The Effects of Temperature on the Hydrothermal Synthesis of Hydroxyapatite-Zeolite Using Blast Furnace Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Test Methods
3. Results and Discussion
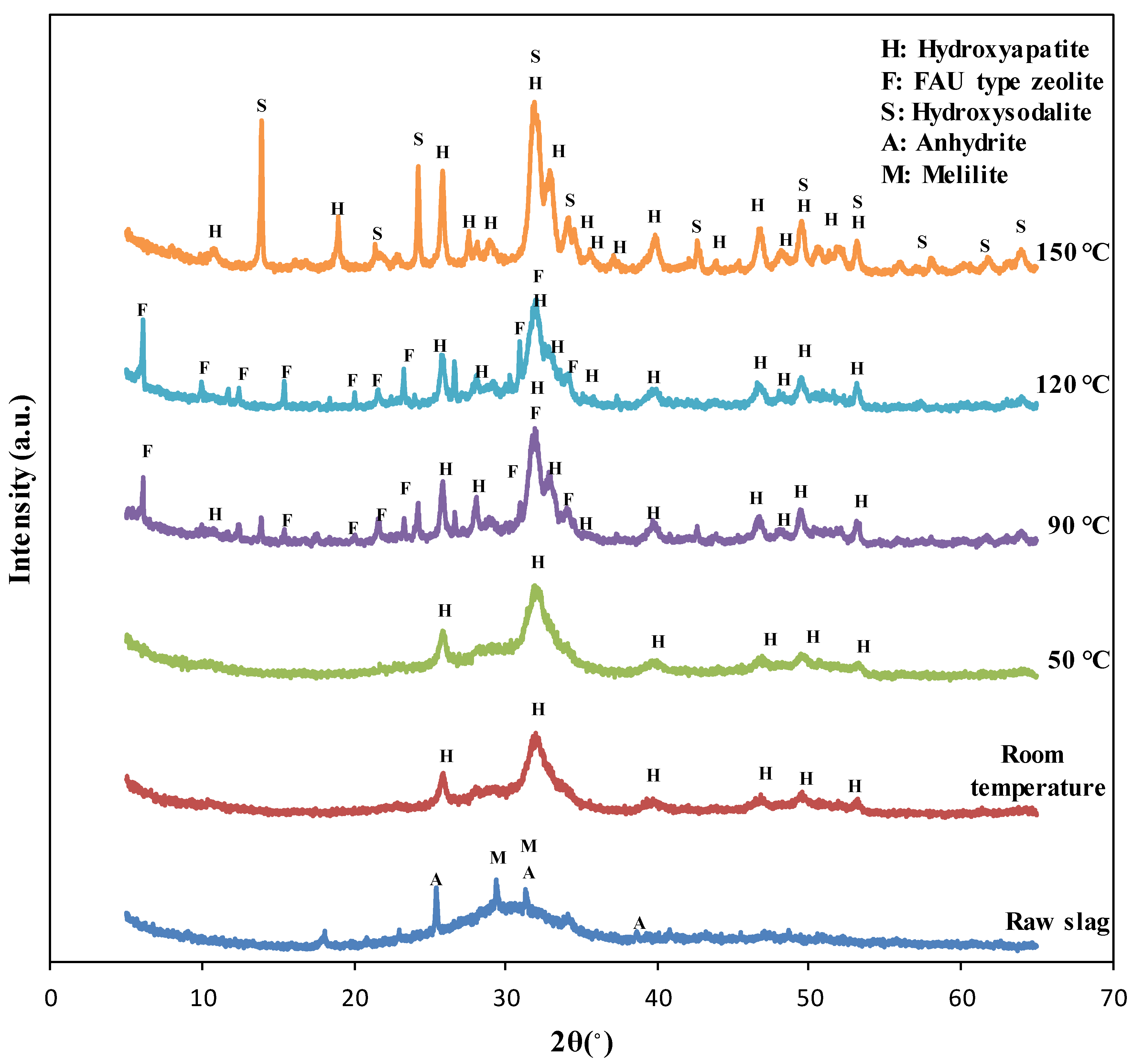
3.1. Crystalline Characteristics
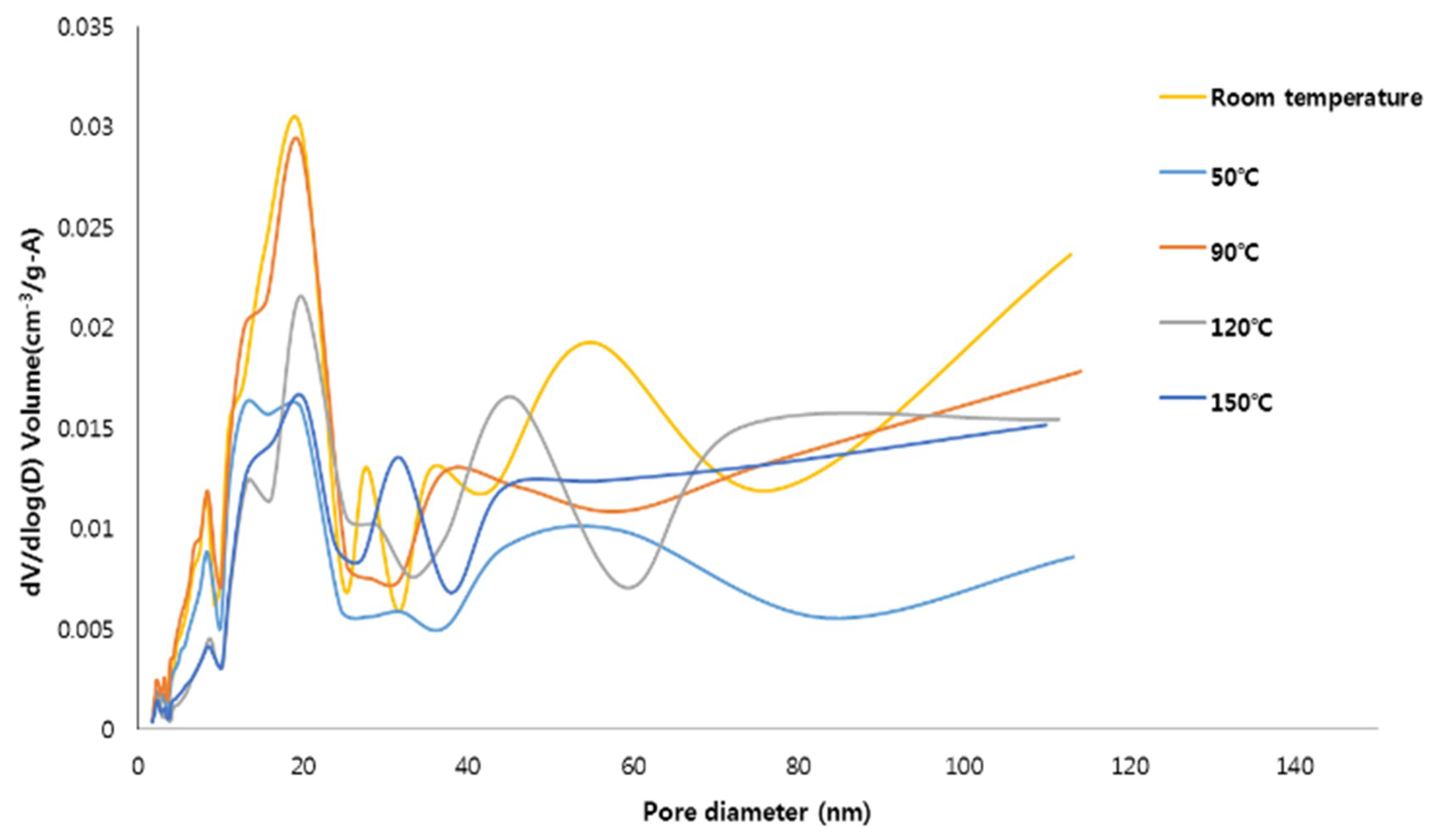
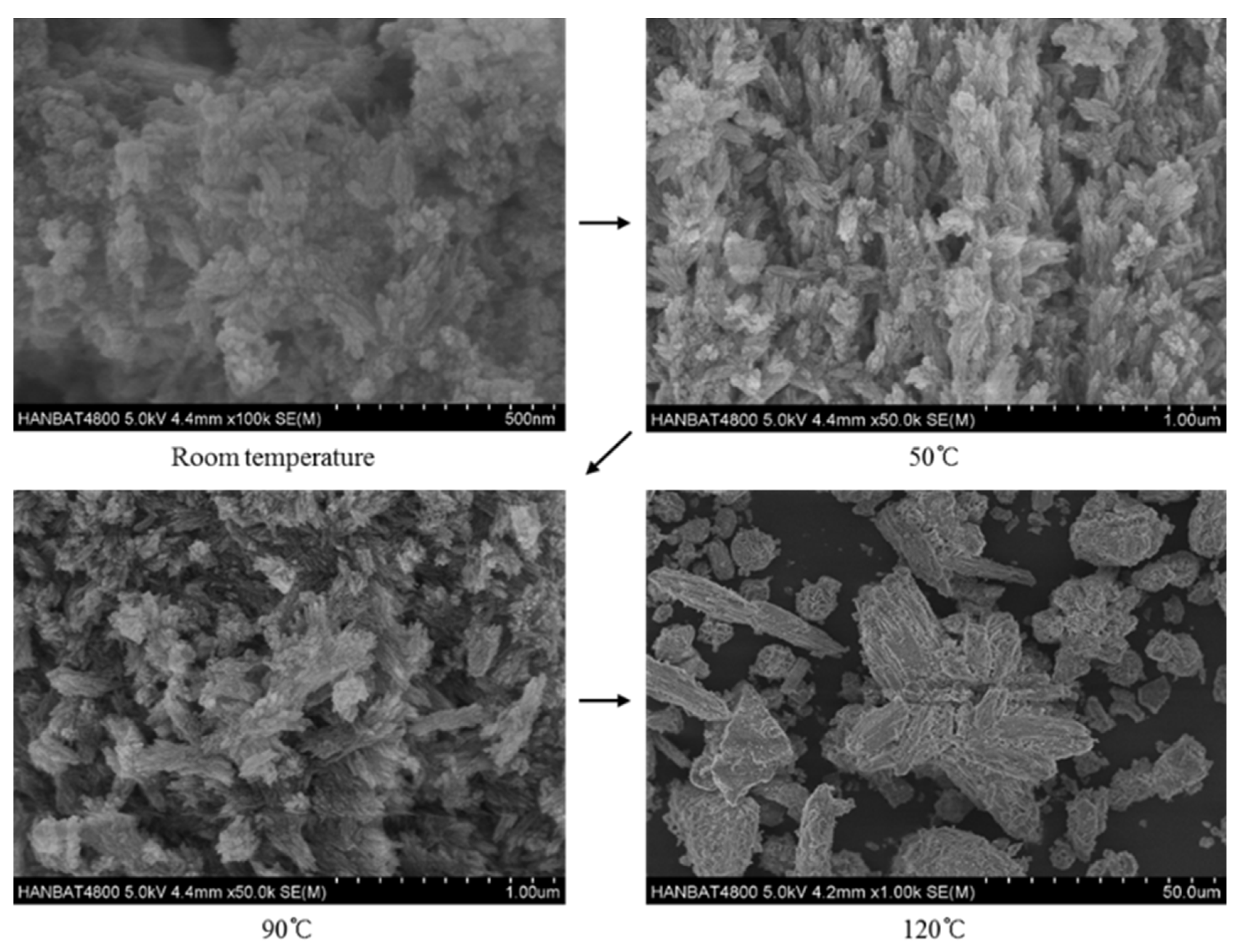
3.2. Microstructural Characteristics
