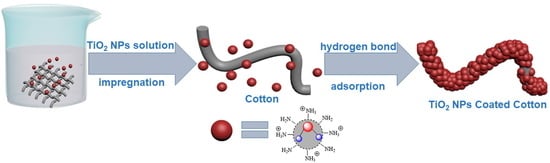
Study on the Photocatalytic and Antibacterial Properties of TiO2 Nanoparticles-Coated Cotton Fabrics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amino-Capped TiO2 NPs
2.3. Preparation of TiO2 NPs-Coated Cotton Fabric
2.4. Characterization of the Amino-Capped TiO2 NPs and Treated Cotton Fabric
3. Results
3.1. Synthesis and Characterization of Amino-Capped TiO2 NPs
3.2. Preparation and Characterization of the Cotton Fabric Coated by the Amino-Capped TiO2 NPs
3.3. Photocatalytic Activity of the TiO2 NPs-Coated Cotton Fabric
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hossam, E.E.; Saleh, N.H.; Khaled, S.N.; Zahran, M.K. Functionalization of medical cotton by direct incorporation of silver nanoparticles. Int. J. Biol. Macromol. 2015, 78, 249–256. [Google Scholar]
- Subbiah, D.K.; Mani, G.K.; Babu, K.J. Nanostructured ZnO on cotton fabrics-A novel flexible gas sensor & UV filter. J. Clean. Prod. 2018, 194, 372–382. [Google Scholar]
- Sahito, I.A.; Sun, K.C.; Arbab, A.A. Integrating high electrical conductivity and photocatalytic activity in cotton fabric by cationizing for enriched coating of negatively charged graphene oxide. Carbohydr. Polym. 2015, 130, 299–306. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Q.; Yuan, J. Hydrophobic modification of cotton fabric with octadecylamine via laccase/TEMPO mediated grafting. Carbohydr. Polym. 2016, 137, 549–555. [Google Scholar] [CrossRef]
- Akhavan Sadr, F.; Montazer, M. In situ sonosynthesis of nano TiO2 on cotton fabric. Ultrason. Sonochem. 2014, 21, 681–691. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Q.; Lai, Q.; Li, P. Biomass-Templated Fabrication of Metallic Materials for Photocatalytic and Bactericidal Applications. Materials 2019, 12, 1271. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A. Self-cleaning cotton functionalized with TiO2/SiO2: Focus on the role of silica. J. Colloid. Interface Sci. 2013, 401, 1–7. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Frache, A.; Malucelli, G. Layer by Layer coatings assembled through dipping, vertical or horizontal spray for cotton flame retardancy. Carbohydr. Polym. 2013, 92, 114–119. [Google Scholar] [CrossRef]
- Chen, C.M.; Zhao, D.C.; Zhou, Q.X.; Wu, Y.L.; Zhou, X.; Wang, H.Y. Facile Preparation and Characterization of Polyaniline and CeO2 Co-Decorated TiO2 Nanotube Array and its Highly Efficient Photoelectrocatalytic Activity. Nanoscale Res. Lett. 2019, 14, 60. [Google Scholar] [CrossRef]
- Li, S.H.; Zhu, T.X.; Huang, J.Y. Durable antibacterial and UV-protective Ag/TiO2@fabrics for sustainable biomedical application. Int. J. Nanomed. 2017, 12, 2593–2606. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, X.; Chen, F. Highly anti-UV properties of silk fiber with uniform and conformal nanoscale TiO2 coatings via atomic layer deposition. ACS Appl. Mater. Interfaces 2015, 7, 21326–21333. [Google Scholar] [CrossRef]
- Rassam, G.; Abdi, Y.; Abdi, A. Deposition of TiO2 nano-particles on wood surfaces for UV and moisture protection. J. Exp. Nanosci. 2012, 7, 468–476. [Google Scholar] [CrossRef]
- Li, F.; Li, Q.; Kim, H. Spray deposition of electrospun TiO2 nanoparticles with self-cleaning and transparent properties onto glass. Appl. Surf. Sci. 2013, 276, 390–396. [Google Scholar] [CrossRef]
- Song, T.; Paik, U. TiO2 as an Active or Supplemental Material for Lithium Batteries. J. Mater. Chem. A 2015, 4, 14–31. [Google Scholar] [CrossRef]
- Moafi, H.F.; Shojaie, A.F.; Zanjanchi, M. Flame-retardancy and photocatalytic properties of cellulosic fabric coated by nano-sized titanium dioxide. J. Therm. Anal. Calorim. 2011, 104, 717–724. [Google Scholar] [CrossRef]
- Fisher, M.B.; Keane, D.A.; Fernandez-Ibanez, P.; Colreavy, J.; Hinder, S.J.; McGuigan, K.G.; Pillai, S.C. Nitrogen and Copper doped solar light active TiO2 photocatalyst for water decontamination. Appl. Catal. B Environ. 2013, 130, 8–13. [Google Scholar] [CrossRef]
