Femtosecond Laser Nano/Micro Textured Ti6Al4V Surfaces—Effect on Wetting and MG-63 Cell Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Pretreatment
2.2. Micromachining
2.3. Surface Characterization
2.4. Wetting Properties
2.5. Cell Culture
2.6. Morphology and Spreading of MG-63 Cells
2.7. Actin Cytoskeleton Organization
3. Results and Discussion
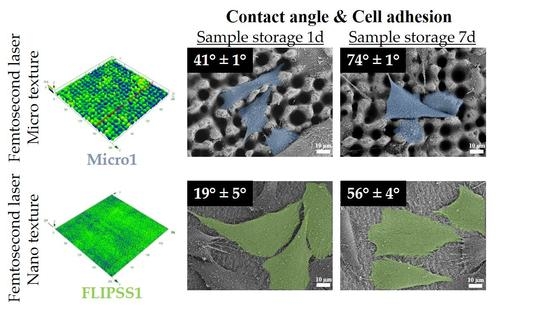
3.1. Surface Characterization
3.2. Wetting Properties of Ti6Al4V Surfaces
3.3. Cellular Response
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Ponche, A.; Bigerelle, M. Relative influence of surface topography and surface chemistry on cell response to bone implant materials. Part 2: Biological aspects. Proc. Inst. Mech. Eng. H 2010, 224, 1487–1507. [Google Scholar] [CrossRef] [PubMed]
- Rychly, J.; Nebe, B. Cell-material interaction. BioNanoMaterials 2013, 14. [Google Scholar] [CrossRef]
- Zhao, C.; Cao, P.; Ji, W.; Han, P.; Zhang, J.; Zhang, F.; Jiang, Y.; Zhang, X. Hierarchical titanium surface textures affect osteoblastic functions. J. Biomed. Mater. Res. A 2011, 99, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Lofti, M.; Nejib, M.; Naceur, M. Cell Adhesion to Biomaterials: Concept of Biocompatibility. In Advances in Biomaterials Science and Biomedical Applications; Pignatello, R., Ed.; IntechOpen: London, UK, 2013; Chapter 8. [Google Scholar] [CrossRef] [Green Version]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Brunette, D.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Jones, F. Teeth and bones: Applications of surface science to dental materials and related biomaterials. Surf. Sci. Rep. 2001, 42, 75–205. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behaviour. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Cunha, A.; Serro, A.P.; Oliveira, V.; Almeida, A.; Vilar, R.; Durrieu, M.-C. Wetting behaviour of femtosecond laser textured Ti–6Al–4V surfaces. Appl. Surf. Sci. 2013, 265, 688–696. [Google Scholar] [CrossRef]
- Kietzig, A.-M.; Hatzikiriakos, S.G.; Englezos, P. Patterned superhydrophobic metallic surfaces. Langmuir 2009, 25, 4821–4827. [Google Scholar] [CrossRef]
- Jagdheesh, R.; Pathiraj, B.; Karatay, E.; Römer, G.R.B.E.; Huis in’t Veld, A.J. Laser-induced nanoscale superhydrophobic structures on metal surfaces. Langmuir 2011, 27, 8464–8469. [Google Scholar] [CrossRef]
- Cunha, A.; Oliveira, V.; Serro, A.P.; Zouani, O.E.-F.; Almeida, A.; Durrieu, M.-C.; Vilar, R. Ultrafast laser texturing of Ti-6Al-4V surfaces for biomedical applications. In Proceedings of the International Congress on Applications of Lasers & Electro-Optics, Miami, FL, USA, 6–10 October 2013; Laser Institute of America: Orlando, FL, USA, 2013; pp. 910–918. [Google Scholar] [CrossRef]
- Miller, P.R.; Aggarwal, R.; Doraiswamy, A.; Lin, Y.J.; Lee, Y.-S.; Narayan, R.J. Laser micromachining for biomedical applications. JOM 2009, 61, 35–40. [Google Scholar] [CrossRef]
- Staehlke, S.; Koertge, A.; Nebe, B. Intracellular calcium dynamics in dependence on topographical features of titanium. Biomaterials 2015, 46, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Aso, T.; Sasaki, R.; Ashida, M.; Tsutsumi, Y.; Doi, H.; Hanawa, T. Adhesion and differentiation behaviors of mesenchymal stem cells on titanium with micrometer and nanometer-scale grid patterns produced by femtosecond laser irradiation. J. Biomed. Mater. Res. A 2018, 106, 2735–2743. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, R.; Emanuelsson, L.; Palmquist, A.; Thomsen, P. Bone response to laser-induced micro- and nano-size titanium surface features. Nanomedicine 2011, 7, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.; Elie, A.-M.; Plawinski, L.; Serro, A.P.; Botelho do Rego, A.M.; Almeida, A.; Urdaci, M.C.; Durrieu, M.-C.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial retention on superhydrophobic titanium surfaces fabricated by femtosecond laser ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Zhong, M.; Zhang, H.; Fan, P. Superhydrophilicity to superhydrophobicity transition of picosecond laser microstructured aluminum in ambient air. J. Colloid Interface Sci. 2015, 441, 1–9. [Google Scholar] [CrossRef]
- Raimbault, O.; Benayoun, S.; Anselme, K.; Mauclair, C.; Bourgade, T.; Kietzig, A.-M.; Girard-Lauriault, P.-L.; Valette, S.; Donnet, C. The effects of femtosecond laser-textured Ti-6Al-4V on wettability and cell response. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 311–320. [Google Scholar] [CrossRef]
- SAE International. AMS4911: Titanium Alloy, Sheet, Strip, and Plate, 6Al - 4V, Annealed. Available online: https://saemobilus.sae.org/content/AMS4911/ (accessed on 8 July 2019).