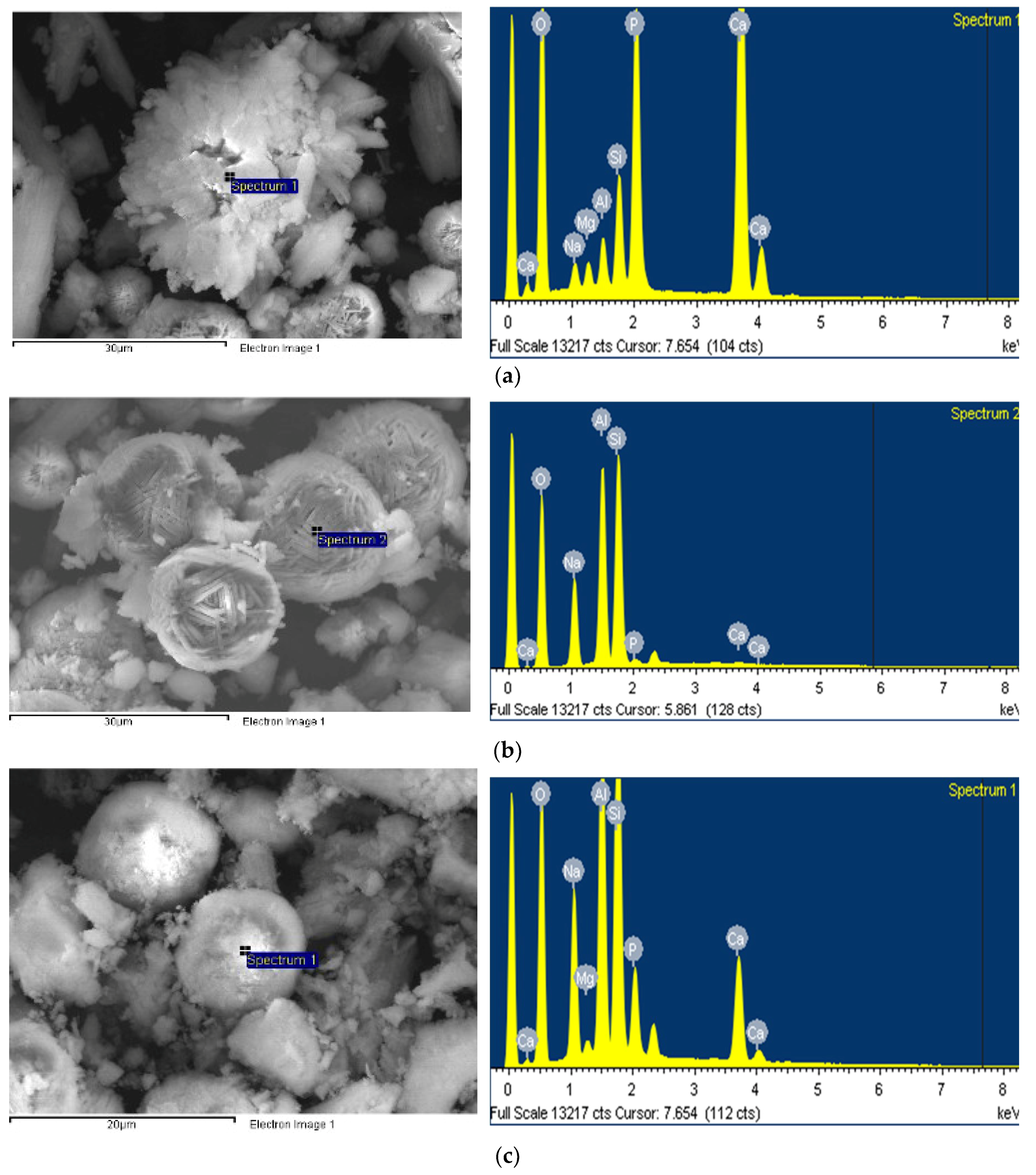
3.3. SEM/EDX Test
4. Concluding Remarks
- Up to 50 °C reaction temperature, only hydroxyapatite was synthesized, while FAU type zeolite was formed at 90 °C and 120 °C in addition to hydroxyapatite. With further increase in temperature to 150 °C, hydroxysodalite was synthesized instead of FAU type zeolite along with hydroxyapatite.
- As the reaction temperature increased, the crystallinity of the specimens tended to increase, and the content of crystalline phases also changed. The content of hydroxyapatite increased with temperature up to 90 °C, while no change was observed with further increment in the temperature.
- The specimens synthesized at 90 °C had the highest specific surface area of 98.7 m2/g, and the specimen synthesized at 150 °C had the lowest specific surface area (51.3) potentially due to the formation of the hydroxysodalite, which has a relatively small specific surface area.
- The pore size distributions of the specimens prepared at room temperature and 90 °C were largely in the mesoporous range, which is advantageous for adsorbing contaminants.
