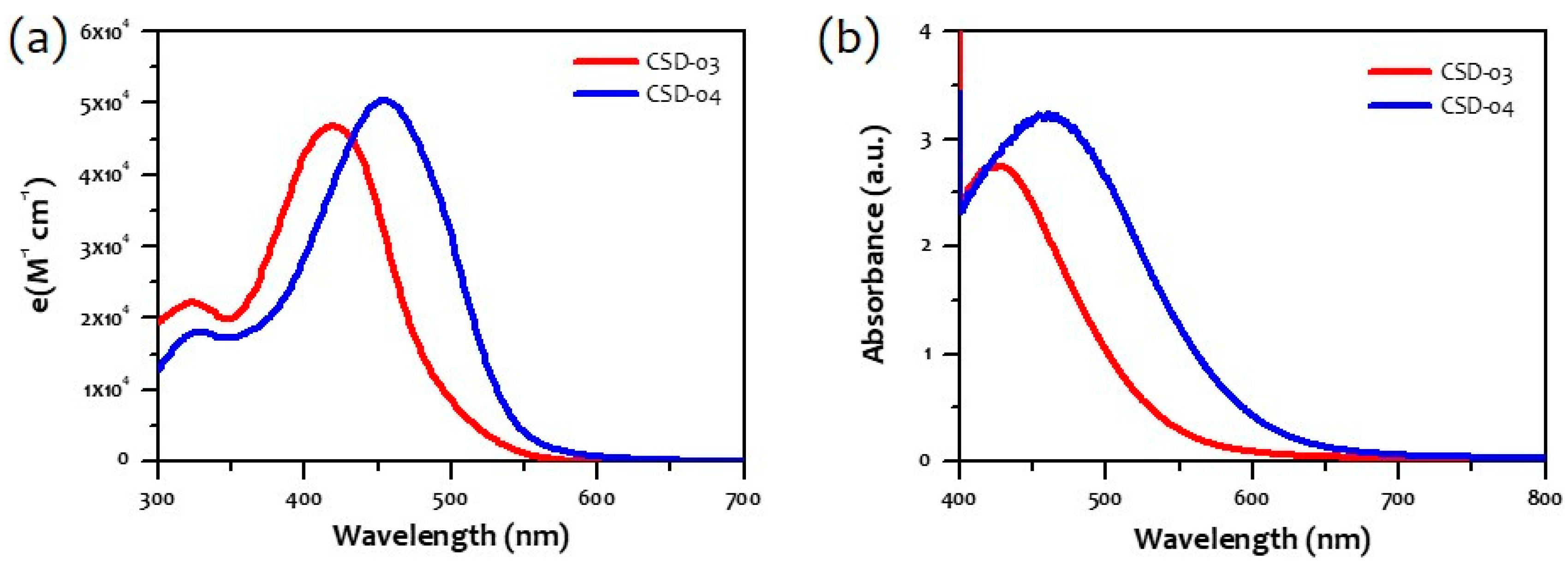
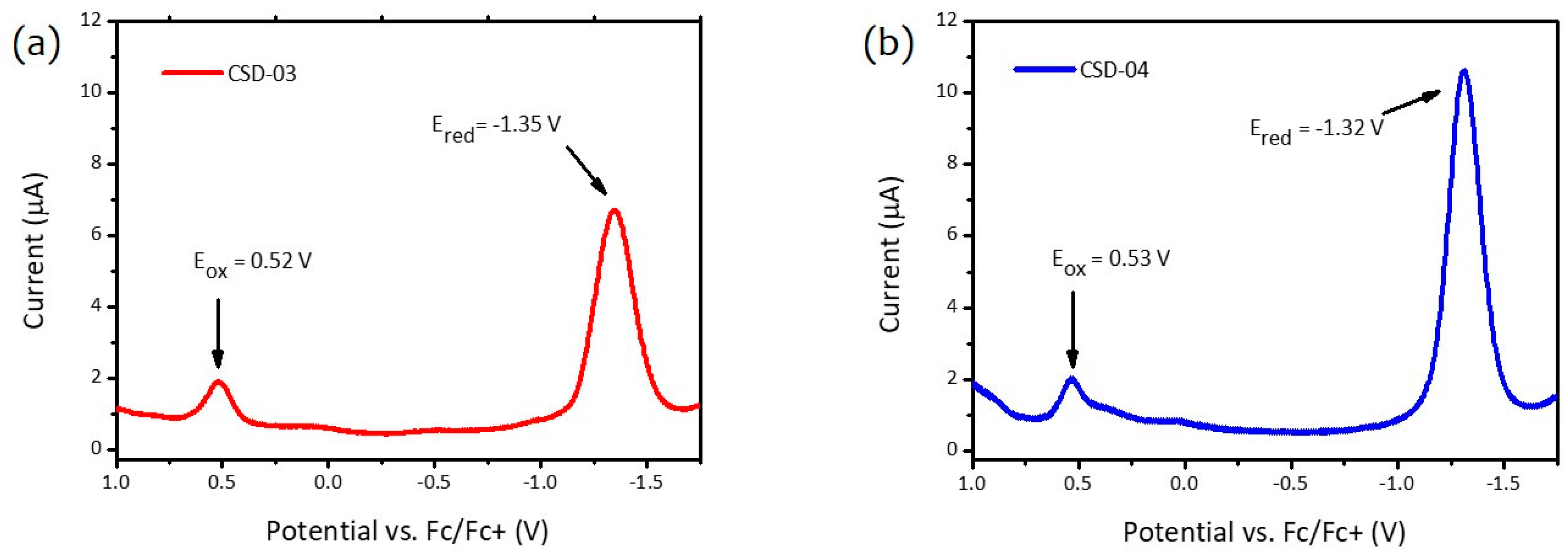

| Dyes | λmax/λonset (nm) | Eg (optical) (eV) | Eox (V) | Ered (V) | IP (eV) | EA (eV) | Eg (e-chem) (eV) |
|---|---|---|---|---|---|---|---|
| CSD-03 | 419/499 | 2.48 | 0.52 | −1.35 | −5.32 | −3.45 | 1.87 |
| CSD-04 | 455/550 | 2.25 | 0.53 | −1.32 | −5.34 | −3.48 | 1.85 |
| Dyes (Soaking Time) | Voc (V) | Jsc (mA/cm2) | FF | η (%) |
|---|---|---|---|---|
| CSD-03 (3 h) | 0.627 ± 0.013 | 12.55 ± 0.18 | 69.4 ± 1.1 | 5.46 ± 0.03 |
| CSD-03 (6 h) | 0.619 ± 0.022 | 12.64 ± 0.68 | 69.1 ± 0.2 | 5.41 ± 0.08 |
| CSD-03 (12 h) | 0.6111 ± 0.004 | 11.15 ± 0.51 | 68.7 ± 0.3 | 4.69 ± 0.27 |
| CSD-04 (3 h) | 0.587 ± 0.004 | 14.85 ± 0.29 | 58.2 ± 1.4 | 5.07 ± 0.18 |
| CSD-04 (6 h) | 0.576 ± 0.009 | 13.86 ± 0.35 | 65.2 ± 1.0 | 5.20 ± 0.03 |
| CSD-04 (12 h) | 0.587 ± 0.016 | 12.974 ± 0.42 | 64.1 ± 0.9 | 4.88 ± 0.01 |
| N719 (3 h) | 0.608 ± 0.003 | 14.74 ± 0.47 | 62.1 ± 0.1 | 5.57 ± 0.21 |
| N719 (24 h) | 0.671 ± 0.001 | 14.95 ± 0.39 | 59.1 ± 0.6 | 5.92 ± 0.01 |
| Dyes | Rtr (Ω) | Rrec (Ω) | Cμ (mF) | τe (ms) | ηcc (%) |
|---|---|---|---|---|---|
| CSD-03 (3 h) | 5.68 | 84.46 | 0.08 | 6.34 | 93.70 |
