Heterocycle Effects on the Liquid Crystallinity of Terthiophene Analogues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
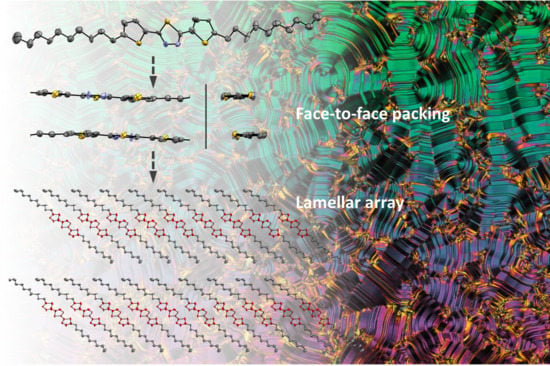
2.2. Phase Behaviour
2.3. Molecular Opto-Electronic Properties
3. Conclusions
4. Experimental Part
4.1. Materials and Methods
| Compound | Oxidation (V) | Reduction (V) | Energy Levels (eV) [calc.] | Band Gap (eV) | ||
|---|---|---|---|---|---|---|
| E11/2 | E11/2 | HOMO a | LUMO b | EgCV c | Egcalc. d | |
| Th-Oxd-Th(10) | – | −3.11 | [−5.59] | −1.69 [−1.62] | – | 3.97 |
| Th-Thd-Th(6) | 1.18 | −2.41 | −5.98 [−5.54] | −2.39 [−1.90] | 3.59 | 3.64 |
| Th3(6)* | 1.51 | −2.14 | −5.92 [−4.87] | −2.70 [−1.52] | 3.22 | 3.35 |
4.2. Synthesis
4.2.1. Precursors and Literature Compounds
4.2.2. Th-Oxd-Th(10)
4.2.3. Th-Thd-Th(10)
4.2.4. General procedure for Th-Thd-Th(n) Derivatives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, E.K.; Lee, M.Y.; Park, C.H.; Lee, H.R.; Oh, J.H. Toward Environmentally Robust Organic Electronics: Approaches and Applications. Adv. Mater. 2017, 29, 1703638. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef] [PubMed]
- Orgiu, E.; Samorì, P. 25th Anniversary Article: Organic Electronics Marries Photochromism: Generation of Multifunctional Interfaces, Materials, and Devices. Adv. Mater. 2014, 26, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, K.-H.; Kim, E.; Moon, C.-K.; Kim, Y.-H.; Kim, J.-J.; Yoo, S. Lensfree OLEDs with over 50% external quantum efficiency via external scattering and horizontally oriented emitters. Nat. Commun. 2018, 9, 3207. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, J.; Zhao, Z.; Wang, L.; Yang, H.; Chang, Q.; Jiang, N.; Liu, Z.; Bian, Z.; Liu, W.; et al. Deep Blue Phosphorescent Organic Light-Emitting Diodes with CIEy Value of 0.11 and External Quantum Efficiency up to 22.5%. Adv. Mater. 2018, 30, 1705005. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.-S.; Ruan, S.-B.; Bencheikh, F.; Nagata, R.; Zhang, L.; Inada, K.; Nakanotani, H.; Liao, L.-S.; Adachi, C. Long-lived efficient delayed fluorescence organic light-emitting diodes using n-type hosts. Nat. Commun. 2017, 8, 2250. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef]
- Ji, D.; Li, T.; Zou, Y.; Chu, M.; Zhou, K.; Liu, J.; Tian, G.; Zhang, Z.; Zhang, X.; Li, L.; et al. Copolymer dielectrics with balanced chain-packing density and surface polarity for high-performance flexible organic electronics. Nat. Commun. 2018, 9, 2339. [Google Scholar] [CrossRef]
- Baumgarten, M.; Hu, B.-L.; Zhang, K.; An, C.; Schollmeyer, D.; Pisula, W. Layered Thiadiazoloquinoxaline Containing Long Pyrene-fused N-Heteroacenes. Angew. Chem. Int. Ed. 2018, 57, 12375–12379. [Google Scholar]
- Hu, D.; Wang, X.; Chen, H.; Guo, T. High Performance Flexible Nonvolatile Memory Based on Vertical Organic Thin Film Transistor. Adv. Funct. Mater. 2017, 27, 1703541. [Google Scholar] [CrossRef]
