On the Microstructure and Isothermal Oxidation of the Si-22Fe-12Cr-12Al-10Ti-5Nb (at.%) Alloy
Abstract
:1. Introduction
2. Selection of Alloy Composition
3. Experimental
4. Results
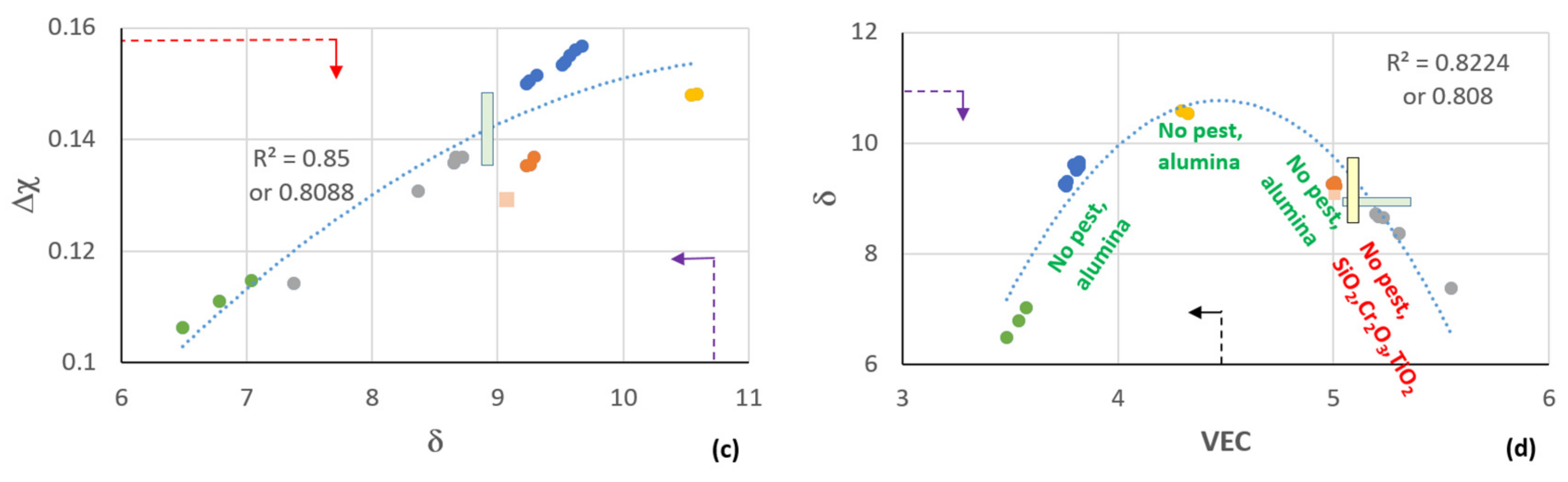
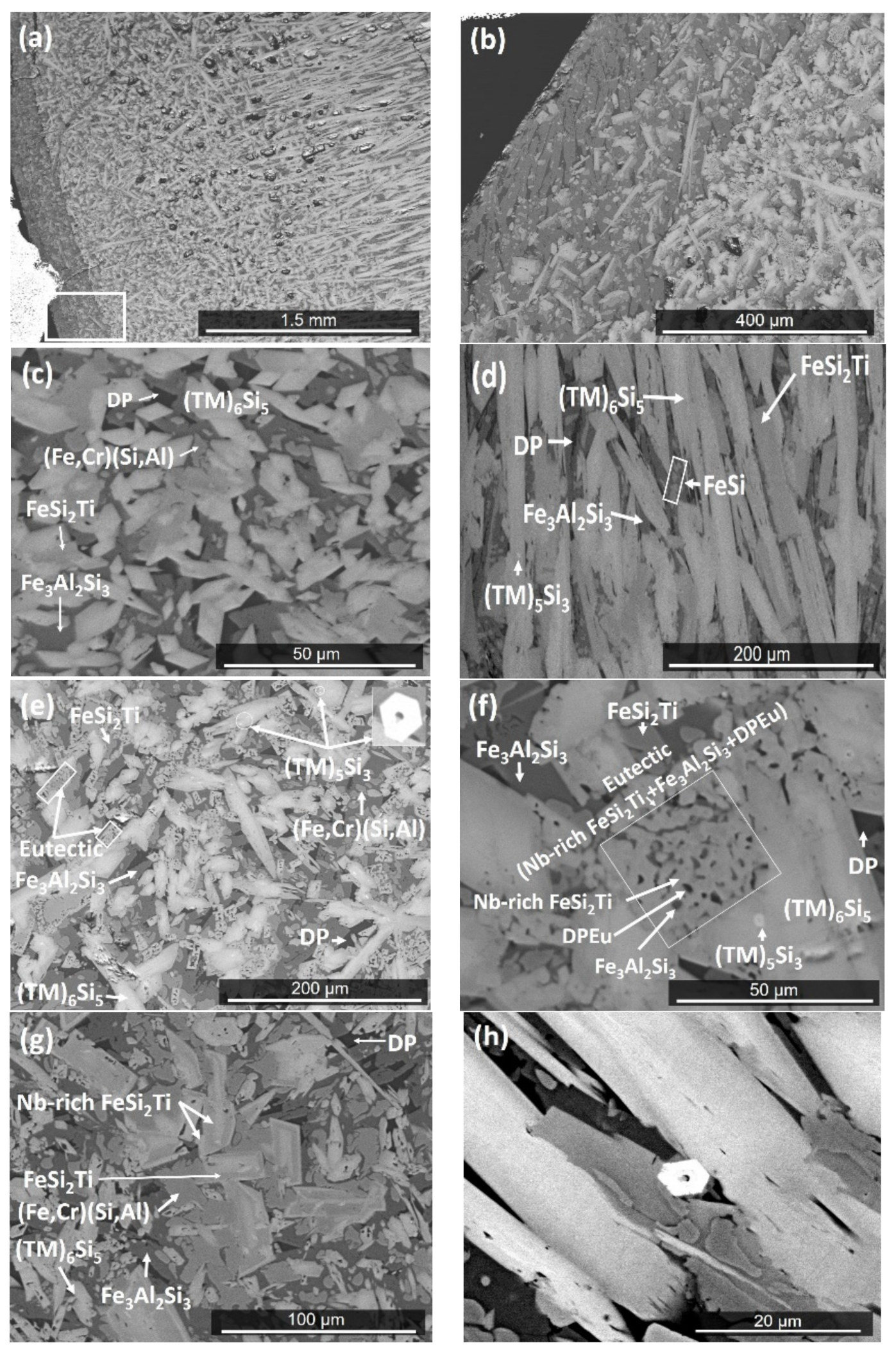
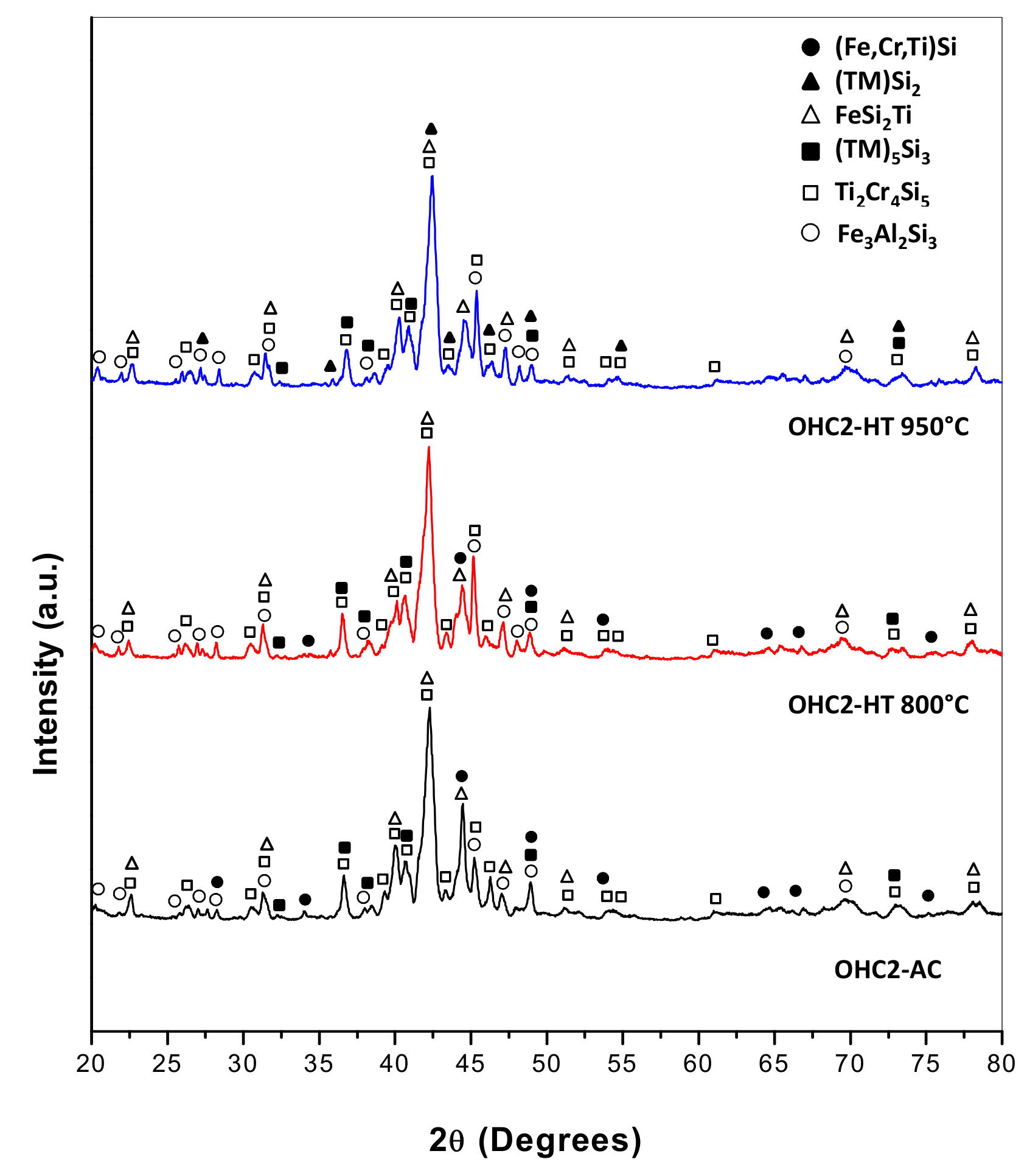
4.1. Cast Alloy
4.2. Heat Treated Alloy
4.3. Oxidation
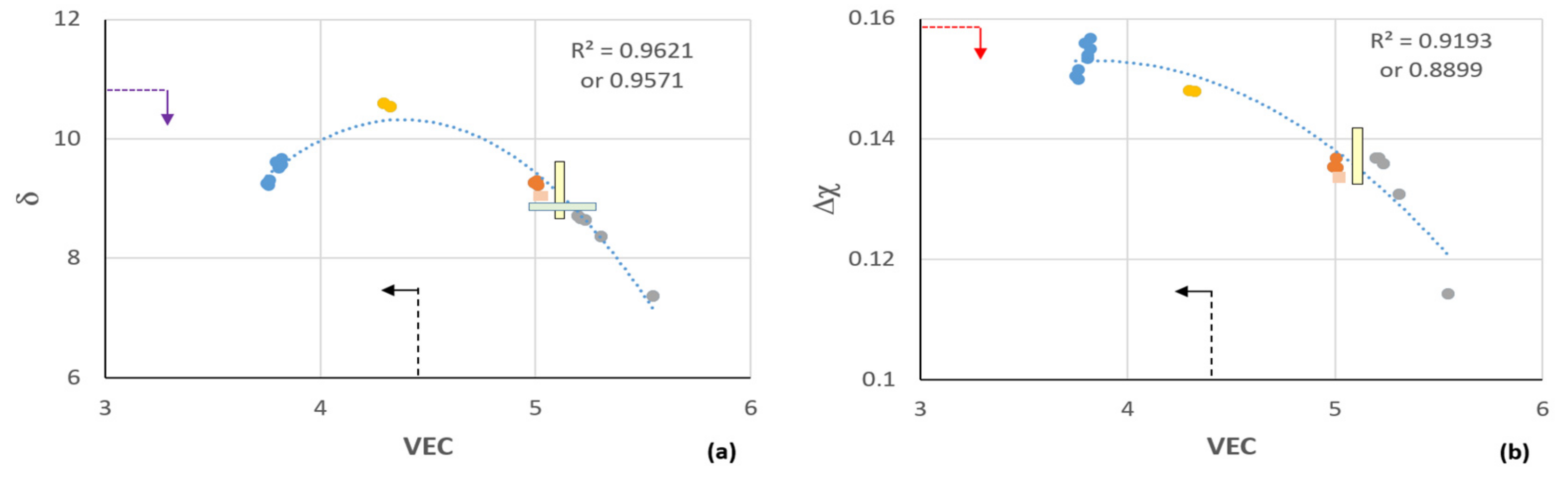
5. Discussion
5.1. Microstructure
5.2. Oxidation
6. Summary and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Subramanian, P.; Mendiratta, M.; Dimiduk, D.; Stucke, M. Advanced intermetallic alloys—beyond gamma titanium aluminides. Mater. Sci. Eng. A 1997, 239, 1–13. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Jackson, M.R.; Subramanian, P.R.; Zhao, J.-C. A review of very-high-temperature Nb-silicide-based composites. Met. Mater. Trans. A 2003, 34, 2043–2052. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On Nb Silicide Based Alloys: Alloy Design and Selection. Materials 2018, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.R.; Rowe, R.G.; Skelly, D.W. Oxidation of some intermetallic compounds and intermetallic matrix composites. MRS Online Proc. Lib. Arch. 1995, 364, 1339. [Google Scholar] [CrossRef]
- Bewlay, B.; Jackson, M.; Zhao, J.-C. Coatings, Method of Manufacture and the articles Derived Therefrom. U.S. Patent US6767653B2, 27 July 2004. [Google Scholar]
- Olson, G.B.; Bryan, D.J.; Misra, A. Oxidation Resistant Niobium Based Alloys. U.S. Patent 2006/0172142A1, 3 August 2006. [Google Scholar]
- Ghadyani, M.; Utton, C.; Tsakiropoulos, P. Microstructures and isothermal oxidation of the alumina scale forming Nb1.7Si2.4Ti2.4Al3Hf0.5 and Nb1.3Si2.4Ti2.4Al3.5Hf0.4 alloys. Materials 2019, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Ghadyani, M.; Utton, C.; Tsakiropoulos, P. Microstructures and isothermal oxidation of the alumina scale forming Nb1.45Si2.7Ti2.25Al3.25Hf0.35 and Nb1.35Si2.3Ti2.3Al3.7Hf0.35 alloys. Materials 2019, 12, 759. [Google Scholar] [CrossRef]
- Hernández-Negrete, O.; Tsakiropoulos, P. On the microstructure and isothermal oxidation of silica and alumina scale forming Si-23Fe-15Cr-15Ti-1Nb and Si-25Nb-5Al-5Cr-5Ti (at.%) silicide alloys. Materials 2019, 12, 1091. [Google Scholar] [CrossRef]
- Jackson, M.R.; Ritter, A.M. Oxidation-Resistant Coating for Niobium-Base Alloys. U.S. Patent 5721061A, 24 February 1998. [Google Scholar]