| CSD-04 (3 h) | 11.99 | 111.20 | 0.05 | 5.65 | 90.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, D.-S.; Park, K.-W.; Wiles, A.A.; Hong, J. Metal-Free Organic Chromophores Featuring an Ethynyl-Thienothiophene Linker with an n-Hexyl Chain for Translucent Dye-Sensitized Solar Cells. Materials 2019, 12, 1741. https://doi.org/10.3390/ma12111741
Lim D-S, Park K-W, Wiles AA, Hong J. Metal-Free Organic Chromophores Featuring an Ethynyl-Thienothiophene Linker with an n-Hexyl Chain for Translucent Dye-Sensitized Solar Cells. Materials. 2019; 12(11):1741. https://doi.org/10.3390/ma12111741
Chicago/Turabian StyleLim, Dong-Suk, Kwang-Won Park, Alan A. Wiles, and Jongin Hong. 2019. "Metal-Free Organic Chromophores Featuring an Ethynyl-Thienothiophene Linker with an n-Hexyl Chain for Translucent Dye-Sensitized Solar Cells" Materials 12, no. 11: 1741. https://doi.org/10.3390/ma12111741
APA StyleLim, D.-S., Park, K.-W., Wiles, A. A., & Hong, J. (2019). Metal-Free Organic Chromophores Featuring an Ethynyl-Thienothiophene Linker with an n-Hexyl Chain for Translucent Dye-Sensitized Solar Cells. Materials, 12(11), 1741. https://doi.org/10.3390/ma12111741