- Daoud, W.A.; Xin, J.H. Nucleation and growth of anatase crystallites on cotton fabrics at low temperature. J. Am. Ceram. Soc. 2004, 87, 953–955. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.; Lin, H.; Lu, Y. Synthesis of an amino-terminated amino polymer and its application in reactive dyeing on cotton as a salt-free dyeing auxiliary. Color Technol. 2010, 123, 351–357. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Liu, Y.; Morikawa, H. Application of ZnO nanoparticles to enhance the antimicrobial activity and ultraviolet protective property of bamboo pulp fabric. Cellulose 2013, 20, 1877–1884. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.Y.; Lin, H.; Zhang, D.S. Performance of cotton fabric treated with an amino-terminated amino polymer. Fiber Polym. 2008, 9, 515–520. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhuang, L.H.; Sun, J.; Zheng, C.L. Salt-free dyeing of ramie fabric with an amino-terminated amino polymer. Cellulose 2014, 21, 3725–3736. [Google Scholar] [CrossRef]
- Lei, W.; Sun, Y.; Huang, B. Synthesis and Application of Polyurethane-Modified Silicone as Finishing Agent for Cotton Fabric. Fibers Polym. 2018, 19, 1024–1031. [Google Scholar] [CrossRef]
- Khan, M.Z.; Baheti, V.; Ashraf, M. Development of UV Protective, Superhydrophobic and Antibacterial Textiles Using ZnO and TiO2 Nanoparticles. Fibers Polym. 2018, 198, 1647–1654. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhang, D.S.; Chen, Y.Y.; Lin, H. Amino Polymer Functional TiO2 Nanoparticles: Synthesis and Its Application for the anti-UV Finishing of Silk Fabric. Fiber Polym. 2015, 16, 503–509. [Google Scholar] [CrossRef]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Photostable Self-Cleaning Cotton by a Copper (II) Porphyrin/TiO2 Visible-Light Photocatalytic System. ACS Appl. Mater. Interfaces 2013, 5, 4753–4759. [Google Scholar] [CrossRef]
- Nocuń, M.; Kwaśny, S.; Zontek, J. Photodecomposition of Rhodamine B on TiO2/SiO2 thin films prepared by sol-gel method. Mater Sci.-Poland 2013, 31, 88–94. [Google Scholar]
- Zheng, L.; Mi, L.; Wang, P.N.; Chen, J.Y. Study on the visible-light-induced photokilling effect of nitrogen-doped TiO2 nanoparticles on cancer cells. Nanoscale Res. Lett. 2011, 6, 356–362. [Google Scholar]
- Xu, S.J.; Zhang, F.; Song, J.C.; Kishimoto, Y.; Morikawa, H. Preparation of silver nanoparticle-coated calcium alginate fibers by amino poly(amidoamine) mediated assembly and their antibacterial activity. Text. Res. J. 2015, 86, 878–886. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, H.; Yang, M. TiO2 nanoparticles supported on PMMA nanofibers for photocatalytic degradation of methyl orange. J. Colloid Interf. Sci. 2017, 508, 500–507. [Google Scholar] [CrossRef]
- Cano-Casanova, L.; Amorós-Pérez, A.; Lillo-Ródenas, M. Effect of the preparation method (sol-gel or hydrothermal) and conditions on the TiO2 properties and activity for propene oxidation. Materials 2018, 11, 2227. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, J.; Hu, Z. Preparation and Photocatalytic Activity of TiO2-Wrapped Cotton Nanofiber Composite Catalysts. BioResources 2017, 12, 6062–6081. [Google Scholar] [CrossRef]
- Charpentier, P.A.; Burgess, K.; Wang, L.; Chowdhury, R.R.; Lotus, A.F.; Moula, G. Nano-TiO2/polyurethane composites for antibacterial and self-cleaning coatings. Nanotechnology 2012, 23, 425606. [Google Scholar] [CrossRef]
- Manjunath, K.; Yadav, L.S.R.; Jayalakshmi, T.; Reddy, V.; Rajanaika, H.; Nagaraju, G. Ionic liquid assisted hydrothermal synthesis of TiO2 nanoparticles: Photocatalytic and antibacterial activity. J. Mater. Res. Technol. 2018, 7, 7–13. [Google Scholar] [CrossRef]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Wang, D.; Yan, J.; Xiao, Y.; Gu, W.; Zang, C. Study on the Photocatalytic and Antibacterial Properties of TiO2 Nanoparticles-Coated Cotton Fabrics. Materials 2019, 12, 2010. https://doi.org/10.3390/ma12122010
Zhang G, Wang D, Yan J, Xiao Y, Gu W, Zang C. Study on the Photocatalytic and Antibacterial Properties of TiO2 Nanoparticles-Coated Cotton Fabrics. Materials. 2019; 12(12):2010. https://doi.org/10.3390/ma12122010
Chicago/Turabian StyleZhang, Guangyu, Dao Wang, Jiawei Yan, Yao Xiao, Wenyan Gu, and Chuanfeng Zang. 2019. "Study on the Photocatalytic and Antibacterial Properties of TiO2 Nanoparticles-Coated Cotton Fabrics" Materials 12, no. 12: 2010. https://doi.org/10.3390/ma12122010