- Beuth Verlag GmbH. WL 3.7164-1: Aerospace; titanium alloy with approx. 6Al-4V; sheet and plate. Available online: https://www.beuth.de/de/norm/wl-3-7164-1/1616956 (accessed on 8 July 2019).
- Staehlke, S.; Rebl, H.; Nebe, B. Phenotypic stability of the human MG-63 osteoblastic cell line at different passages. Cell Biol. Int. 2019, 43, 22–32. [Google Scholar] [CrossRef]
- Matschegewski, C.; Staehlke, S.; Birkholz, H.; Lange, R.; Beck, U.; Engel, K.; Nebe, J. Automatic actin filament quantification of osteoblasts and their morphometric analysis on microtextured silicon-titanium arrays. Materials 2012, 5, 1176–1195. [Google Scholar] [CrossRef]
- Matschegewski, C.; Birkholz, H.; Staehlke, S.; Loeffler, R.; Kern, P.D.; Engel, K.; Nebe, J.B. Quantitative analysis of the cellular actin cytoskeleton on geometrically designed surface topography. Mater. Sci. Forum 2012, 706–709, 543–548. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Sánchez, F.; Morenza, J.L.; Aguiar, R.; Delgado, J.C.; Varela, M. Dynamics of the hydrodynamical growth of columns on silicon exposed to ArF excimer-laser irradiation. Appl. Phys. A Mater. Sci. Process. 1998, 66, 83–86. [Google Scholar] [CrossRef]
- Zuhlke, C.A.; Anderson, T.P.; Alexander, D.R. Understanding the formation of self-organized micro/nanostructures on metal surfaces from femtosecond laser ablation using stop-motion SEM imaging. In Proceedings of the Laser-Based Micro- and Nanoprocessing VIII, San Francisco, CA, USA, 1–6 February 2014; Klotzbach, U., Washio, K., Arnold, C.B., Eds.; SPIE: Bellingham, WA, USA, 2014; Volume 89680C. [Google Scholar]
- Oliveira, V.; Ausset, S.; Vilar, R. Surface micro/nanostructuring of titanium under stationary and non-stationary femtosecond laser irradiation. Appl. Surf. Sci. 2009, 255, 7556–7560. [Google Scholar] [CrossRef]
- Cardoso, J.T.; Garcia-Girón, A.; Romano, J.M.; Huerta-Murillo, D.; Jagdheesh, R.; Walker, M.; Dimov, S.S.; Ocaña, J.L. Influence of ambient conditions on the evolution of wettability properties of an IR-, ns-laser textured aluminium alloy. RSC Adv. 2017, 7, 39617–39627. [Google Scholar] [CrossRef] [Green Version]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Kunzler, T.P.; Drobek, T.; Schuler, M.; Spencer, N.D. Systematic study of osteoblast and fibroblast response to roughness by means of surface-morphology gradients. Biomaterials 2007, 28, 2175–2182. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2012, 28, 843–863. [Google Scholar] [CrossRef]
- Belaud, V.; Petithory, T.; Ponche, A.; Mauclair, C.; Donnet, C.; Pieuchot, L.; Benayoun, S.; Anselme, K. Influence of multiscale and curved structures on the migration of stem cells. Biointerphases 2018, 13, 06D408. [Google Scholar] [CrossRef]
- Kieswetter, K.; Schwartz, Z.; Hummert, T.W.; Cochran, D.L.; Simpson, J.; Dean, D.D.; Boyan, B.D. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J. Biomed. Mater. Res. 1996, 32, 55–63. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Berg, M.E.; Schneider, J.M.; Hotchkiss, K.; Schwartz, Z.; Boyan, B.D. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann. Biomed. Eng. 2014, 42, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Lincks, J.; Boyan, B.D.; Blanchard, C.R.; Lohmann, C.H.; Liu, Y.; Cochran, D.L.; Dean, D.D.; Schwartz, Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials 1998, 19, 2219–2232. [Google Scholar] [CrossRef]
