Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Fluoride-Containing Bioactive Glass (FBAG)
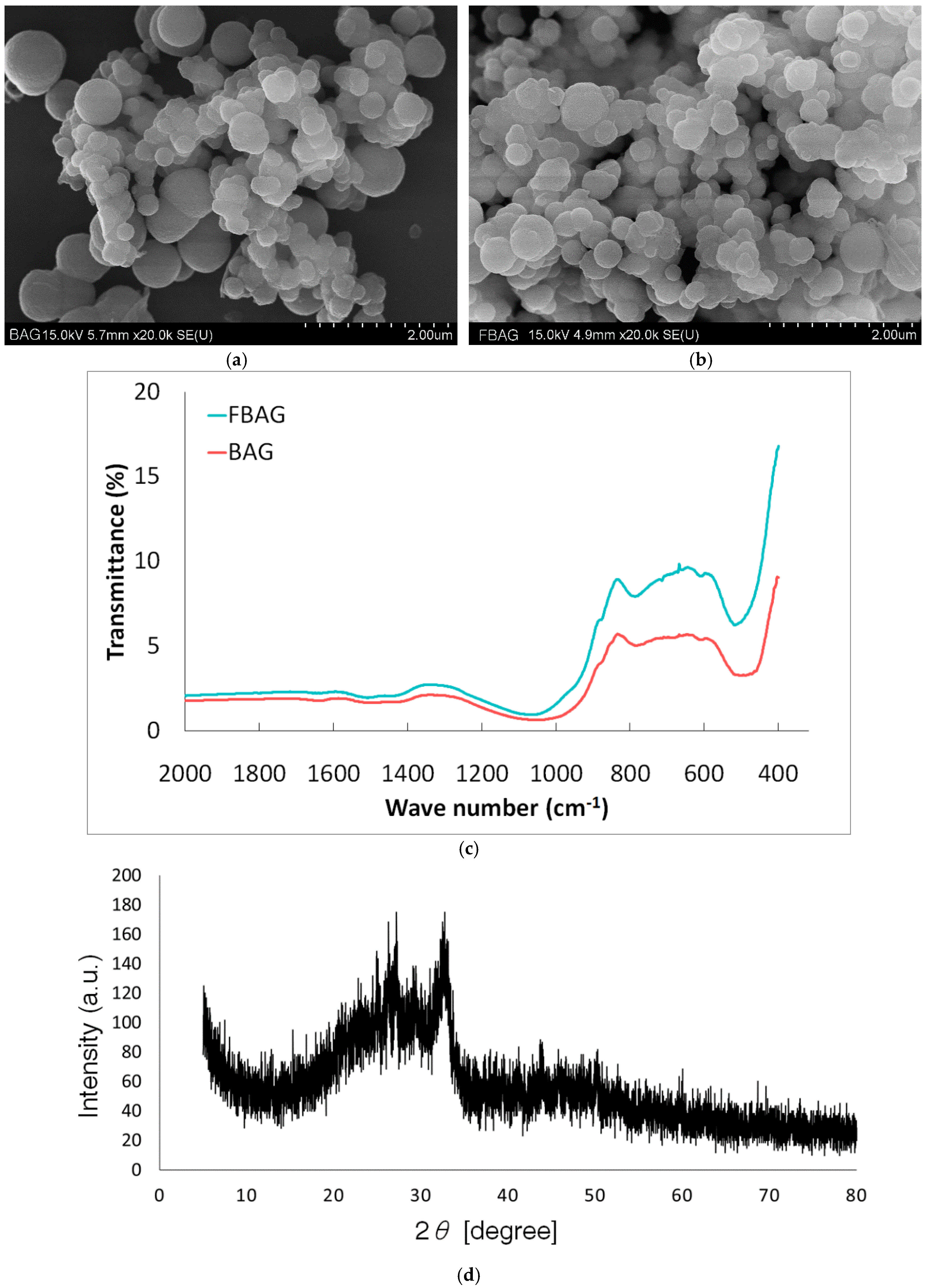
2.2. Characterization of FBAG
2.3. Preparation of a Resin Disk Containing FBAG
2.4. Vickers Hardness
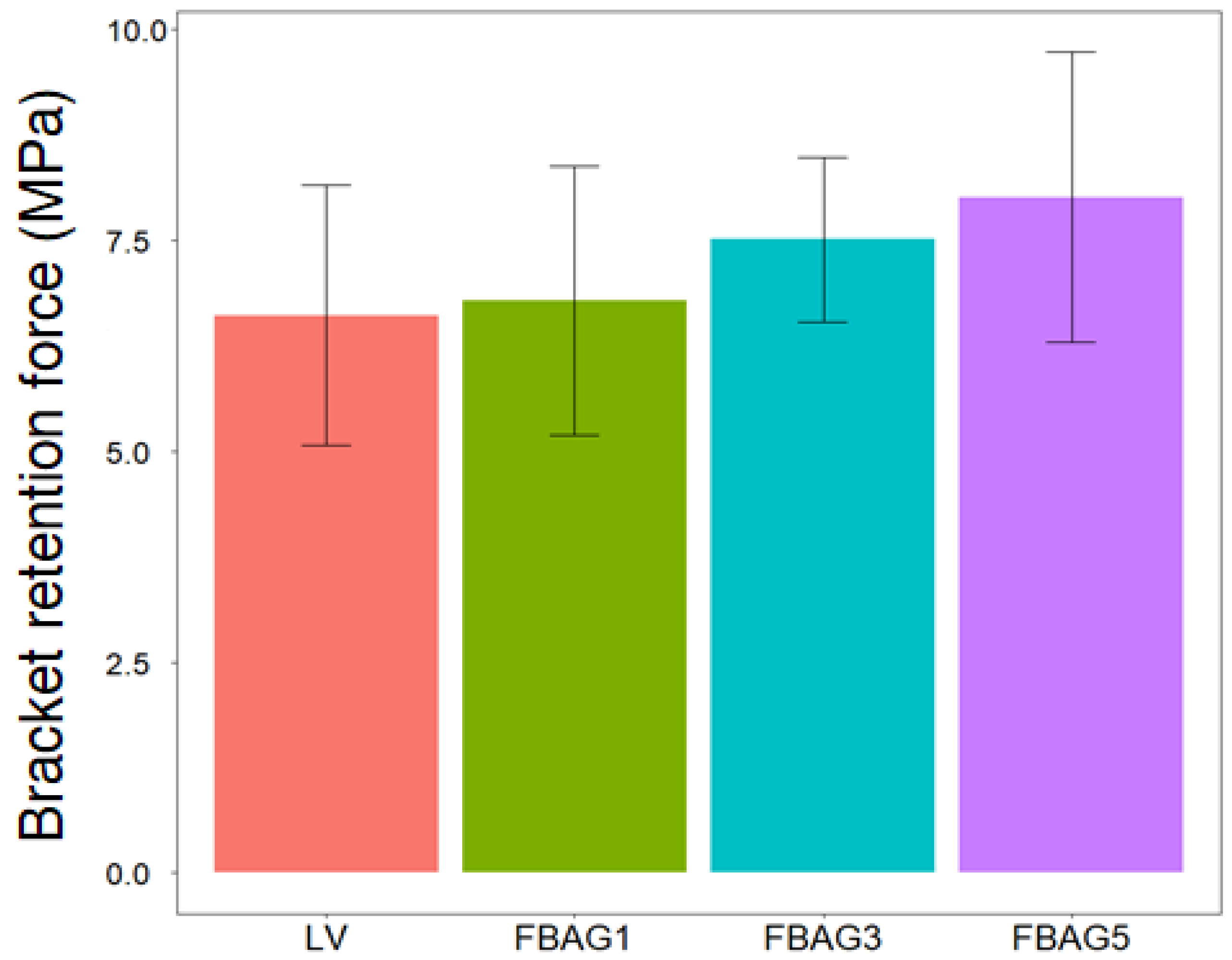
2.5. Bracket Retention Test
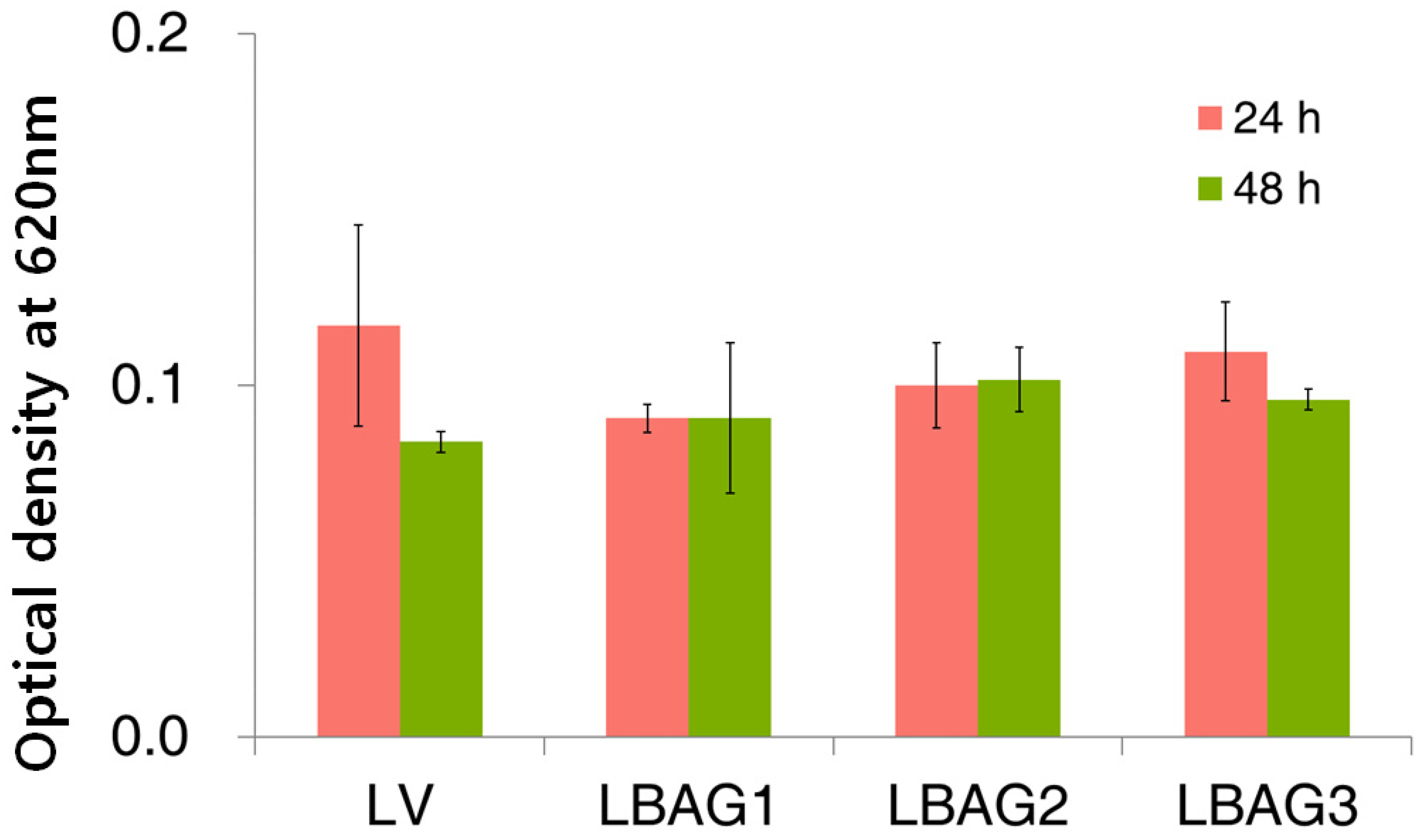
2.6. Antibacterial Properties
2.7. Cell Viability Assay
2.8. In Vitro F Dissolution Test
2.9. Remineralization Properties
2.10. Statistical Analysis
2.11. Ethics Statement
3. Results
3.1. Characterization
3.2. Vickers Hardness
3.3. Bracket Retention Test
3.4. Adhesive Remnant Index (ARI) Score
3.5. Cell Viability Test
3.6. Antibacterial Test
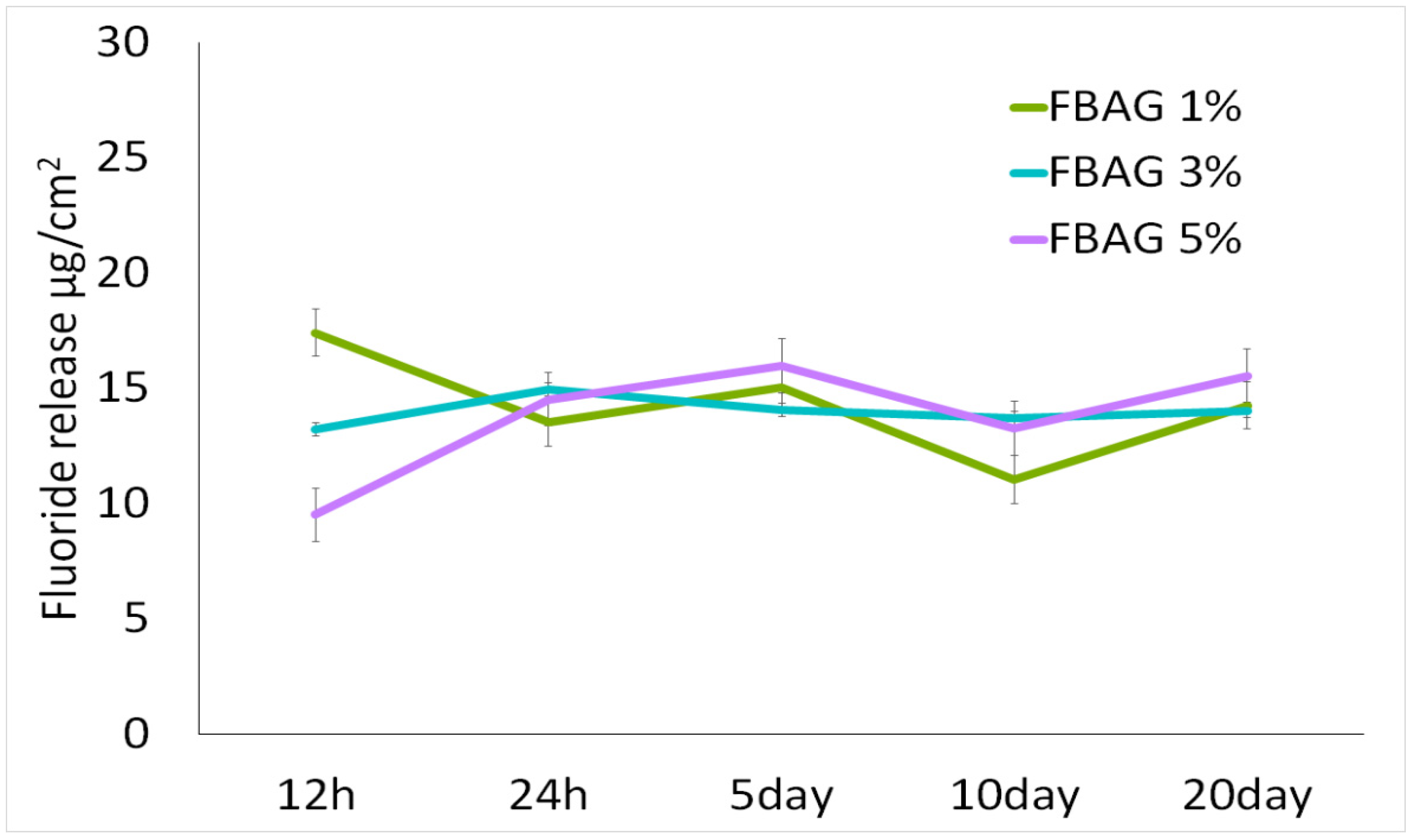
3.7. In Vitro F Dissolution Test
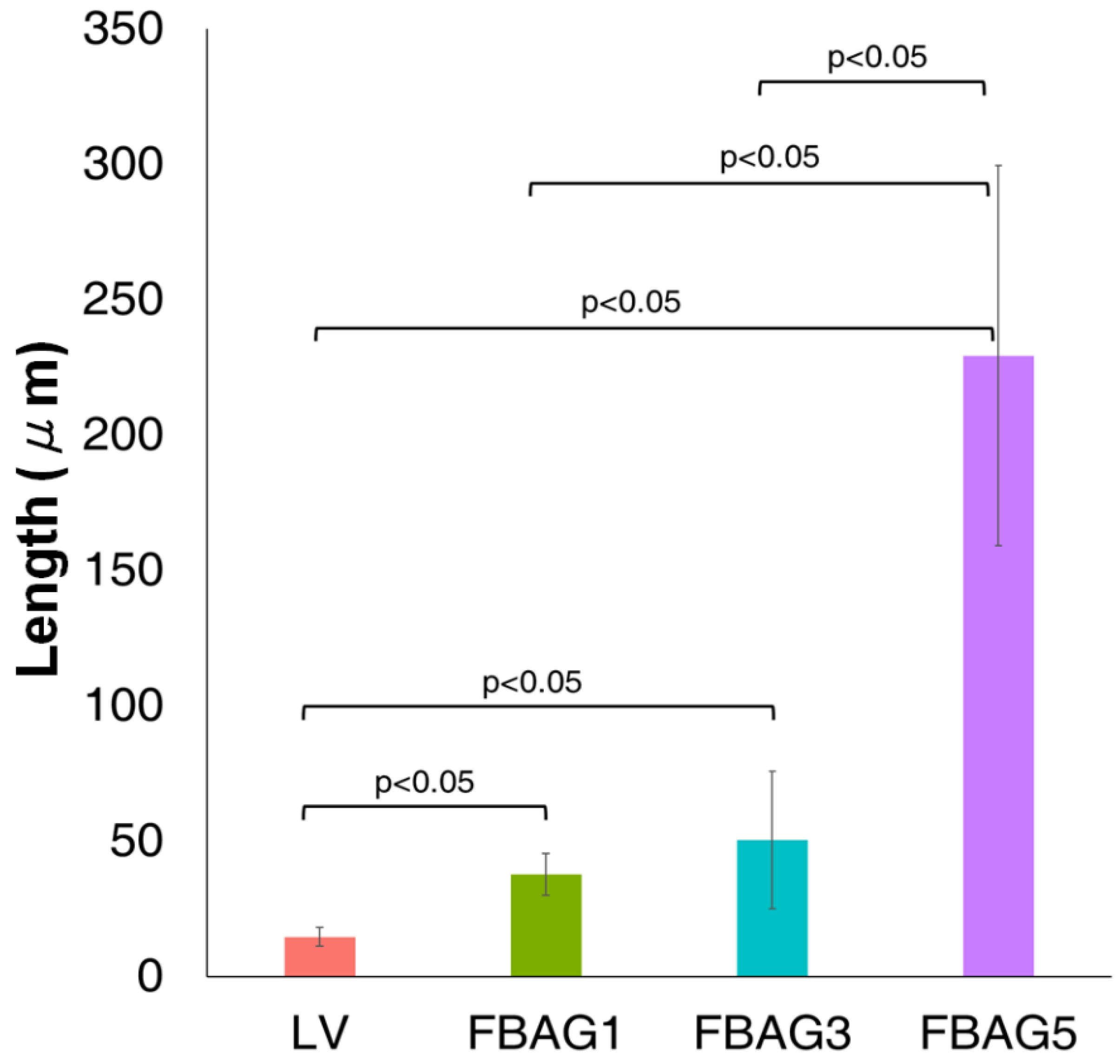
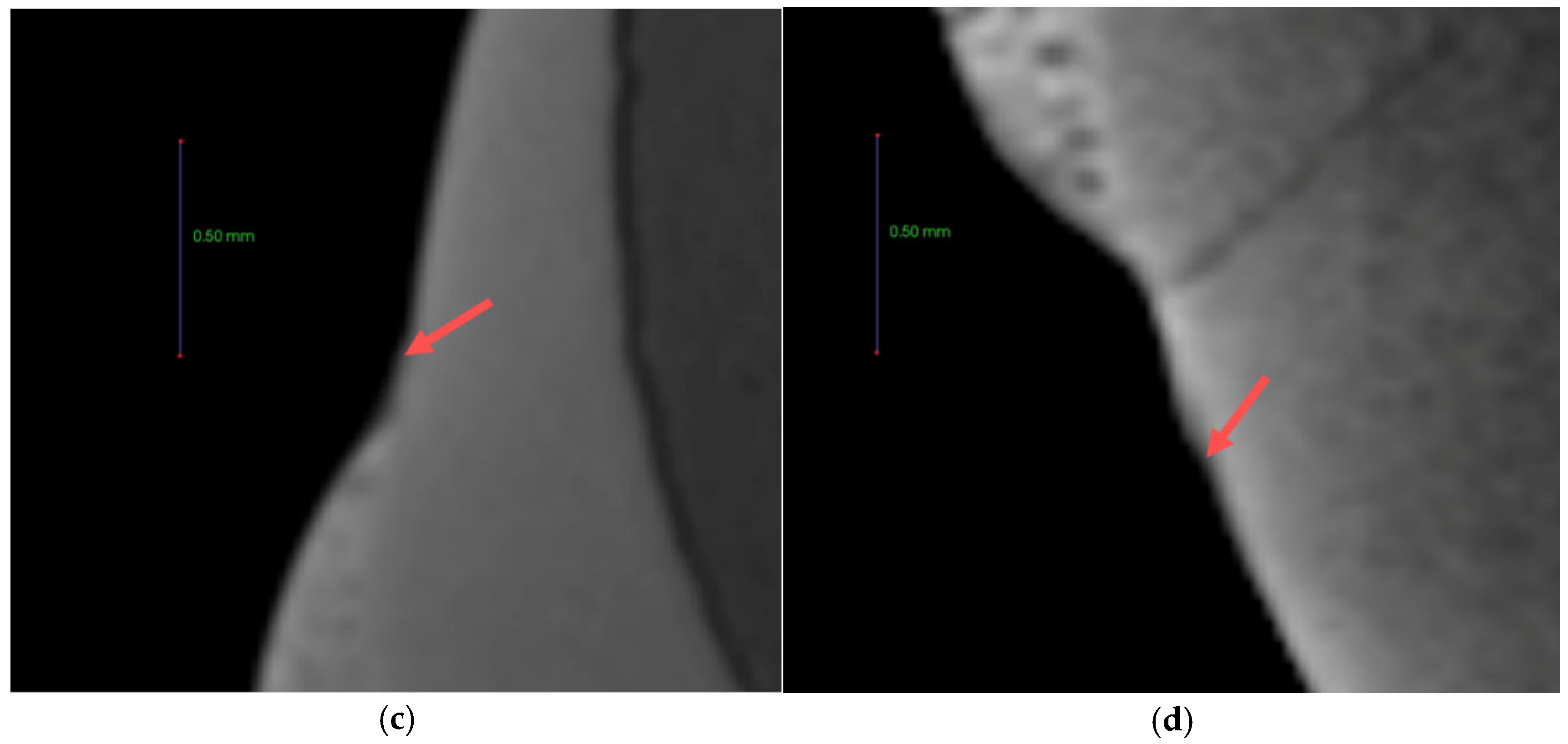
3.8. Anti-demineralization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chambers, C.; Stewart, S.; Su, B.; Sandy, J.; Ireland, A. Prevention and treatment of demineralisation