- National Research Council (US). Committee on Coatings. High-Temperature Oxidation-Resistant Coatings: Coatings for Protection from Oxidation of Superalloys, Refractory Metals, and Graphite; National Academies: Washington, DC, USA, 1970; ISBN 978-0-309-01769-5. [Google Scholar] [CrossRef]
- Kirkwood, B.L.; Picha, E.M.W. Silica-Enriched Protective Coating for Hypersonic Flight Vehicles and Method of Applying Same, Including Field Repair. U.S. Patent 5431961A, 11 July 1995. [Google Scholar]
- Weber, R.; Bouvier, J.; Slama, G. Failure mechanisms of aluminide and silicide coatings on a niobium alloy subjected to thermal cycling in air. J. Less-Common Met. 1973, 32, 1–20. [Google Scholar] [CrossRef]
- Wahl, G. Coating composition and the formation of protective oxide layers at high temperatures. Thin Solid Films 1983, 107, 417–426. [Google Scholar] [CrossRef]
- Tsirlin, M.S.; Anurova, G.M.; Aliev, A.D. Structure and oxidation resistance of a slurry-diffusion coating on Nb. Powder Metall. Met. Ceram. 1981, 20, 801–804. [Google Scholar]
- Portebois, L.; Mathieu, S.; Knittel, S.; Aranda, L.; Vilasi, M. Protective coatings for niobium alloys: Manufacture, characterization and oxidation behaviour of (TiXCr)7Si6 with X = Fe, Co and Ni. Oxid. Met. 2013, 80, 243–255. [Google Scholar] [CrossRef]
- Adachi, T.; Meier, G.H. Oxidation of iron-silicon alloys. Oxid. Met. 1987, 27, 347–366. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X. Oxidation behaviour of an Al-modified silicide coating on an Nb-silicide based ultrahigh-temperature alloy. Corrosion 2010, 66, 025003-025003-6. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, X. Preparation and oxidation resistance of silicide/aluminide composite coatings on an Nb–Ti–Si based alloy. Surf. Coat. Technol. 2015, 274, 18–25. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, S.N.; Zhou, C.G.; Sha, J.B.; Zhao, X.Q.; Jia, L.N. Research progress on ultra-high-temperature Nb-silicide-based alloys. Acta Aeronaut. Astronaut. Sin. 2014, 35, 2756–2766. [Google Scholar]
- Zhao, J.-C.; Jackson, M.R.; Bewlay, B.P. Oxidation Resistant Coatings for Niobium Based Silicide Composites. U.S. Patent 6,645,560 B2, 11 November 2003. [Google Scholar]
- Jackson, M.R.; Subramanian, P.; Zhao, J.-C.; Bewlay, B.P.; Darolia, R.; Schafrik, R. Turbine Blade for Extreme Temperature Conditions. U.S. Patent 7189459B2, 13 March 2007. [Google Scholar]