- Kanagasekaran, T.; Shimotani, H.; Shimizu, R.; Hitosugi, T.; Tanigaki, K. A new electrode design for ambipolar injection in organic semiconductors. Nat. Commun. 2017, 8, 999. [Google Scholar] [CrossRef]
- Hailey, A.K.; Petty, A.J.; Washbourne, J.; Thorley, K.J.; Parkin, S.R.; Anthony, J.E.; Loo, Y.-L. Understanding the Crystal Packing and Organic Thin-Film Transistor Performance in Isomeric Guest–Host Systems. Adv. Mater. 2017, 29, 1700048. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zhou, Z.; Zhang, D.; Zhang, Y.; Xu, S.; Zhu, X. Design of a New Fused-Ring Electron Acceptor with Excellent Compatibility to Wide-Bandgap Polymer Donors for High-Performance Organic Photovoltaics. Adv. Mater. 2018, 30, 1800403. [Google Scholar] [CrossRef]
- Nam, M.; Cha, M.; Lee, H.H.; Hur, K.; Lee, K.-T.; Yoo, J.; Han, I.K.; Kwon, S.J.; Ko, D.-H. Long-term efficient organic photovoltaics based on quaternary bulk heterojunctions. Nat. Commun. 2017, 8, 14068. [Google Scholar] [CrossRef] [Green Version]
- Baran, D.; Ashraf, R.S.; Hanifi, D.A.; Abdelsamie, M.; Gasparini, N.; Röhr, J.A.; Holliday, S.; Wadsworth, A.; Lockett, S.; Neophytou, M.; et al. Reducing the efficiency–stability–cost gap of organic photovoltaics with highly efficient and stable small molecule acceptor ternary solar cells. Nat. Mater. 2017, 16, 363–369. [Google Scholar] [CrossRef]
- Nian, L.; Gao, K.; Jiang, Y.; Rong, Q.; Hu, X.; Yuan, D.; Liu, F.; Peng, X.; Russell, T.P.; Zhou, G. Small-Molecule Solar Cells with Simultaneously Enhanced Short-Circuit Current and Fill Factor to Achieve 11% Efficiency. Adv. Mater. 2017, 29, 1700616. [Google Scholar] [CrossRef]
- Sutton, C.; Risko, C.; Brédas, J.-L. Noncovalent Intermolecular Interactions in Organic Electronic Materials: Implications for the Molecular Packing vs. Electronic Properties of Acenes. Chem. Mater. 2016, 28, 3–16. [Google Scholar] [CrossRef]
- Pfattner, R.; Bromley, S.T.; Rovira, C.; Mas-Torrent, M. Tuning Crystal Ordering, Electronic Structure, and Morphology in Organic Semiconductors: Tetrathiafulvalenes as a Model Case. Adv. Funct. Mater. 2016, 26, 2256–2275. [Google Scholar] [CrossRef]
- Ryno, S.M.; Risko, C.; Brédas, J.-L. Impact of Molecular Packing on Electronic Polarization in Organic Crystals: The Case of Pentacene vs TIPS-Pentacene. J. Am. Chem. Soc. 2014, 136, 6421–6427. [Google Scholar] [CrossRef]
- Rivnay, J.; Jimison, L.H.; Northrup, J.E.; Toney, M.F.; Noriega, R.; Lu, S.; Marks, T.J.; Facchetti, A.; Salleo, A. Large modulation of carrier transport by grain-boundary molecular packing and microstructure in organic thin films. Nat. Mater. 2009, 8, 952–958. [Google Scholar] [CrossRef]
- McDowell, C.; Abdelsamie, M.; Toney, M.F.; Bazan, G.C. Solvent Additives: Key Morphology-Directing Agents for Solution-Processed Organic Solar Cells. Adv. Mater. 2018, 30, 1707114. [Google Scholar] [CrossRef]
