Field Test of Self-Cleaning Zr-Modified-TiO2-SiO2 Films on Glass with a Demonstration of Their Anti-Fogging Effect
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Materials
2.2. Field Tests
- Ljubljana (abbreviated LJ): large city in the urban area with industrial complexes, moderate continental climate;
- Nova Gorica (abbreviated NG): city in the urban area, moderate Mediterranean climate;
- Žiri (abbreviated ZI): a small industrial complex in the rural and remote area, moderate continental climate.
2.3. Characterization
3. Results and Discussion
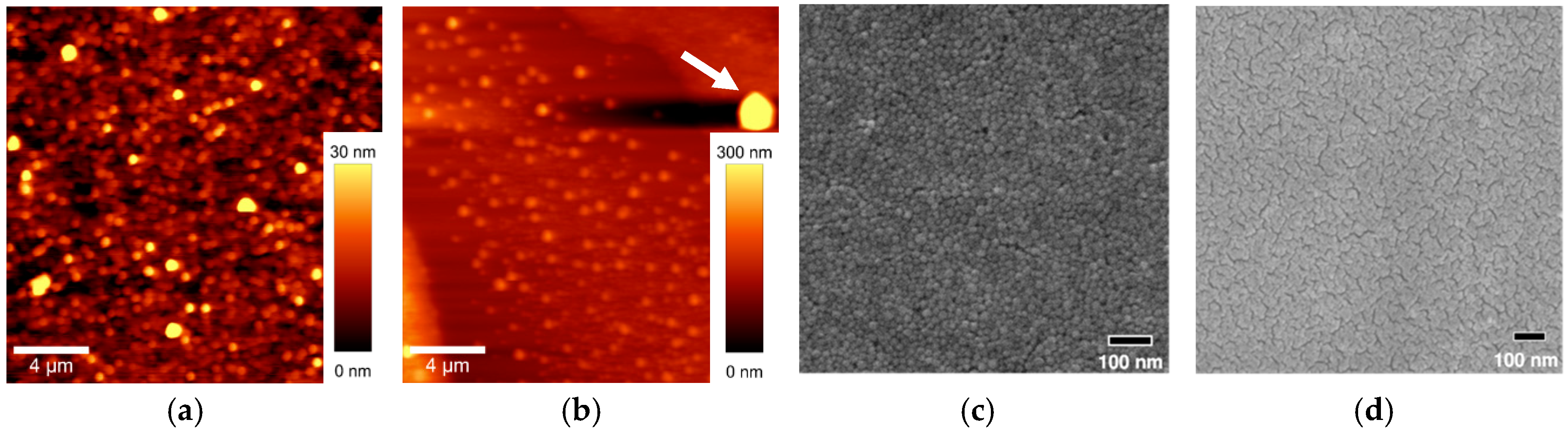
3.1. Coatings Characterization
3.2. Field Test Results
- -
- Similar trends for float and TiZr specimen;
- -
- BioClean reveals lower transmittance values (higher 1-R) due to a light bluish tint of this glass;
- -
- The smallest variations are found for Žiri (ZI) location (rural and remote area);
- -
- The inlet of Saharan sand at ca. 480th day was significantly contributing to obstruction of transmitted light, the largest at Nova Gorica (NG) location with Mediterranean climate;
- -
- No correlation could be seen with solar irradiance (number of sun hours per day) in any of the inclinations;
- -
- Angles of exposure did not influence the measured parameters significantly.
- -
- The temporal changes for all types of samples overlap significantly;
- -
- ΔE* values generally increase with time of exposure;
- -
- The inlet of Saharan sand contributed to a more expressed increase of ΔE* at all three locations;
- -
- Solar irradiance correlated with bigger changes in ΔE* values;
- -
- Differences among angles of exposure are well seen for ΔE* measurements—the samples exposed at 45° and 90°-roof exhibit the highest increase in ΔE*.
- -
- The curves for all types of samples overlap significantly;
- -
- Haze increases with time of exposure, the lowest values were found for the remote area of ZI (the samples remain cleaner—forestry area around);
- -
- The inlet of Saharan sand contributed to a more expressed increase of haze at NG and Ljubljana (LJ), much less for location in ZI;
- -
- Solar irradiance correlated with bigger changes in haze values;
- -
- Differences among angles of exposure confirm that the rain flow cleans the surface dirt more efficiently when exposed at 90°.