- Tsakiropoulos, P. On the alloying and properties of tetragonal Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Tsakiropoulos, P. Alloying and properties of C14–NbCr2 and A15–Nb3X (X = Al, Ge, Si, Sn) in Nb–silicide-based alloys. Materials 2018, 11, 395. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. Alloying and Hardness of eutectics with Nbss and Nb5Si3 in Nb-silicide based alloys. Materials 2018, 11, 592. [Google Scholar] [CrossRef]
- Lysenko, L.A.; Markiv, V.Y.; Tsybukh, O.V.; Gladyshevski, E.I. The system titanium-chromium-silicon. Inorg. Mater. 1971, 7, 157–159. [Google Scholar]
- Raghavan, V. The Fe-Si-Ti (iron-silicon-titanium) system, phase diagrams ternary iron alloys, Indian Inst. Met. 1987, 1, 65–72. [Google Scholar]
- Raghavan, V. The Cr-Fe-Si (chromium-iron-silicon) system, phase diagrams ternary iron alloys. Indian Inst. Met. 1987, 1, 31–42. [Google Scholar]
- David, N.; Cartigny, Y.; Belmonte, T.; Fiorani, J.; Vilasi, M. Thermodynamic description of the Cr–Nb–Si isothermal section at 1473 K. Intermetallics 2006, 14, 464–473. [Google Scholar] [CrossRef]
- Ghosh, G. Aluminium-Iron-Silicon, Ternary Alloys; VCH: Weinheim, Germany, 1992. [Google Scholar]
- Weitzer, F.; Schuster, J.C.; Naka, M.; Stein, F.; Palm, M. On the reaction scheme and liquidus surface in the ternary system Fe–Si–Ti. Intermetallics 2008, 16, 273–282. [Google Scholar] [CrossRef]
- Krendelsberger, N.; Weitzer, F.; Schuster, J.C. On the Reaction Scheme and Liquidus Surface in the Ternary System Al-Fe-Si. Met. Mater. Trans. B 2007, 38, 1681–1691. [Google Scholar] [CrossRef]
- Kofstad, P. High Temperature Oxidation of Metals; John Wiley & Son: New York, NY, USA, 1966. [Google Scholar]
- Kofstad, P. High-Temperature Oxidation; Elsevier: London, UK, 1988. [Google Scholar]
- Raghavan, V.; Ghosh, G. The Fe-Nb-Si (iron-niobium-silicon) system. Trans. Indian Inst. Met. 1984, 37, 421–425. [Google Scholar]
- Marker, M.C.; Skolyszewska-Kühberger, B.; Effenberger, H.S.; Schmetterer, C.; Richter, K.W. Phase equilibria and structural investigations in the system Al–Fe–Si. Intermetallics 2011, 19, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Schuster, J.C. Experimental investigation and thermodynamic description of the Cr-Si-Ti system. Scand. J. Met. 2002, 31, 25–33. [Google Scholar] [CrossRef]
- ASM Alloy Phase Diagram Database, Editor in Chief P Villars, Section Editors H Okamoto, K Cenzual, 2006–2019. Available online: https://matdata.asminternational.org/apd/index.aspx (accessed on 3 June 2019).