- Zhang, X.; Jie, J.; Deng, W.; Shang, Q.; Wang, J.; Wang, H.; Chen, X.; Zhang, X. Alignment and Patterning of Ordered Small-Molecule Organic Semiconductor Micro-/Nanocrystals for Device Applications. Adv. Mater. 2016, 28, 2475–2503. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xu, X.; Zhang, W.; Meng, X.; Ma, W.; Yartsev, A.; Inganäs, O.; Andersson, M.R.; Janssen, R.A.J.; Wang, E. High Performance All-Polymer Solar Cells by Synergistic Effects of Fine-Tuned Crystallinity and Solvent Annealing. J. Am. Chem. Soc. 2016, 138, 10935–10944. [Google Scholar] [CrossRef]
- Deng, D.; Zhang, Y.; Zhang, J.; Wang, Z.; Zhu, L.; Fang, J.; Xia, B.; Wang, Z.; Lu, K.; Ma, W.; et al. Fluorination-enabled optimal morphology leads to over 11% efficiency for inverted small-molecule organic solar cells. Nat. Commun. 2016, 7, 13740. [Google Scholar] [CrossRef]
- Yang, J.; Yan, D.; Jones, T.S. Molecular Template Growth and Its Applications in Organic Electronics and Optoelectronics. Chem. Rev. 2015, 115, 5570–5603. [Google Scholar] [CrossRef]
- Niazi, M.R.; Li, R.; Li, E.Q.; Kirmani, A.R.; Abdelsamie, M.; Wang, Q.; Pan, W.; Payne, M.M.; Anthony, J.E.; Smilgies, D.-M.; et al. Solution-printed organic semiconductor blends exhibiting transport properties on par with single crystals. Nat. Commun. 2015, 6, 8598. [Google Scholar] [CrossRef]
- Lee, C.; Kang, H.; Lee, W.; Kim, T.; Kim, K.-H.; Woo, H.Y.; Wang, C.; Kim, B.J. High-Performance All-Polymer Solar Cells Via Side-Chain Engineering of the Polymer Acceptor: The Importance of the Polymer Packing Structure and the Nanoscale Blend Morphology. Adv. Mater. 2015, 27, 2466–2471. [Google Scholar] [CrossRef]
- Choi, D.; Chang, M.; Reichmanis, E. Controlled Assembly of Poly(3-hexylthiophene): Managing the Disorder to Order Transition on the Nano- through Meso-Scales. Adv. Funct. Mater. 2015, 25, 920–927. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Naitoh, Y.; Ishida, T. Morphological Change and Mobility Enhancement in PEDOT:PSS by Adding Co-solvents. Adv. Mater. 2013, 25, 2831–2836. [Google Scholar] [CrossRef]
- Keum, C.M.; Liu, S.; Al-Shadeedi, A.; Kaphle, V.; Callens, M.K.; Han, L.; Neyts, K.; Zhao, H.; Gather, M.C.; Bunge, S.D.; et al. Tuning charge carrier transport and optical birefringence in liquid-crystalline thin films: A new design space for organic light-emitting diodes. Sci. Rep. 2018, 8, 699. [Google Scholar] [CrossRef]
- Nickmans, K.; Schenning, A.P.H.J. Directed Self-Assembly of Liquid-Crystalline Molecular Building Blocks for Sub-5 nm Nanopatterning. Adv. Mater. 2018, 30, 1703713. [Google Scholar] [CrossRef]