- Boyan, B.D.; Lossdörfer, S.; Wang, L.; Zhao, G.; Lohmann, C.H.; Cochran, D.L.; Schwartz, Z. Osteoblasts generate an osteogenic microenvironment when grown on surfaces with rough microtopographies. Cell Mater. 2003, 24, 22–27. [Google Scholar] [CrossRef]
- Dowling, D.P.; Miller, I.S.; Ardhaoui, M.; Gallagher, W.M. Effect of surface wettability and topography on the adhesion of osteosarcoma cells on plasma-modified polystyrene. J. Biomater. Appl. 2011, 26, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Van Wachem, P.B.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.P.; van Aken, W.G. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 1985, 6, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.Y.; Liu, X.; Vogler, E.A.; Donahue, H.J. Systematic variation in osteoblast adhesion and phenotype with substratum surface characteristics. J. Biomed. Mater. Res. A 2004, 68, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, M.M.; Gentleman, E. The role of surface free energy in osteoblast–biomaterial interactions. Int. Mater. Rev. 2014, 59, 417–429. [Google Scholar] [CrossRef]
- Hallab, N.J.; Bundy, K.J.; O’Connor, K.; Moses, R.L.; Jacobs, J.J. Evaluation of metallic and polymeric biomaterial surface energy and surface roughness characteristics for directed cell adhesion. Tissue Eng. 2001, 7, 55–71. [Google Scholar] [CrossRef]
- Moerke, C.; Rebl, H.; Finke, B.; Dubs, M.; Nestler, P.; Airoudj, A.; Roucoules, V.; Schnabelrauch, M.; Koertge, A.; Anselme, K.; et al. Abrogated cell contact guidance on amino-functionalized micro-grooves. ACS Appl. Mater. Interfaces 2017, 9, 10461–10471. [Google Scholar] [CrossRef]
- Fadeeva, E.; Deiwick, A.; Chichkov, B.; Schlie-Wolter, S. Impact of laser-structured biomaterial interfaces on guided cell responses. Interface Focus 2014, 4, 20130048. [Google Scholar] [CrossRef] [PubMed]
- Coathup, M.J.; Blunn, G.W.; Mirhosseini, N.; Erskine, K.; Liu, Z.; Garrod, D.R.; Li, L. Controlled laser texturing of titanium results in reliable osteointegration. J. Orthop. Res. 2017, 35, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Hasan, A.; Pattanayek, S.K.; Pandey, L.M. Effect of Functional Groups of Self-Assembled Monolayers on Protein Adsorption and Initial Cell Adhesion. ACS Biomater. Sci. Eng. 2018, 4, 3224–3233. [Google Scholar] [CrossRef]
- Hasan, A.; Waibhaw, G.; Pandey, L.M. Conformational and Organizational Insights into Serum Proteins during Competitive Adsorption on Self-Assembled Monolayers. Langmuir 2018, 34, 8178–8194. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef] [PubMed]



| Structure | Laser Parameters | |||
|---|---|---|---|---|
| Pulse Energy (µJ) | Fluence in Focus (J/cm²) | Line Overlap (%) | Procedure of Laser Passages | |
| Micro1 | 50 | 9.82 | 60 | Grid |