- Shao, G. Thermodynamic assessment of the Nb–Si–Al system. Intermetallics 2004, 12, 655–664. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Jackson, M.R.; Lipsitt, H.A. The Nb-Ti-Si ternary phase diagram: Evaluation of liquid- solid phase equilibria in Nb-and Ti-rich alloys. J. Phase Equilibria Diffus. 1997, 18, 264–278. [Google Scholar] [CrossRef]
- Zhan, Y.; Sun, Z.; Jiang, J.; Ma, J.; Zhang, X.; Zhuang, Y. The 773K isothermal section of the ternary phase diagram of the Nb–Ti–Si system. J. Alloy Compd. 2009, 468, 150–153. [Google Scholar] [CrossRef]
- Zhao, J.-C.; Jackson, M.; Peluso, L. Determination of the Nb–Cr–Si phase diagram using diffusion multiples. Acta Mater. 2003, 51, 6395–6405. [Google Scholar] [CrossRef]
- Shao, G. Thermodynamic modelling of the Cr–Nb–Si system. Intermetallics 2005, 13, 69–78. [Google Scholar] [CrossRef]
- Lindholm, M. A thermodynamic description of the Fe-Cr-Si system with emphasis on the equilibria of the sigma (G) phase. J. Phase Equilib. 1997, 18, 432. [Google Scholar] [CrossRef]
- Chen, H.-L.; Weitzer, F.; Schuster, J.C.; Du, Y.; Xu, H. The Isothermal Section of the Al—Cr—Si System at 800 °C and the Crystal Structure of τ2 (Cr3Al9Si). J. Alloy Compd. 2007, 38, 313–318. [Google Scholar] [CrossRef]