- Uchida, J.; Yoshio, M.; Sato, S.; Yokoyama, H.; Fujita, M.; Kato, T. Self-Assembly of Giant Spherical Liquid-Crystalline Complexes and Formation of Nanostructured Dynamic Gels that Exhibit Self-Healing Properties. Angew. Chem. Int. Ed. 2017, 56, 14085–14089. [Google Scholar] [CrossRef]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Feng, X.; Liu, M.; Pisula, W.; Takase, M.; Li, J.; Müllen, K. Supramolecular Organization and Photovoltaics of Triangle-shaped Discotic Graphenes with Swallow-tailed Alkyl Substituents. Adv. Mater. 2008, 20, 2684–2689. [Google Scholar] [CrossRef]
- McCulloch, I.; Heeney, M.; Bailey, C.; Genevicius, K.; MacDonald, I.; Shkunov, M.; Sparrowe, D.; Tierney, S.; Wagner, R.; Zhang, W.; et al. Liquid-crystalline semiconducting polymers with high charge-carrier mobility. Nat. Mater. 2006, 5, 328–333. [Google Scholar] [CrossRef]
- van Breemen, A.J.J.M.; Herwig, P.T.; Chlon, C.H.T.; Sweelssen, J.; Schoo, H.F.M.; Setayesh, S.; Hardeman, W.M.; Martin, C.A.; de Leeuw, D.M.; Valeton, J.J.P.; et al. Large Area Liquid Crystal Monodomain Field-Effect Transistors. J. Am. Chem. Soc. 2006, 128, 2336–2345. [Google Scholar] [CrossRef]
- Iguarbe, V.; Concellón, A.; Termine, R.; Golemme, A.; Barberá, J.; Serrano, J.L. Making Coaxial Wires Out of Janus Dendrimers for Efficient Charge Transport. ACS Macro Lett. 2018, 7, 1138–1143. [Google Scholar] [CrossRef]
- Chico, R.; de Domingo, E.; Domínguez, C.; Donnio, B.; Heinrich, B.; Termine, R.; Golemme, A.; Coco, S.; Espinet, P. High One-Dimensional Charge Mobility in Semiconducting Columnar Mesophases of Isocyano-Triphenylene Metal Complexes. Chem. Mater. 2017, 29, 7587–7595. [Google Scholar] [CrossRef] [Green Version]
- Wöhrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J.C.; Staffeld, P.; Baro, A.; Giesselmann, F.; et al. Discotic Liquid Crystals. Chem. Rev. 2016, 116, 1139–1241. [Google Scholar] [CrossRef]
- García-Frutos, E.M.; Pandey, U.K.; Termine, R.; Omenat, A.; Barberá, J.; Serrano, J.L.; Golemme, A.; Gómez-Lor, B. High Charge Mobility in Discotic Liquid-Crystalline Triindoles: Just a Core Business? Angew. Chem. Int. Ed. 2011, 50, 7399–7402. [Google Scholar] [CrossRef]
- Thiebaut, O.; Bock, H.; Grelet, E. Face-on Oriented Bilayer of Two Discotic Columnar Liquid Crystals for Organic Donor−Acceptor Heterojunction. J. Am. Chem. Soc. 2010, 132, 6886–6887. [Google Scholar] [CrossRef]
- Pisula, W.; Feng, X.; Müllen, K. Tuning the Columnar Organization of Discotic Polycyclic Aromatic Hydrocarbons. Adv. Mater. 2010, 22, 3634–3649. [Google Scholar] [CrossRef]
- Feng, X.; Marcon, V.; Pisula, W.; Hansen, M.R.; Kirkpatrick, J.; Grozema, F.; Andrienko, D.; Kremer, K.; Müllen, K. Towards high charge-carrier mobilities by rational design of the shape and periphery of discotics. Nat. Mater. 2009, 8, 421–426. [Google Scholar] [CrossRef]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Iino, H.; Hanna, J. Liquid crystalline organic semiconductors for organic transistor applications. Polym. J. 2017, 49, 23–30. [Google Scholar] [CrossRef]