| Micro2 | 50 | 9.82 | 30 | Grid |
| FLIPSS1 | 15 | 2.95 | 90 | Line |
| FLIPSS2 | 5 | 0.98 | 80 | Grid |
| Sample | Roughness | Pillar/Profile Dimension | |
|---|---|---|---|
| Sa (µm) | Depth (µm) | Width (µm) | |
| Micro1 | 2.65 ± 0.19 | 9.57 ± 1.15 | 13.59 ± 1.23 |
| 2.69 ± 0.10 | |||
| Micro2 | 1.09 ± 0.02 | 4.26 ± 0.32 | 25.13 ± 0.99 |
| 1.13 ± 0.04 | |||
| FLIPSS1 | 0.14 ± 0.01 | 0.52 ± 0.05 | – |
| 0.13 ± 0.004 | |||
| FLIPSS2 | 0.14 ± 0.01 | 0.50 ± 0.02 | – |
| 0.15 ± 0.01 | |||
| Reference | 0.08 ± 0.02 | 0.48 ± 0.02 | – |
| 0.09 ± 0.01 | |||
| Sample | Contact Angle (°) | |
|---|---|---|
| Storage Time 1 Day | Storage Time 7 Days | |
| Micro1 | 40.97 ± 1.01 | 73.53 ± 1.14 |
| 39.93 ± 1.59 | 79.60 ± 2.5 | |
| Micro2 | 40.30 ± 3.16 | 98.60 ± 2.69 |
| 43.73 ± 1.41 | 100.13 ± 4.42 | |
| FLIPSS1 | 18.80 ± 5.16 | 55.67 ± 4.27 |
| ~ 0 | 59.70 ± 3.75 | |
| FLIPSS2 | 78.00 ± 1.94 | 92.50 ± 4.49 |
| 75.17 ± 1.35 | 81.80 ± 5.06 | |
| Reference | 88.80 ± 1.80 | 83.73 ± 2.09 |
| 89.37 ± 1.91 | 89.20 ± 2.03 | |
| Sample | Actin Filament Number | Total Filament Length (µm) | Mean Filament Length (µm) | Max. Filament Length (µm) | Orientation Dispersion (°) |
|---|---|---|---|---|---|
| Storage time 1 day | |||||
| Micro1 | 10.9 ± 2.9 a,b,c | 92.5 ± 38.5 a | 4.5 ± 0.7 a | 23.4 ± 9.4 | 17.5 ± 1.7 |
| Micro2 | 20.2 ± 4.3 | 105.4 ± 21.3 a | 5.6 ± 1.1 a | 18.1 ± 6.6 | 22.8 ± 2.2 |
| FLIPSS1 | 56.2 ± 14,6 | 387.5 ± 120.3 | 8.1 ± 1.1 | 33.5 ± 7.1 | 22.5 ± 2.0 |
| FLIPSS2 | 49 ± 8.9 | 359.2 ± 43.9 | 7.8 ± 1.4 | 39.4 ± 8.3 | 21.8 ± 1.3 |
| Reference | 49.5 ± 8.6 | 552.1 ± 89.5 | 10.4 ± 0.2 | 52.5 ± 10.7 | 21.5 ± 1.7 |
| Storage time 7 days | |||||
| Micro1 | 18.1 ± 3.2 b | 105.5 ±24.7 a,c | 3.7 ± 1.4 | 17.0 ± 5.6 | 24.5 ± 1.0 |
| Micro2 | 21.7 ± 2.6 | 104.4 ±11.9 a,c | 3.6 ± 1.2 | 15.5 ± 4.0 | 22.0 ± 1.7 |
| FLIPSS1 | 40.4 ± 4.1 | 213.7 ± 25.8 | 7.4 ± 4.3 | 40.3 ± 7.1 | 21.7 ± 2.1 |
| FLIPSS2 | 35.8 ± 5.1 | 427.5 ± 43.9 | 6.4 ± 3.7 | 46.5 ± 6.1 | 20.4 ± 1.3 |
| Reference | 35.7 ± 7.4 | 552.1 ± 89.5 | 6.7 ± 3.9 | 47.4 ± 13.3 | 22.4 ± 2.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schnell, G.; Staehlke, S.; Duenow, U.; Nebe, J.B.; Seitz, H. Femtosecond Laser Nano/Micro Textured Ti6Al4V Surfaces—Effect on Wetting and MG-63 Cell Adhesion. Materials 2019, 12, 2210. https://doi.org/10.3390/ma12132210
Schnell G, Staehlke S, Duenow U, Nebe JB, Seitz H. Femtosecond Laser Nano/Micro Textured Ti6Al4V Surfaces—Effect on Wetting and MG-63 Cell Adhesion. Materials. 2019; 12(13):2210. https://doi.org/10.3390/ma12132210
Chicago/Turabian StyleSchnell, Georg, Susanne Staehlke, Ulrike Duenow, J. Barbara Nebe, and Hermann Seitz. 2019. "Femtosecond Laser Nano/Micro Textured Ti6Al4V Surfaces—Effect on Wetting and MG-63 Cell Adhesion" Materials 12, no. 13: 2210. https://doi.org/10.3390/ma12132210
APA StyleSchnell, G., Staehlke, S., Duenow, U., Nebe, J. B., & Seitz, H. (2019). Femtosecond Laser Nano/Micro Textured Ti6Al4V Surfaces—Effect on Wetting and MG-63 Cell Adhesion. Materials, 12(13), 2210. https://doi.org/10.3390/ma12132210