- SEM test results showed that hydroxyapatite and FAU type zeolite phases could be clearly observed at the reaction temperature of 120 °C, while hydroxysodalite phase was observed at the reaction temperature of 150 °C instead of FAU type zeolite.
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Kuwahara, Y.; Ohmichi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. Synthesis of Hydroxyapatite–Zeolite Composite Material from Disposed Steel Slag and Investigation of Its Structural and Physicochemical Characteristics. Chem. Lett. 2009, 38, 626–627. [Google Scholar] [CrossRef]
- Saito, M.; Maruoka, A.; Mori, T.; Sugano, N.; Hino, K. Experimental studies on a new bioactive bone cement: Hydroxyapatite composite resin. Biomaterials 1994, 15, 156–160. [Google Scholar] [CrossRef]
- Roy, D.M.; GLinnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef]
- He, J.; Zhang, K.; Wu, S.; Cai, X.; Chen, K.; Li, Y.; Sun, B.; Jia, Y.; Meng, F.; Jin, Z.; et al. Performance of novel hydroxyapatite nanowires in treatment of fluoride contaminated water. J. Hazard. Mater. 2016, 303, 119–130. [Google Scholar] [CrossRef]
- Kendrick, E.; Dann, S. Synthesis, properties and structure of ion exchanged hydrosodalite. J. Solid State Chem. 2004, 177, 1513–1519. [Google Scholar] [CrossRef]
- Gómez del Río, J.A.; Morando, P.J.; Cicerone, D.S. Natural materials for treatment of industrial effluents: Comparative study of the retention of Cd, Zn and Co by calcite and hydroxyapatite. Part I: Batch experiments. J. Environ. Manag. 2004, 71, 169–177. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Adsorption characteristics of cesium onto mesoporous geopolymers containing nano-crystalline zeolites. Microporous Mesoporous Mater. 2017, 242, 238–244. [Google Scholar] [CrossRef]
- Corami, A.; Mignardi, S.; Ferrini, V. Copper and zinc decontamination from single- and binary-metal solutions using hydroxyapatite. J. Hazard. Mater. 2007, 146, 164–170. [Google Scholar] [CrossRef]
- Choi, S.; Jeong, Y. The removal of heavy metals in aqueous solution by hydroxyapatite/cellulose composite. Fibers Polym. 2008, 9, 267–270. [Google Scholar] [CrossRef]
- Bailliez, S.; Nzihou, A.; Bèche, E.; Flamant, G. Removal of lead (Pb) by hydroxyapatite sorbent. Process Saf. Environ. Prot. 2004, 82, 175–180. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Ohmichi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. A novel synthetic route to hydroxyapatite-zeolite composite material from steel slag: Investigation of synthesis mechanism and evaluation of physicochemical properties. J. Mater. Chem. 2009, 19, 7263–7272. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Lei, J.; Zhang, X.; Sun, Z.; Zheng, S. One-step hydrothermal synthesis of zeolite X powder from natural low-grade diatomite. Materials (Basel) 2018, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Wei, K.; Zhao, N.; Chen, J.; Wang, X. Hydrothermal synthesis of hydroxyapatite nanopowders using cationic surfactant as a template. Mater. Lett. 2006, 60, 1484–1487. [Google Scholar] [CrossRef]
- Ma, M.G. Hierarchically nanostructured hydroxyapatite: Hydrothermal synthesis, morphology control, growth mechanism, and biological activity. Int. J. Nanomed. 2012, 7, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Querol, X.; Moreno, N.; Alastuey, A.; Herna´ndez, E.; Lo´pez-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: an overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Ren, F.; Ding, Y.; Ge, X.; Lu, X.; Wang, K.; Leng, Y. Growth of one-dimensional single-crystalline hydroxyapatite nanorods. J. Cryst. Growth 2012, 349, 75–82. [Google Scholar] [CrossRef]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of steel slag for Portland cement clinker production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef]