- He, Y.; Sezen, M.; Zhang, D.; Li, A.; Yan, L.; Yu, H.; He, C.; Goto, O.; Loo, Y.-L.; Meng, H. High Performance OTFTs Fabricated Using a Calamitic Liquid Crystalline Material of 2-(4-Dodecyl phenyl)[1]benzothieno[3,2-b][1]benzothiophene. Adv. Electron. Mater. 2016, 2, 1600179. [Google Scholar] [CrossRef]
- Iino, H.; Usui, T.; Hanna, J. Liquid crystals for organic thin-film transistors. Nat. Commun. 2015, 6, 6828. [Google Scholar] [CrossRef]
- Roncali, J.; Leriche, P.; Cravino, A. From One- to Three-Dimensional Organic Semiconductors: In Search of the Organic Silicon? Adv. Mater. 2007, 19, 2045–2060. [Google Scholar] [CrossRef] [Green Version]
- Boucher, N.; Leroy, J.; Sergeyev, S.; Pouzet, E.; Lemaur, V.; Lazzaroni, R.; Cornil, J.; Geerts, Y.H.; Sferrazza, M. Mesomorphism of dialkylterthiophene homologues. Synth. Met. 2009, 159, 1319–1324. [Google Scholar] [CrossRef]
- Leroy, J.; Boucher, N.; Sergeyev, S.; Sferrazza, M.; Geerts, Y.H. Symmetrical and Nonsymmetrical Liquid Crystalline Oligothiophenes: Convenient Synthesis and Transition-Temperature Engineering. Eur. J. Org. Chem. 2007, 2007, 1256–1261. [Google Scholar] [CrossRef]
- Leroy, J.; Levin, J.; Sergeyev, S.; Geerts, Y. Practical One-step Synthesis of Symmetrical Liquid Crystalline Dialkyloligothiophenes for Molecular Electronic Applications. Chem. Lett. 2006, 35, 166–167. [Google Scholar] [CrossRef]
- Funahashi, M.; Hanna, J. High ambipolar carrier mobility in self-organizing terthiophene derivative. Appl. Phys. Lett. 2000, 76, 2574–2576. [Google Scholar] [CrossRef]
- Byron, D.J.; Matharu, A.S.; Wilson, R.C.; Wright, G. The Synthesis and Liquid Crystal Properties of Certain 5,5″-Disubstituted 2,2′:5′,2″-Terthienyls. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1995, 264, 227–230. [Google Scholar] [CrossRef]
- Byron, D.; Matharu, A.; Wilson, R.; Wright, G. The Synthesis and Liquid Crystal Properties of Certain 5,5″-Disubstituted 2,2′:5′,2″-Terthiophenes. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1995, 265, 61–76. [Google Scholar] [CrossRef]
- Hanna, J.; Ohno, A.; Iino, H. Charge carrier transport in liquid crystals. Thin Solid Films 2014, 554, 58–63. [Google Scholar] [CrossRef]
- Funahashi, M.; Yasuda, T.; Kato, T. Liquid Crystal Semiconductors: Oligothiophene and Related Materials. In Handbook of Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, E.P., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; Volume 8, pp. 675–708. ISBN 978-3-527-67140-3. [Google Scholar]
- Pisula, W.; Zorn, M.; Chang, J.Y.; Müllen, K.; Zentel, R. Liquid Crystalline Ordering and Charge Transport in Semiconducting Materials. Macromol. Rapid Commun. 2009, 30, 1179–1202. [Google Scholar] [CrossRef]
- Iino, H.; Hanna, J. Availability of Liquid Crystallinity in Solution Processing for Polycrystalline Thin Films. Adv. Mater. 2011, 23, 1748–1751. [Google Scholar] [CrossRef]
- Iino, H.; Hanna, J.-I. Liquid Crystalline Materials for Organic Polycrystalline Field Effect Transistors. Mol. Cryst. Liq. Cryst. 2011, 542, 237–243. [Google Scholar] [CrossRef]