- Raghavan, V. Al-Si-Ti (Iron-silicon-titanium). J. Phase Equilib. Diffus. 2009, 30, 82–83. [Google Scholar] [CrossRef]
- Vellios, N.; Tsakiropoulos, P. Study of the role of Fe and Sn additions in the microstructure of Nb–24Ti–18Si–5Cr silicide based alloys. Intermetallics 2010, 18, 1729–1736. [Google Scholar] [CrossRef]
- Marker, M.C.; Duarte, L.I.; Leinenbach, C.; Richter, K.W. Characterisation of the Fe-rich corner of Al-Fe-Si-Ti. Intermetallics 2013, 39, 38–49. [Google Scholar] [CrossRef]
- Gupta, S.P. Formation of intermetallic compounds in the Cr-Al-Si ternary system. Mater. Charact. 2004, 52, 255–370. [Google Scholar] [CrossRef]
- Li, M.H.; Sun, X.F.; Li, J.G.; Zhang, Z.Y.; Jin, T.; Guan, H.R.; Hu, Z.Q. Oxidation behaviour of single-crystal Ni-base superalloy in air. I: At 800 and 900 °C. Oxid. Met. 2003, 59, 591–605. [Google Scholar] [CrossRef]
- González-Carrasco, J.L.; Garcia-Alonso, M.C.; Montealegre, M.A.; Escudero, M.L.; Chao, J. Comparative study of the alumina-scale integrity on MA 956 and PM 2000 Alloys. Oxid. Met. 2001, 55, 209–221. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; González-Carrasco, J.L.; Escudero, M.L.; Chao, J. Oxidation Behaviour of fine-grain MA 956 superalloy. Oxid. Met. 2000, 53, 77–98. [Google Scholar] [CrossRef]