3.3. Anti-Fogging Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minella, M.; Minero, C. Quantification of the Photocatalytic Self-Cleaning Ability of Non-Transparent Materials. Materials (Basel) 2019, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Soklič, A.; Tasbihi, M.; Kete, M.; Štangar, U.L. Deposition and possible influence of a self-cleaning thin TiO2/SiO2 film on a photovoltaic module efficiency. Catal. Today 2015, 252, 54–60. [Google Scholar] [CrossRef]
- Midtdal, K.; Jelle, B.P. Self-cleaning glazing products: A state-of-the-art review and future research pathways. Sol. Energy Mater. Sol. Cells 2013, 109, 126–141. [Google Scholar] [CrossRef] [Green Version]
- Chabas, A.; Lombardo, T.; Cachier, H.; Pertuisot, M.H.; Oikonomou, K.; Falcone, R.; Verità, M.; Geotti-Bianchini, F. Behaviour of self-cleaning glass in urban atmosphere. Build. Environ. 2008, 43, 2124–2131. [Google Scholar] [CrossRef]
- Jelle, B.P.; Hynd, A.; Gustavsen, A.; Arasteh, D.; Goudey, H.; Hart, R. Fenestration of today and tomorrow: A state-of-the-art review and future research opportunities. Sol. Energy Mater. Sol. Cells 2012, 96, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Gläser, H.J.; Ulrich, S. Condensation on the outdoor surface of window glazing—Calculation methods, key parameters and prevention with low-emissivity coatings. Thin Solid Film. 2013, 532, 127–131. [Google Scholar] [CrossRef]
- Dzik, P.; Veselý, M.; Kete, M.; Pavlica, E.; Štangar, U.L.; Neumann-Spallart, M. Properties and Application Perspective of Hybrid Titania-Silica Patterns Fabricated by Inkjet Printing. ACS Appl. Mater. Interfaces 2015, 7, 16177–16190. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Chen, P.; Wang, H.; Chen, R. Calcination-free synthesis of high strength self-cleaning anti-reflective MgF2-TiO2 coating. Mater. Lett. 2019, 240, 17–20. [Google Scholar] [CrossRef]
- Faustini, M.; Ceratti, D.R.; Louis, B.; Boudot, M.; Albouy, P.A.; Boissière, C.; Grosso, D. Engineering functionality gradients by dip coating process in acceleration mode. ACS Appl. Mater. Interfaces 2014, 6, 17102–17110. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Boudot, M.; Boissiere, C.; Grosso, D.; Faustini, M. Suppressing Structural Colors of Photocatalytic Optical Coatings on Glass: The Critical Role of SiO2. ACS Appl. Mater. Interfaces 2017, 9, 14093–14102. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Mosquera, M.J. Photocatalytic activity of TiO2–SiO2 nanocomposites applied to buildings: Influence of particle size and loading. Appl. Catal. B Environ. 2013, 134–135, 205–221. [Google Scholar] [CrossRef]
- Štangar, U.L.; Černigoj, U.; Trebše, P.; Maver, K.; Gross, S. Photocatalytic TiO2 Coatings: Effect of Substrate and Template. Monatshefte für Chemie Chem. Mon. 2006, 137, 647–655. [Google Scholar] [CrossRef]
- Vodišek, N.; Šuligoj, A.; Korte, D.; Lavrenčič Štangar, U. Transparent Photocatalytic Thin Films on Flexible Polymer Substrates. Materials (Basel) 2018, 11, 1945. [Google Scholar] [CrossRef] [PubMed]
- Estekhraji, S.A.Z.; Amiri, S. Sol–gel preparation and characterization of antibacterial and self-cleaning hybrid nanocomposite coatings. J. Coat. Technol. Res. 2017, 14, 1335–1343. [Google Scholar] [CrossRef]
- Maver, K.; Štangar, U.L.; Černigoj, U.; Gross, S.; Cerc Korošec, R. Low-temperature synthesis and characterization of TiO2 and TiO2–ZrO2 photocatalytically active thin films. Photochem. Photobiol. Sci. 2009, 8, 657–662. [Google Scholar] [CrossRef]
- Han, S.; Choi, S.-H.; Kim, S.-S.; Cho, M.; Jang, B.; Kim, D.-Y.; Yoon, J.; Hyeon, T. Low-Temperature Synthesis of Highly Crystalline TiO2 Nanocrystals and their Application to Photocatalysis. Small 2005, 1, 812–816. [Google Scholar] [CrossRef]
- Hakki, A.; Yang, L.; Wang, F.; Macphee, D.E. The Effect of Interfacial Chemical Bonding in TiO2-SiO2 Composites on Their Photocatalytic NOx Abatement Performance. J. Vis. Exp. 2017, e56070. [Google Scholar] [CrossRef]
- ASTM. ASTM D1003 Standard Test Method for Haze and Luminous Transmittance of Transparent Plastic; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Printer Technical Service. Defining and Communicating Color: The CIELAB System; Sappi Fine Paper North America Inc.: Skowhegan, ME, USA, 2013. [Google Scholar]