- Mei, J.; Diao, Y.; Appleton, A.L.; Fang, L.; Bao, Z. Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 2013, 135, 6724–6746. [Google Scholar] [CrossRef]
- Funahashi, M.; Hanna, J.-I. High Carrier Mobility up to 0.1 cm2 V–1 s–1 at Ambient Temperatures in Thiophene-Based Smectic Liquid Crystals. Adv. Mater. 2005, 17, 594–598. [Google Scholar] [CrossRef]
- Funahashi, M. Electronic Carrier Transport in Liquid Crystalline Semiconductors–Increase in Carrier Mobility and Extension of Mesomorphic Temperature Range. Mol. Cryst. Liq. Cryst. 2006, 458, 3–10. [Google Scholar] [CrossRef]
- Funahashi, M.; Zhang, F.; Tamaoki, N.; Hanna, J. Ambipolar Transport in the Smectic E Phase of 2-Propyl-5′′-Hexynylterthiophene Derivative over a Wide Temperature Range. ChemPhysChem 2008, 9, 1465–1473. [Google Scholar] [CrossRef]
- Funahashi, M.; Hanna, J.-I. Mesomorphic behaviors and charge carrier transport in terthiophene derivatives. Mol. Cryst. Liq. Cryst. 2004, 410, 1–12. [Google Scholar] [CrossRef]
- Zhang, F.; Funahashi, M.; Tamaoki, N. High-performance thin film transistors from semiconducting liquid crystalline phases by solution processes. Appl. Phys. Lett. 2007, 91, 63515. [Google Scholar] [CrossRef]
- Ghosh, T.; Lehmann, M. Recent advances in heterocycle-based metal-free calamitics. J. Mater. Chem. C 2017, 5, 12308–12337. [Google Scholar] [CrossRef]
- Tuzimoto, P.; Santos, D.M.P.O.; Moreira, T.D.S.; Cristiano, R.; Bechtold, I.H.; Gallardo, H. Luminescent liquid crystals containing a sulphur-based heterocyclic core. Liq. Cryst. 2014, 41, 1097–1108. [Google Scholar] [CrossRef]
- Han, J. 1,3,4-Oxadiazole based liquid crystals. J. Mater. Chem. C 2013, 1, 7779–7797. [Google Scholar] [CrossRef]
- Kaur, S.; Tian, L.; Liu, H.; Greco, C.; Ferrarini, A.; Seltmann, J.; Lehmann, M.; Gleeson, H.F. The elastic and optical properties of a bent-core thiadiazole nematic liquid crystal: The role of the bend angle. J. Mater. Chem. C 2013, 1, 2416–2425. [Google Scholar] [CrossRef]
- Seed, A. Synthesis of self-organizing mesogenic materials containing a sulfur-based five-membered heterocyclic core. Chem. Soc. Rev. 2007, 36, 2046–2069. [Google Scholar] [CrossRef]
- Oikawa, K.; Tsuchiya, K.; Yamazaki, H. Fluorescent and Liquid Crystalline Thiophenes, and Their Compositions and Polymers. Patent JP 2004269424, 30 September 2004. [Google Scholar]
- Kuo, C.Y.; Liu, Y.; Yarotski, D.; Li, H.; Xu, P.; Yen, H.J.; Tretiak, S.; Wang, H.L. Synthesis, electrochemistry, STM investigation of oligothiophene self-assemblies with superior structural order and electronic properties. Chem. Phys. 2016, 481, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Pu, S.; Zhu, S.; Rao, Y.; Liu, G.; Wei, H. Photochromism of 1,2-bis(2-n-alkyl-5-formyl-3-thienyl)perfluorocyclopentene derivatives. J. Mol. Struct. 2009, 921, 89–100. [Google Scholar] [CrossRef]