- Brumm, M.W.; Grabke, H.J. The oxidation behaviour of NiAl-I. Phase transformation s in the alumina scale during oxidation of NiAl and NiAl-Cr alloys. Corros. Sci. 1992, 33, 1677–1690. [Google Scholar] [CrossRef]
- Novak, P.; Zelinková, M.; Šerák, J.; Michalcová, A.; Novak, M.; Vojtěch, D. Oxidation resistance of SHS Fe–Al–Si alloys at 800 °C in air. Intermetallics 2011, 19, 1306–1312. [Google Scholar] [CrossRef]
- Pownceby, M.I.; Constanti-Carey, K.K.; Fisher-White, M.J. Fisher-white, sub-solidus phase relationships in the system Fe2O3–Al2O3–TiO2 between 1000 °C and 1300 °C. J. Am. Ceram. Soc. 2003, 86, 975–980. [Google Scholar] [CrossRef]
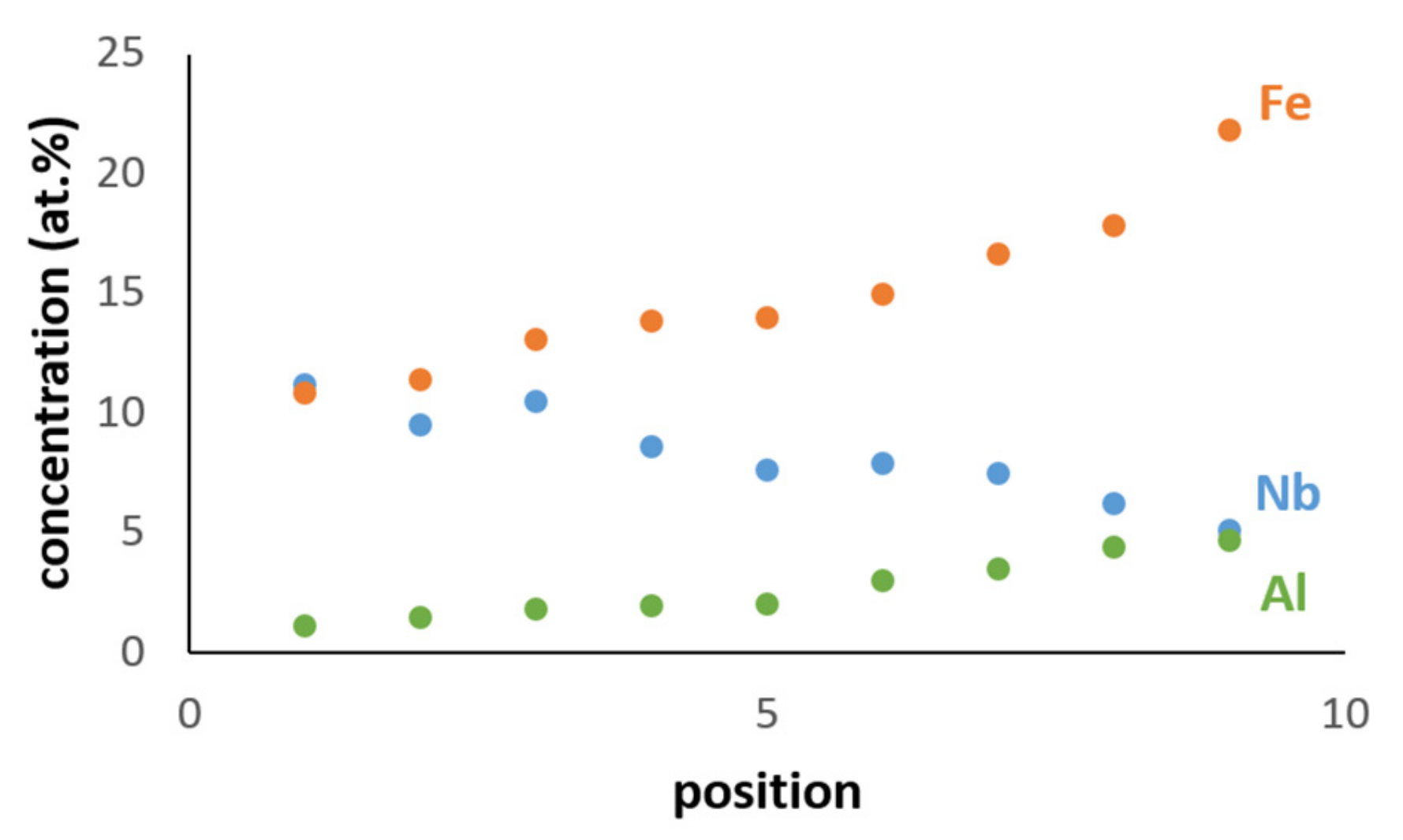
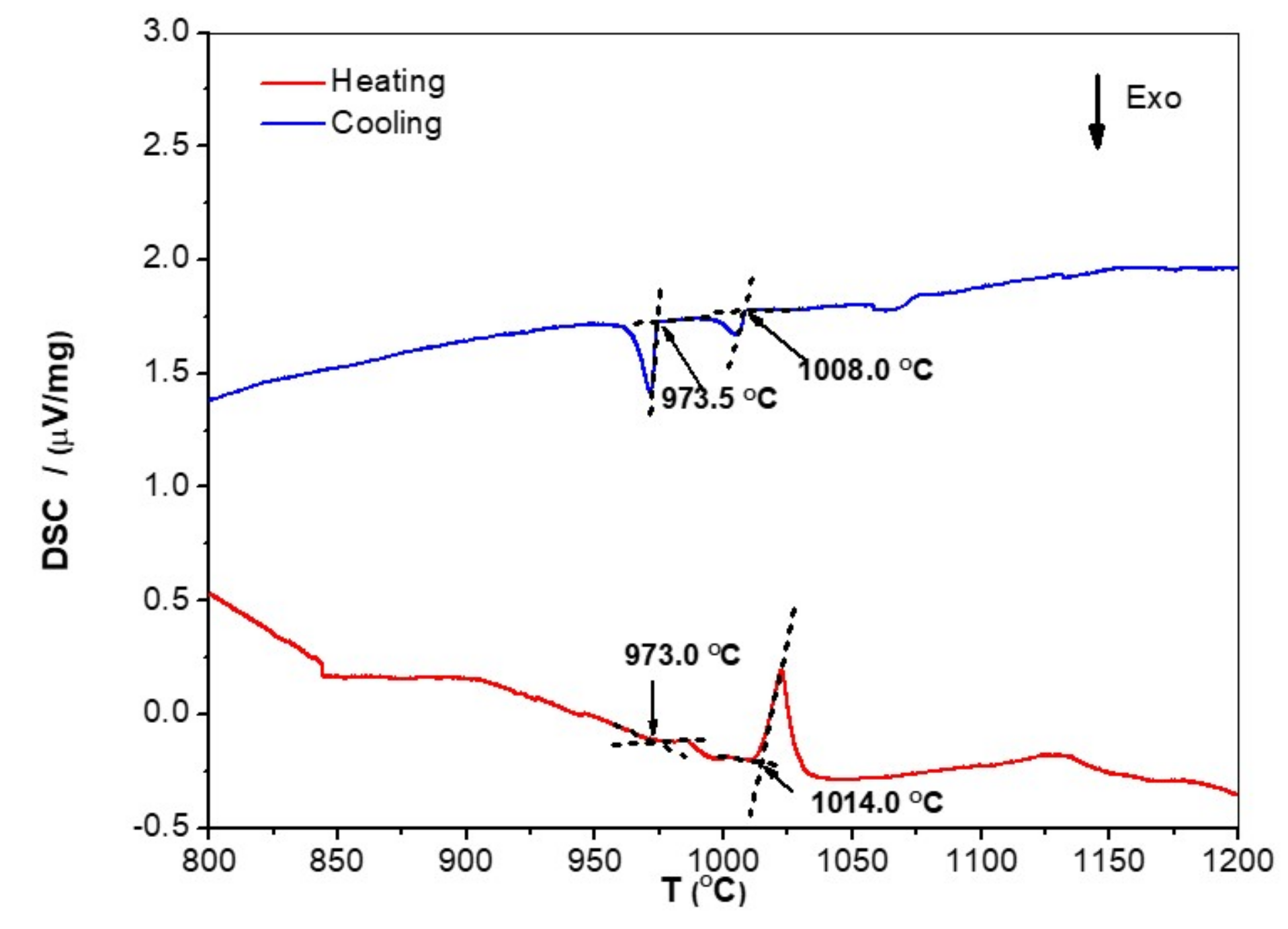
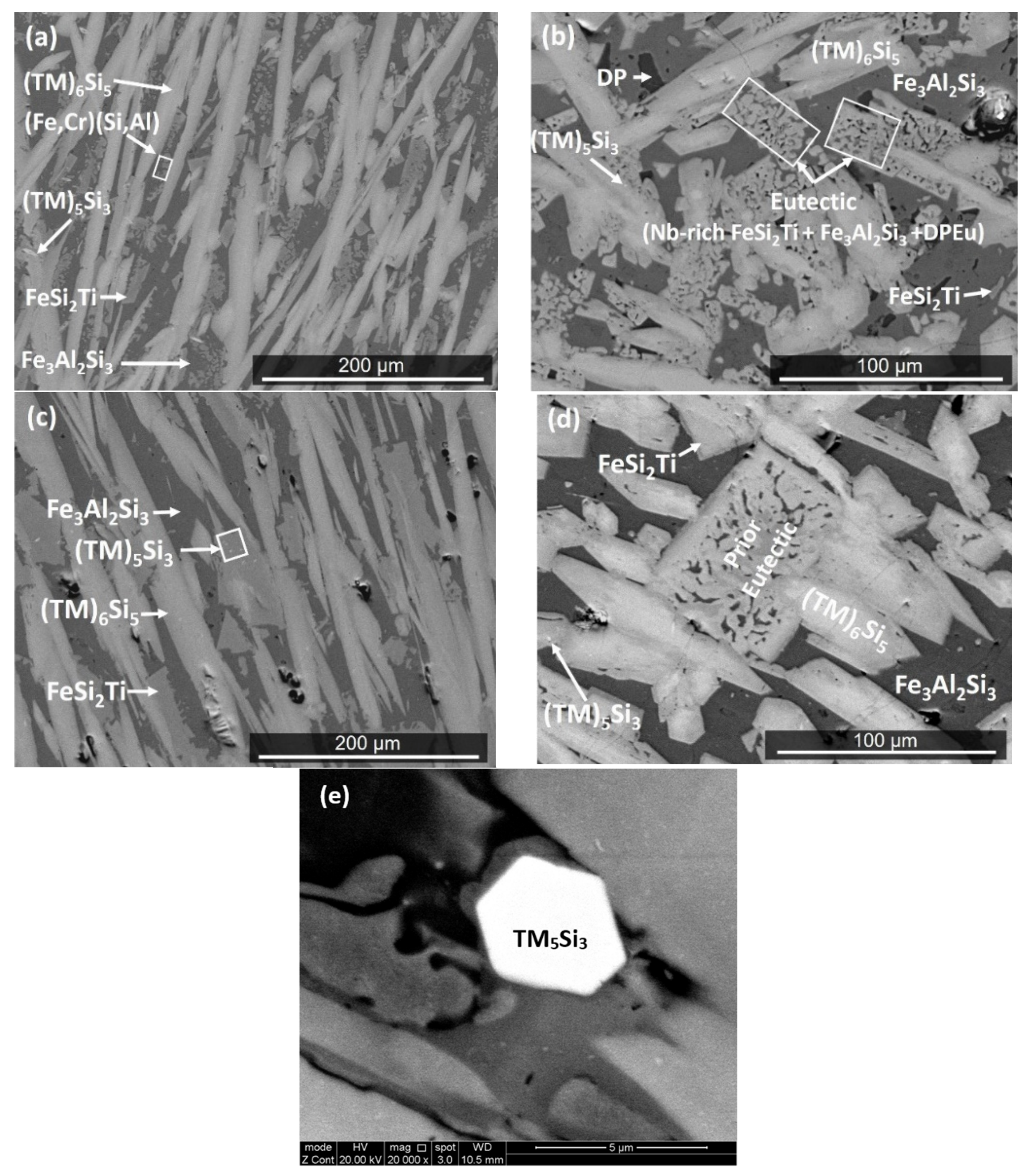

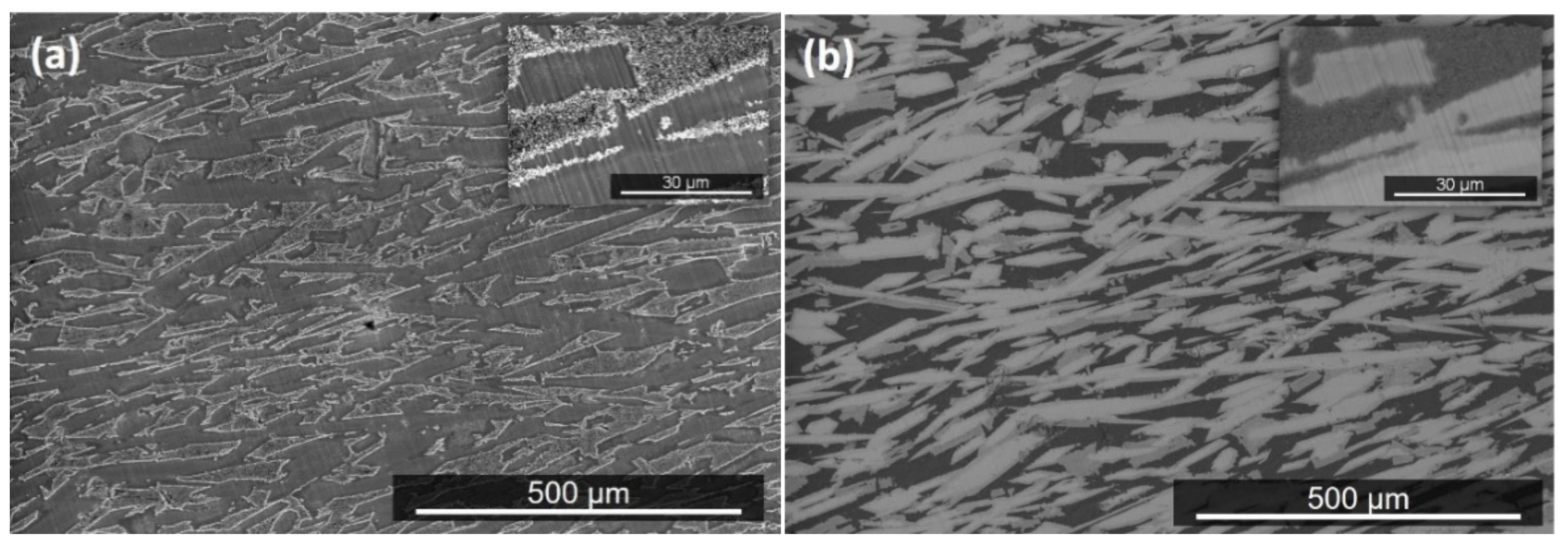
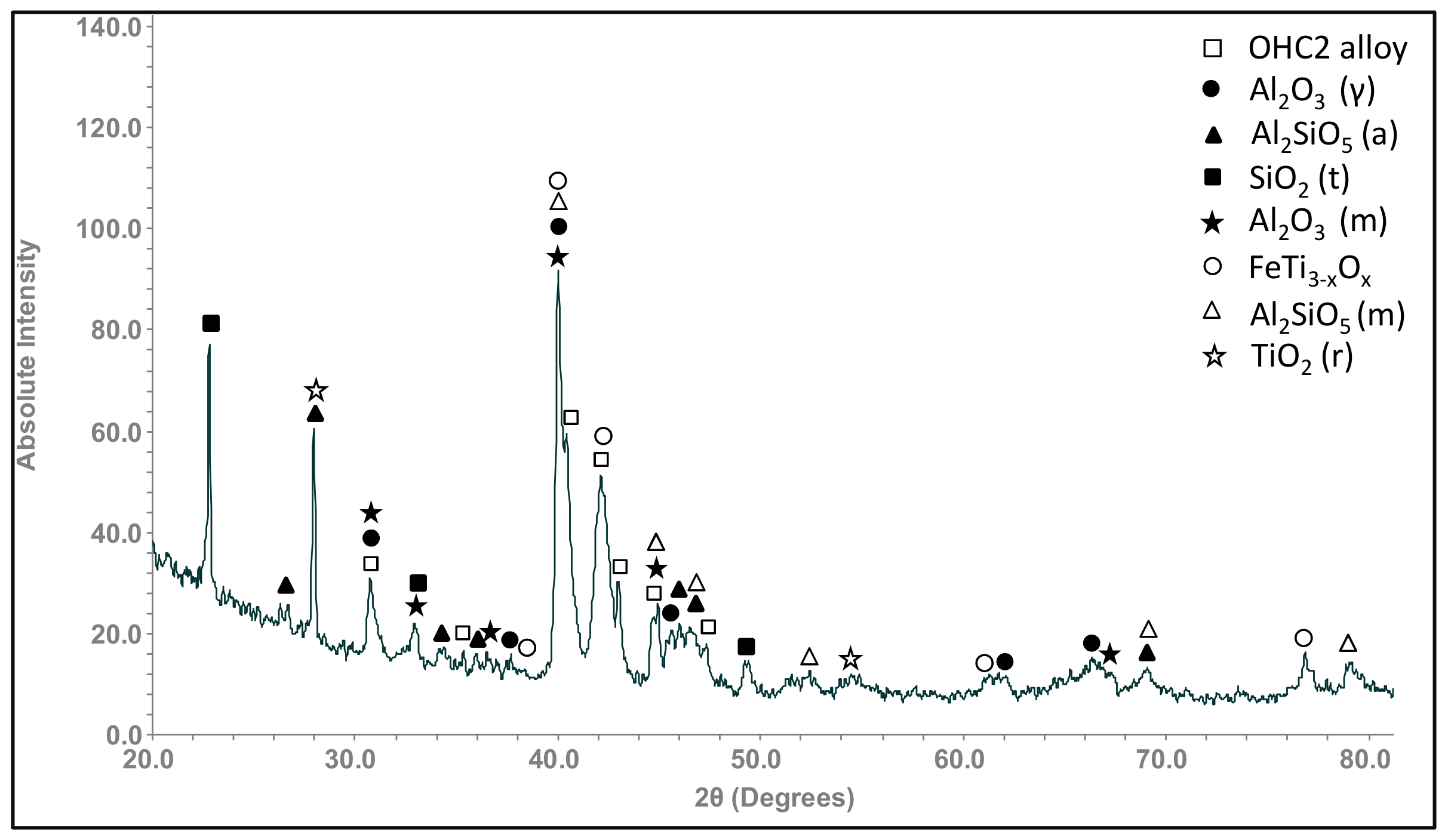
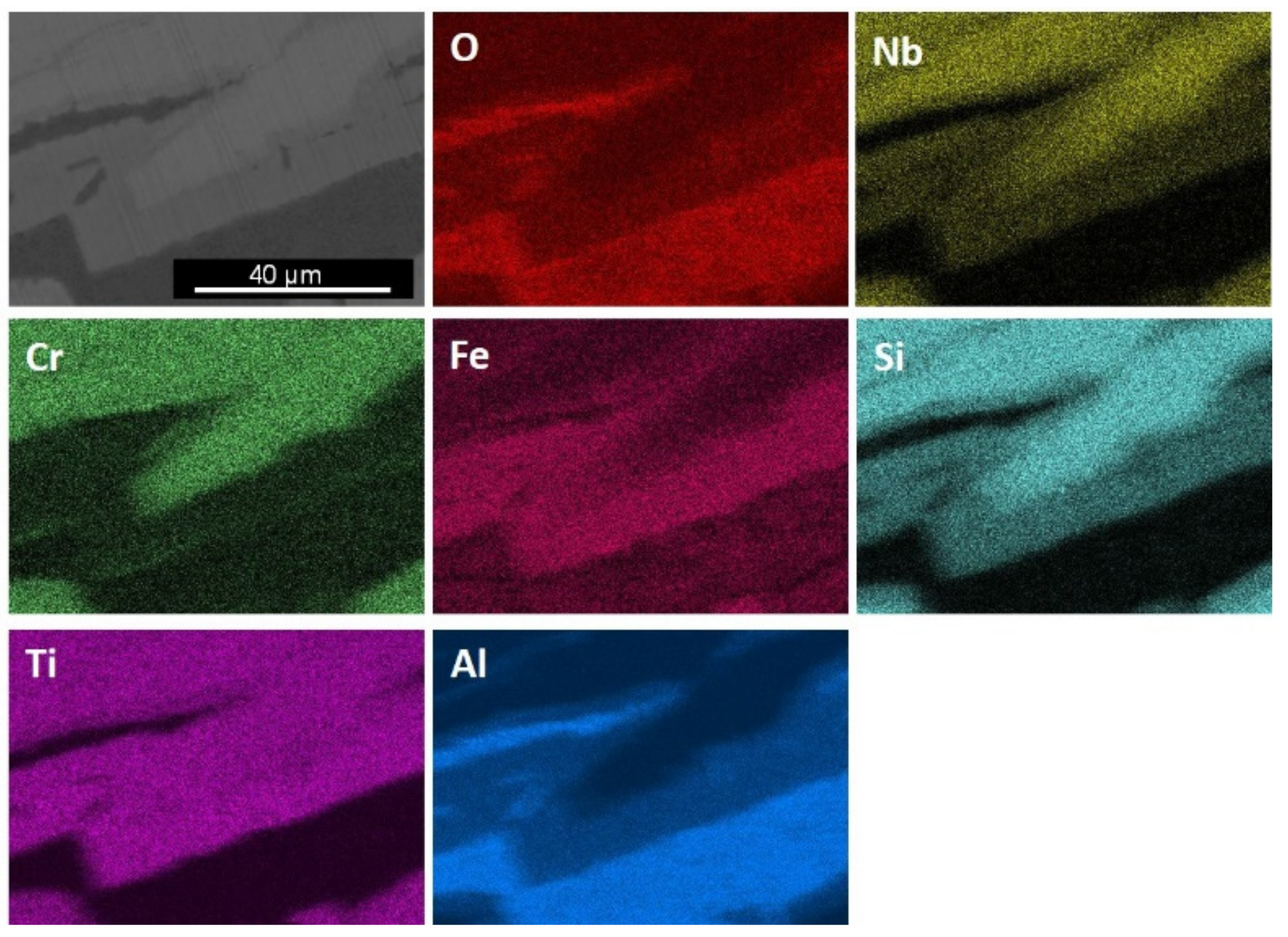
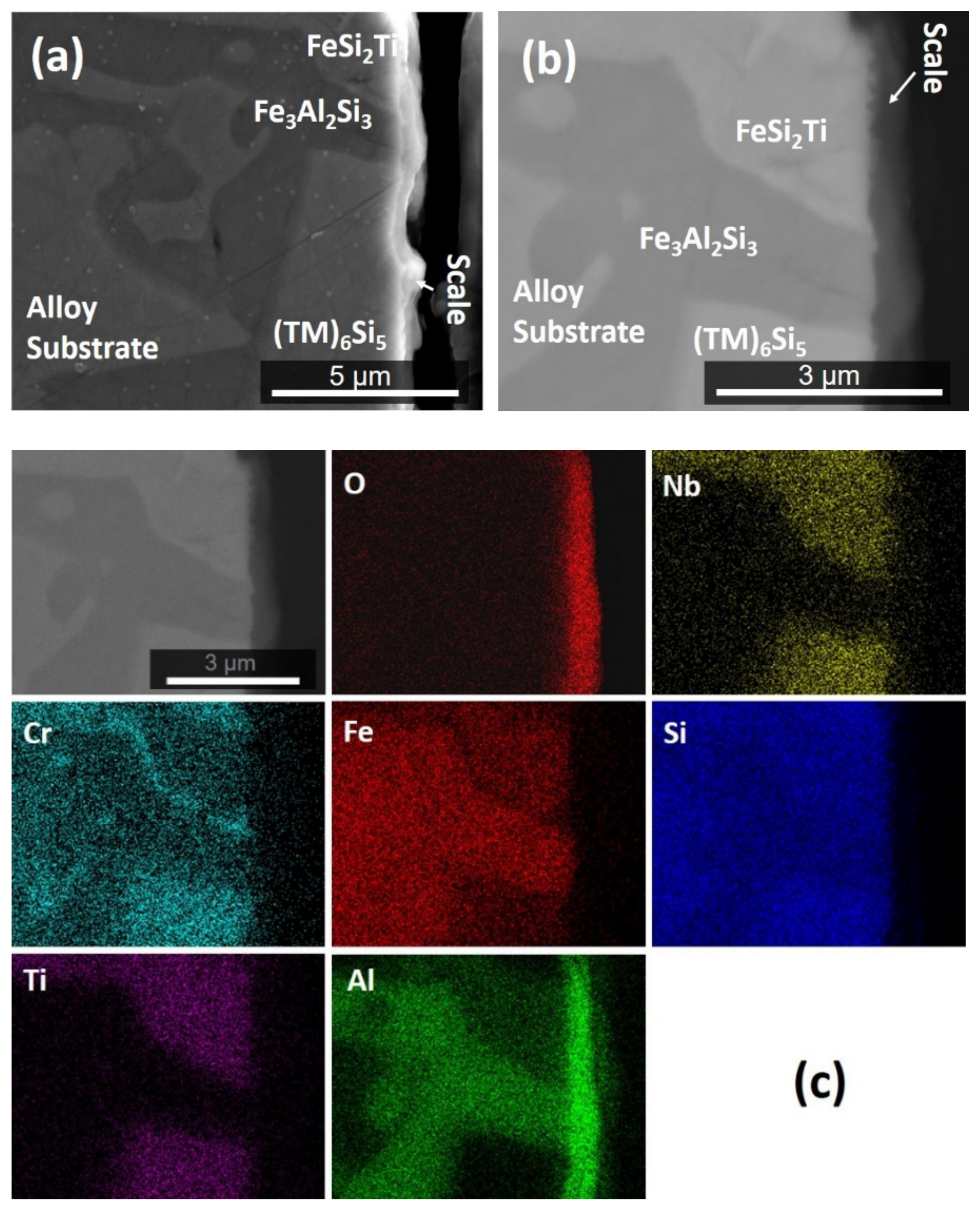
| Phase | Nb (at.%) | Ti (at.%) | Cr (at.%) | Fe (at.%) | Al (at.%) | Si (at.%) |
|---|---|---|---|---|---|---|