during fixed appliance therapy: A review of current methods and future applications. Br. Dent. J. 2013, 215, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Bishara, S.E.; Ostby, A.W. White spot lesions: Formation, prevention, and treatment. Semin. Orthodont. 2008, 14, 174–182. [Google Scholar] [CrossRef]
- Willmot, D. White spot lesions after orthodontic treatment. Semin. Orthodont. 2008, 14, 209–219. [Google Scholar] [CrossRef]
- Khalaf, K. Factors affecting the formation, severity and location of white spot lesions during orthodontic treatment with fixed appliances. J. Oral. Maxillofac. Res. 2014, 5, e4. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Gherlone, E. Prevalence of white-spot lesions before and during orthodontic treatment with fixed appliances. Eur. J. Orthod. 2013, 35, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Knösel, M.; Bojes, M.; Jung, K.; Ziebolz, D. Increased susceptibility for white spot lesions by surplus orthodontic etching exceeding bracket base area. Am. J. Orthodont. Dentofac. Orthop. 2012, 141, 574–582. [Google Scholar] [CrossRef]
- Rosenbloom, R.G.; Tinanoff, N. Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am. J. Orthodont. Dentofac. Orthop. 1991, 100, 35–37. [Google Scholar] [CrossRef]
- Featherstone, J.D. Prevention and reversal of dental caries role of low level fluoride. Community Dent. Oral. Epidemiol. 1999, 27, 31–40. [Google Scholar]
- Wiltshire, W.A. Shear bond strengths of a glass ionomer for direct bonding in orthodontics. Am. J. Orthod. Dentofac. Orthop. 1994, 106, 127–130. [Google Scholar]
- Oz, A.Z.; Oz, A.A.; Yazicioglu, S. In vivo effect of antibacterial and fluoride-releasing adhesives on enamel demineralization around brackets: A micro-CT study. Angle Orthodont. 2017, 87, 841–846. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, I.-R.; Park, B.-S.; Lee, D.; Ko, C.-C.; Son, W.-S.; Kim, Y.-I. Remineralization Property of an orthodontic primer containing a bioactive glass with silver and zinc. Materials 2017, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Ko, C.