- Kim, G.M.; Khalid, H.R.; Kim, H.J.; Lee, H.K. Alkali activated slag pastes with surface-modified blast furnace slag. Cem. Concr. Compos. 2017, 76, 39–47. [Google Scholar] [CrossRef]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Synthesis of mesoporous geopolymers containing zeolite phases by a hydrothermal treatment. Microporous Mesoporous Mater. 2016, 229, 22–30. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Choudhry, I.; Wang, Z.; Lee, H.K. Evolution of zeolite crystals in geopolymer-supported zeolites: Effects of composition of starting materials. Mater. Lett. 2019, 239, 33–36. [Google Scholar] [CrossRef]
- Guo, H.; Tang, L.; Yan, B.; Wan, K.; Li, P. NaA zeolite derived from blast furnace slag: Its application for ammonium removal. Water Sci. Technol. 2017, 76, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal synthesis of mixtures of NaA zeolite and sodalite from Ti-bearing electric arc furnace slag. RSC Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Huang, L.Y.; Xu, K.W.; Lu, J. A study of the process and kinetics of electrochemical deposition and the hydrothermal synthesis of hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 2000, 11, 667–673. [Google Scholar] [CrossRef]
- Jenkins, R.; Snyder, R.L. Introduction to X-Ray Powder Diffractometry; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Belviso, C.; Cavalcante, F.; Fiore, S. Synthesis of zeolite from Italian coal fly ash: Differences in crystallization temperature using seawater instead of distilled water. Waste Manag. 2010, 30, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Golbad, S.; Khoshnoud, P.; Abu-Zahra, N. Hydrothermal synthesis of hydroxy sodalite from fly ash for the removal of lead ions from water. Int. J. Environ. Sci. Technol. 2017, 14, 135–142. [Google Scholar] [CrossRef]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L. Si-substituted hydroxyapatite nanopowders- Synthesis, thermal stability and sinterability. Mater. Res. Bull. 2009, 44, 345–354. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; Tenhuisen, K.S. Mechanochemical-hydrothermal synthesis of calcium phosphate powders with coupled magnesium and carbonate substitution. J. Solid State Chem. 2004, 177, 793–799. [Google Scholar] [CrossRef]
- Clarkin, O.M.; Towler, M.R.; Insley, G.M.; Murphy, M.E. Phase transformations of calcium phosphates formed in wet field environments. J. Mater. Sci. 2007, 42, 8357–8362. [Google Scholar] [CrossRef]
- Ludvigsson, M.; Lindgren, J.; Tegenfeldt, J. FTIR study of water in cast Nafion films. Electrochim. Acta 2000, 45, 2267–2271. [Google Scholar] [CrossRef]
- Loo, S.C.J.; Siew, Y.E.; Ho, S.; Boey, F.Y.C.; Ma, J. Synthesis and hydrothermal treatment of nanostructured hydroxyapatite of controllable sizes. J. Mater. Sci. Mater. Med. 2008, 19, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.; Wei, M.; Vasiliev, A.; Shaw, M.T. Influence of temperature and concentration on the sintering behavior and mechanical properties of hydroxyapatite. Acta Mater. 2004, 52, 5655–5663. [Google Scholar] [CrossRef]
- Fontes, M.P.F.; Weed, S.B. Phosphate adsorption by clays from Brazilian Oxisols: Relationships with specific surface area and mineralogy. Geoderma 1996, 72, 37–51. [Google Scholar] [CrossRef]