| (TM)5Si3 | 16.2 ± 1.1 | 21.4 ± 1.2 | 16.1 ± 0.7 | 6.7 ± 0.9 | 1.8 ± 0.7 | 37.7 ± 1.0 |
| 17.9–14.3 | 22.9–18.9 | 17.3–15.0 | 8.3–5.4 | 3.3–1.1 | 40.0–36.8 | |
| TM6Si5 | 7.9 ± 2.0 | 12.4 ± 0.8 | 18.5 ± 0.8 | 14.5 ± 2.8 | 2.0 ± 0.4 | 44.8 ± 0.4 |
| 11.2–4.1 | 13.8–10.9 | 20.0–17.0 | 20.0–10.2 | 2.8–1.3 | 46.4–44.1 | |
| FeSi2Ti | 4.3 ± 0.6 | 14.6 ± 0.8 | 5.7 ± 0.3 | 23.3 ± 0.4 | 12.5 ± 1.0 | 39.6 ± 0.6 |
| 5.2–3.1 | 15.9–13.1 | 6.3–5.3 | 23.8–22.7 | 13.8–10.4 | 40.9–38.6 | |
| (Fe,Cr)(Si,Al) | 0.1 | 0.4 | 9.2 ± 0.8 | 37.1 ± 0.7 | 8.5 ± 0.6 | 44.7 ± 0.4 |
| - | - | 10.4–7.2 | 38.8–36.0 | 10.2–7.4 | 45.4–43.7 | |
| Fe3Al2Si3 | 0.1 | 0.3 | 4.6 ± 0.4 | 30.2 ± 0.6 | 32.2 ± 0.8 | 32.6 ± 0.6 |
| - | - | 5.4–3.7 | 31.1–28.6 | 34.2–30.7 | 34.4–31.5 | |
| Dark phase (DP) | 0.1 | 0.3 | 3.5 ± 0.3 | 21.3 ± 0.5 | 57.5 ± 1.7 | 17.3 ± 1.2 |
| - | - | 4.3–2.9 | 22.7–20.6 | 59.3–54.0 | 19.5–16.0 | |
| Eutectic (large area analysis) | 5.5±0.6 | 11.8 ± 0.7 | 5.0 ± 0.6 | 22.5 ± 0.7 | 16.0 ± 2.2 | 39.3 ± 1.0 |
| 6.3–4.2 | 12.9–10.6 | 6.1–4.4 | 23.4–21.6 | 20.8–12.6 | 40.9–37.0 | |
| FeSi2Ti (in eutectic) | 7.1 ± 0.8 | 14.4 ± 0.4 | 5.2 ± 0.7 | 21.9 ± 0.6 | 9.7 ± 0.8 | 41.7 ± 0.6 |
| 8.5–5.8 | 15.5–14.0 | 7.6–4.2 | 23.0–21.0 | 11.6–8.5 | 42.5–40.0 | |