-C.; Son, S.-A.; Kim, Y.-I. Enamel anti-demineralization effect of orthodontic adhesive containing bioactive glass and graphene oxide: An in-vitro study. Materials 2018, 11, 1728. [Google Scholar] [CrossRef] [PubMed]
- Fernando, D.; Attik, N.; Pradelle-Plasse, N.; Jackson, P.; Grosgogeat, B.; Colon, P. Bioactive glass for dentin remineralization: A systematic review. Mater. Sci. Eng. C 2017, 76, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Kohda, N.; Iijima, M.; Kawaguchi, K.; Toshima, H.; Muguruma, T.; Endo, K.; Mizoguchi, I. Inhibition of enamel demineralization and bond-strength properties of bioactive glass containing 4-META/MMA-TBB-based resin adhesive. Eur. J. Oral Sci. 2015, 123, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. Part A 2010, 93, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Al-Eesa, N.A.; Johal, A.; Hill, R.G.; Wong, F.S.L. Fluoride containing bioactive glass composite for orthodontic adhesives—Apatite formation properties. Dent. Mater. 2018, 34, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.; Zahid, S.; Zahid, S.; Kaleem, M.; Khan, A.S.; Shah, A.T. Sol-gel derived fluoride-doped bioactive glass powders: Structural and long-term fluoride release/pH analysis. J. Non Cryst. Solids 2018, 498, 216–222. [Google Scholar] [CrossRef]
- Zheng, K.; Boccaccini, A.R. Sol-gel processing of bioactive glass nanoparticles: A review. Adv. Colloid Interface Sci. 2017, 249, 363–373. [Google Scholar] [CrossRef]
- Stookey, G.K.; Featherstone, J.D.; Rapozo-Hilo, M.; Schemehorn, B.R.; Williams, R.A.; Baker, R.A.; Barker, M.L.; Kaminski, M.A.; McQueen, C.M.; Amburgey, J.S.; et al. The Featherstone laboratory pH cycling model: A prospective, multi-site validation exercise. Am. J. Dent. 2011, 24, 322–328. [Google Scholar]
- Paschos, E.; Kleinschrodt, T.; Clementino-Luedemann, T.; Huth, K.C.; Hickel, R.; Kunzelmann, K.H.; Rudzki-Janson, I. Effect of different bonding agents on prevention of enamel demineralization around orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 603–612. [Google Scholar] [CrossRef]