- Josep, V.K. Paint and Coating Testing Manual; ASTM International: West Conshohocken, PA, USA, 1995; ISBN 0-8031-2060-5. [Google Scholar]
- Vodišek, N.; Ramanujachary, K.; Brezová, V.; Lavrenčič Štangar, U. Transparent titania-zirconia-silica thin films for self-cleaning and photocatalytic applications. Catal. Today 2017, 287, 142–147. [Google Scholar] [CrossRef]
- Elminir, H.K.; Ghitas, A.E.; Hamid, R.H.; El-Hussainy, F.; Beheary, M.M.; Abdel-Moneim, K.M. Effect of dust on the transparent cover of solar collectors. Energy Convers. Manag. 2006, 47, 3192–3203. [Google Scholar] [CrossRef]
- Petronella, F.; Pagliarulo, A.; Truppi, A.; Lettieri, M.; Masieri, M.; Calia, A.; Curri, M.L.; Comparelli, R. TiO2 Nanocrystal Based Coatings for the Protection of Architectural Stone: The Effect of Solvents in the Spray-Coating Application for a Self-Cleaning Surfaces. Coatings 2018, 8, 356. [Google Scholar] [CrossRef]
- Hedayati, M.K.; Elbahri, M. Antireflective Coatings: Conventional Stacking Layers and Ultrathin Plasmonic Metasurfaces, A Mini-Review. Materials (Basel) 2016, 9, 497. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Witzel, R.F.; Burnham, R.W.; Onley, J.W. Threshold and suprathreshold perceptual color differences. J. Opt. Soc. Am. 1973, 63, 615–625. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; D’Orazio, M. Photocatalytic TiO2 Nano-Coating for Biofouling Prevention of Clay Façades. In New Approaches to Building Pathology and Durability; Springer: Berlin, Germany, 2016; pp. 159–175. [Google Scholar]
- Sakhuja, M.; Son, J.; Yang, H.; Bhatia, C.S.; Danner, A.J. Outdoor performance and durability testing of antireflecting and self-cleaning glass for photovoltaic applications. Sol. Energy 2014, 110, 231–238. [Google Scholar] [CrossRef]
- Gholami, A.; Alemrajabi, A.A.; Saboonchi, A. Experimental study of self-cleaning property of titanium dioxide and nanospray coatings in solar applications. Sol. Energy 2017, 157, 559–565. [Google Scholar] [CrossRef]
- Zheng, J.; Bao, S.; Jin, P. TiO2(R)/VO2(M)/TiO2(A) multilayer film as smart window: Combination of energy-saving, antifogging and self-cleaning functions. Nano Energy 2015, 11, 136–145. [Google Scholar] [CrossRef]
- Li, X.; He, J. Synthesis of raspberry-like SiO2-TiO2 nanoparticles toward antireflective and self-cleaning coatings. ACS Appl. Mater. Interfaces 2013, 5, 5282–5290. [Google Scholar] [CrossRef]
- Ramírez-García, R.E.; González-Rodríguez, J.A.; Arroyo-Ortega, M.; Pérez-García, S.A.; Licea-Jiménez, L. Engineered TiO2 and SiO2-TiO2 films on silica-coated glass for increased thin film durability under abrasive conditions. Int. J. Appl. Ceram. Technol. 2017, 14, 39–49. [Google Scholar] [CrossRef]
- Taga, Y.; Yamada, N. Sputter joining of TiO2/SiO2 thin film system. IOP Conf. Ser. Mater. Sci. Eng. 2011, 24, 012011. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuligoj, A.; Pliekhova, O.; Vodišek, N.; Mihelčič, M.; Surca, A.K.; Kunič, R.; Šubic, B.; Starman, J.; Ugovšek, A.; Lavrenčič Štangar, U. Field Test of Self-Cleaning Zr-Modified-TiO2-SiO2 Films on Glass with a Demonstration of Their Anti-Fogging Effect. Materials 2019, 12, 2196. https://doi.org/10.3390/ma12132196
Šuligoj A, Pliekhova O, Vodišek N, Mihelčič M, Surca AK, Kunič R, Šubic B, Starman J, Ugovšek A, Lavrenčič Štangar U. Field Test of Self-Cleaning Zr-Modified-TiO2-SiO2 Films on Glass with a Demonstration of Their Anti-Fogging Effect. Materials. 2019; 12(13):2196. https://doi.org/10.3390/ma12132196
Chicago/Turabian StyleŠuligoj, Andraž, Olena Pliekhova, Nives Vodišek, Mohor Mihelčič, Angelja K. Surca, Roman Kunič, Barbara Šubic, Jernej Starman, Aleš Ugovšek, and Urška Lavrenčič Štangar. 2019. "Field Test of Self-Cleaning Zr-Modified-TiO2-SiO2 Films on Glass with a Demonstration of Their Anti-Fogging Effect" Materials 12, no. 13: 2196. https://doi.org/10.3390/ma12132196
APA StyleŠuligoj, A., Pliekhova, O., Vodišek, N., Mihelčič, M., Surca, A. K., Kunič, R., Šubic, B., Starman, J., Ugovšek, A., & Lavrenčič Štangar, U. (2019). Field Test of Self-Cleaning Zr-Modified-TiO2-SiO2 Films on Glass with a Demonstration of Their Anti-Fogging Effect. Materials, 12(13), 2196. https://doi.org/10.3390/ma12132196