| Fe3Al2Si3 (in eutectic) | 0.9 | 2.4 ± 1.9 | 4.5 ± 0.4 | 28.5 ± 1.4 | 29.2 ± 2.1 | 34.5 ± 0.7 |
| - | 5.7–0.6 | 5.3–4.0 | 30.1–25.6 | 31.6–26.4 | 35.4–33.7 | |
| (DPEu) | 2.5±0.6 | 5.8 ± 1.0 | 3.9 ± 0.3 | 20.7 ± 0.3 | 39.8 ± 3.9 | 27.3 ± 2.4 |
| 3.4–1.7 | 7.4–4.6 | 4.3–3.5 | 21.1–20.3 | 45.3–35.2 | 30.3–23.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Negrete, O.; Tsakiropoulos, P. On the Microstructure and Isothermal Oxidation of the Si-22Fe-12Cr-12Al-10Ti-5Nb (at.%) Alloy. Materials 2019, 12, 1806. https://doi.org/10.3390/ma12111806
Hernández-Negrete O, Tsakiropoulos P. On the Microstructure and Isothermal Oxidation of the Si-22Fe-12Cr-12Al-10Ti-5Nb (at.%) Alloy. Materials. 2019; 12(11):1806. https://doi.org/10.3390/ma12111806
Chicago/Turabian StyleHernández-Negrete, Ofelia, and Panos Tsakiropoulos. 2019. "On the Microstructure and Isothermal Oxidation of the Si-22Fe-12Cr-12Al-10Ti-5Nb (at.%) Alloy" Materials 12, no. 11: 1806. https://doi.org/10.3390/ma12111806
APA StyleHernández-Negrete, O., & Tsakiropoulos, P. (2019). On the Microstructure and Isothermal Oxidation of the Si-22Fe-12Cr-12Al-10Ti-5Nb (at.%) Alloy. Materials, 12(11), 1806. https://doi.org/10.3390/ma12111806