- ElBatal, H.A.; Azooz, M.A.; Khalil, E.M.A.; Soltan Monem, A.; Hamdy, Y.M. Characterization of some bioglass–ceramics. Mater. Chem. Phys. 2003, 80, 599–609. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, D.H.; Song, C.W.; Yoon, S.Y.; Kim, S.Y.; Na, H.S.; Chung, J.; Kim, Y.I.; Kwon, Y.H. Antibacterial and remineralization effects of orthodontic bonding agents containing bioactive glass. Korean J. Orthodont. 2018, 48, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Ylänen, H. Bioactive Glasses: Materials, Properties and Applications; Woodhead Publishing: Saxton, UK, 2017; p. 426. [Google Scholar]
- Al-Eesa, N.A.; Wong, F.S.L.; Johal, A.; Hill, R.G. Fluoride containing bioactive glass composite for orthodontic adhesives—Ion release properties. Dent. Mater. 2017, 33, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.J.; Kim, Y.M.; Kwon, Y.H.; Kim, I.R.; Park, B.S.; Son, W.S.; Lee, S.M.; Kim, Y.I. Enamel Surface Remineralization Effect by Fluorinated Graphite and Bioactive Glass-Containing Orthodontic Bonding Resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R. Calcium phosphates as ion-releasing fillers in restorative resin-based materials. Dent. Mater. 2019, 35, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Randolph, L.D.; Palin, W.M.; Leloup, G.; Leprince, J.G. Filler characteristics of modern dental resin composites and their influence on physico-mechanical properties. Dent. Mater. 2016, 32, 1586–1599. [Google Scholar] [CrossRef]
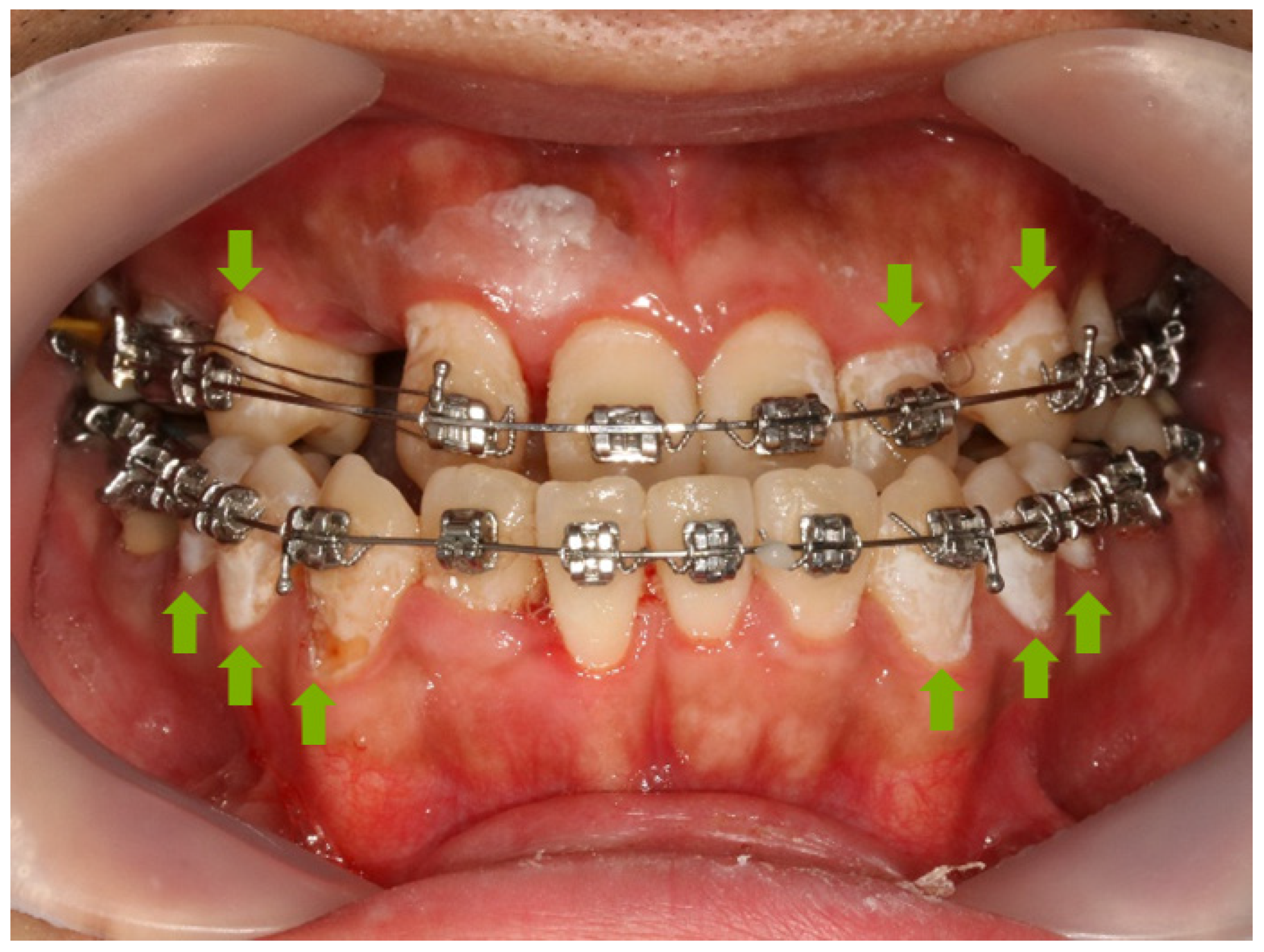





| Score | Evaluation Criteria (Remaining Adhesive on the Tooth) |
|---|---|
| 1 | All |
| 2 | More than 90% |
| 3 | Between 10–90% |
| 4 | Less than 10% |
| 5 | No adhesive |
| The Solutions | Composition |
|---|---|
| Demineralizing solution (pH 4.4, 0.5 L) | Ca(NO3)2∙4H2O 2.0 mM |
| KH2PO4 2.0 mM | |
| CH3COOH 75.0 mM | |
| Remineralizing solution (pH 7.0, 0.5 L) | Ca(NO3)2∙4H2O 1.5 mM |
| KH2PO4 0.9 mM | |
| KCl 130 mM | |