- Chiang, P.C.; You, J.H. Use of sewage sludge for manufacturing adsorbents. Can. J. Chem. Eng. 1987, 65, 922–927. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly ash-based geopolymers: The relationship between composition, pore structure and efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Yang, L.X.; Yin, J.J.; Wang, L.L.; Xing, G.X.; Yin, P.; Liu, Q.W. Hydrothermal synthesis of hierarchical hydroxyapatite: Preparation, growth mechanism and drug release property. Ceram. Int. 2012, 38, 495–502. [Google Scholar] [CrossRef]
- Rioland, G.; Albrecht, S.; Josien, L.; Vidal, L.; Daou, T.J. The influence of the nature of organosilane surfactants and their concentration on the formation of hierarchical FAU-type zeolite nanosheets. New J. Chem. 2015, 39, 2675–2681. [Google Scholar] [CrossRef]
- Smolen, D.; Chudoba, T.; Malka, I.; Kedzierska, A.; Lojkowski, W.; Swieszkowski, W.; Kurzydlowski, K.J.; Kolodziejczyk-Mierzynska, M.; Lewandowska-Szumiel, M. Highly biocompatible, nanocrystalline hydroxyapatite synthesized in a solvothermal process driven by high energy density microwave radiation. Int. J. Nanomed. 2013, 8, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, Z.; Zhu, J.; Zhang, J.; Shi, Y.; Yu, K.; Wang, W.; Wang, X.; Feng, X.; Luo, L.; et al. Hydroxyapatite coating on porous silicon substrate obtained by precipitation process. Appl. Surf. Sci. 2004, 230, 418–424. [Google Scholar]
- Chen, C.W.; Oakes, C.S.; Byrappa, K.; Riman, R.E.; Brown, K.; TenHuisen, K.S.; Janas, V.F. Synthesis, characterization, and dispersion properties of hydroxyapatite prepared by mechanochemical-hydrothermal methods. J. Mater. Chem. 2004, 14, 2425–2432. [Google Scholar] [CrossRef]
- Wang, A.; Yin, H.; Liu, D.; Wu, H.; Ren, M.; Jiang, T.; Cheng, X.; Xu, Y. Size-controlled synthesis of hydroxyapatite nanorods in the presence of organic modifiers. Mater. Lett. 2007, 61, 2084–2088. [Google Scholar] [CrossRef]


| Compound | Proportion (wt.%) |
|---|---|
| Al2O3 | 7.50 |
| SiO2 | 18.20 |
| SO3 | 3.10 |
| K2O | 0.76 |
| CaO | 67.60 |
| TiO2 | 0.95 |
| MnO | 0.44 |
| Fe2O3 | 1.0 |
| NiO | 0.04 |
| CuO | 0.046 |
| SrO | 0.019 |
| ZrO2 | 0.082 |
| Reaction Temperature | Amorphous Phase (%) | Crystallinity (%) | Phases | Quantitative Content (%) |
|---|---|---|---|---|
| Room temperature | 64 | 36 | Hydroxyapatite | 36 |
| 50 °C | 66 | 34 | Hydroxyapatite | 34 |
| 90 °C | 37 | 63 | Hydroxyapatite | 50 |
| FAU type zeolite | 13 | |||
| 120 °C | 43 | 57 | Hydroxyapatite | 50 |
| FAU type zeolite | 7 | |||
| 150 °C | 30 | 71 | Hydroxyapatite | 46 |
| Hydroxysodalite | 25 |
| Reaction Temperature | Room Temperature | 50 °C | 90 °C | 120 °C | 150 °C |
|---|---|---|---|---|---|
| Specific surface area (m2/g) | 84.84 | 69.35 | 98.74 | 78.57 | 51.34 |
| BJH Adsorption cumulative volume of pores (cm3/g) | 0.28 | 0.19 | 0.30 | 0.19 | 0.18 |
| BJH Adsorption average pore diameter (nm) | 11.30 | 9.44 | 9.96 | 13.37 | 13.18 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryu, G.U.; Kim, G.M.; Khalid, H.R.; Lee, H.K. The Effects of Temperature on the Hydrothermal Synthesis of Hydroxyapatite-Zeolite Using Blast Furnace Slag. Materials 2019, 12, 2131. https://doi.org/10.3390/ma12132131
Ryu GU, Kim GM, Khalid HR, Lee HK. The Effects of Temperature on the Hydrothermal Synthesis of Hydroxyapatite-Zeolite Using Blast Furnace Slag. Materials. 2019; 12(13):2131. https://doi.org/10.3390/ma12132131
Chicago/Turabian StyleRyu, G.U., G.M. Kim, Hammad R. Khalid, and H.K. Lee. 2019. "The Effects of Temperature on the Hydrothermal Synthesis of Hydroxyapatite-Zeolite Using Blast Furnace Slag" Materials 12, no. 13: 2131. https://doi.org/10.3390/ma12132131
APA StyleRyu, G. U., Kim, G. M., Khalid, H. R., & Lee, H. K. (2019). The Effects of Temperature on the Hydrothermal Synthesis of Hydroxyapatite-Zeolite Using Blast Furnace Slag. Materials, 12(13), 2131. https://doi.org/10.3390/ma12132131