| NaC2H6AsO2∙3H2O 20.2 mM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Yoo, K.-H.; Yoon, S.-Y.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials 2019, 12, 1813. https://doi.org/10.3390/ma12111813
Nam H-J, Kim Y-M, Kwon YH, Yoo K-H, Yoon S-Y, Kim I-R, Park B-S, Son W-S, Lee S-M, Kim Y-I. Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials. 2019; 12(11):1813. https://doi.org/10.3390/ma12111813
Chicago/Turabian StyleNam, Hyung-Jin, You-Min Kim, Yong Hoon Kwon, Kyung-Hyeon Yoo, Seog-Young Yoon, In-Ryoung Kim, Bong-Soo Park, Woo-Sung Son, Seung-Min Lee, and Yong-Il Kim. 2019. "Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin" Materials 12, no. 11: 1813. https://doi.org/10.3390/ma12111813
APA StyleNam, H.-J., Kim, Y.-M., Kwon, Y. H., Yoo, K.-H., Yoon, S.-Y., Kim, I.-R., Park, B.-S., Son, W.-S., Lee, S.-M., & Kim, Y.-I. (2019). Fluorinated Bioactive Glass Nanoparticles: Enamel Demineralization Prevention and Antibacterial Effect of Orthodontic Bonding Resin. Materials, 12(11), 1813. https://doi.org/10.3390/ma12111813