- Screttas, C.G.; Steele, B.R. Metal alkoxide modified organometallic reagents. Preparation and stability of organolithium reagents in tetrahydrofuran in the presence of magnesium 2-ethoxyethoxide. J. Org. Chem. 1989, 54, 1013–1017. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Z.; Zhao, Z.; Zheng, L.; Tan, S.; Yin, Z.; Zhu, C.; Liu, Y. Synthesis, Structural Characterization, and Field-Effect Transistor Properties of n-Channel Semiconducting Polymers Containing Five-Membered Heterocyclic Acceptors: Superiority of Thiadiazole Compared with Oxadiazole. ACS Appl. Mater. Interfaces 2016, 8, 33051–33059. [Google Scholar] [CrossRef]
- Kurach, E.; Kotwica, K.; Zapala, J.; Knor, M.; Nowakowski, R.; Djurado, D.; Toman, P.; Pfleger, J.; Zagorska, M.; Pron, A. Semiconducting Alkyl Derivatives of 2,5-Bis(2,2′-bithiophene-5-yl)-1,3,4-thiadiazole—Effect of the Substituent Position on the Spectroscopic, Electrochemical, and Structural Properties. J. Phys. Chem. C 2013, 117, 15316–15326. [Google Scholar] [CrossRef]
- Gane, P.A.C.; Leadbetter, A.J.; Moussa, F.; Benattar, J.J.; Lambert, M. Structural correlations in smectic-F and smectic-I phases. Phys. Rev. A 1981, 24, 2694–2700. [Google Scholar] [CrossRef]
- Getmanenko, Y.A.; Kang, S.-W.; Shakya, N.; Pokhrel, C.; Bunge, S.D.; Kumar, S.; Ellman, B.D.; Twieg, R.J. Bis(5-alkylthiophen-2-yl)arene liquid crystals as molecular semiconductors. J. Mater. Chem. C 2014, 2, 2600–2611. [Google Scholar] [CrossRef]
- Getmanenko, Y.A.; Kang, S.-W.; Shakya, N.; Pokhrel, C.; Bunge, S.D.; Kumar, S.; Ellman, B.D.; Twieg, R.J. 5,5′-Bis-(alkylpyridinyl)-2,2′-bithiophenes: Synthesis, liquid crystalline behaviour and charge transport. J. Mater. Chem. C 2013, 2, 256–271. [Google Scholar] [CrossRef]
- Kim, S.; Kim, A.; Jang, K.-S.; Yoo, S.; Ka, J.-W.; Kim, J.; Yi, M.H.; Won, J.C.; Hong, S.-K.; Kim, Y.H. The effect of thermal annealing on the layered structure of smectic liquid crystalline organic semiconductor on polyimide gate insulator and its OFET performance. Synth. Met. 2016, 220, 311–317. [Google Scholar] [CrossRef]
- Ward, J.W.; Goetz, K.P.; Obaid, A.; Payne, M.M.; Diemer, P.J.; Day, C.S.; Anthony, J.E.; Jurchescu, O.D. Low-temperature phase transitions in a soluble oligoacene and their effect on device performance and stability. Appl. Phys. Lett. 2014, 105, 83305. [Google Scholar] [CrossRef] [Green Version]
- Jurchescu, O.D.; Mourey, D.A.; Subramanian, S.; Parkin, S.R.; Vogel, B.M.; Anthony, J.E.; Jackson, T.N.; Gundlach, D.J. Effects of polymorphism on charge transport in organic semiconductors. Phys. Rev. B 2009, 80, 85201. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Goodby, J.W. Structures and Properties of Smectic Liquid Crystals. In Handbook of Liquid Crystals; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; Volume 4, pp. 43–68. ISBN 978-3-527-67140-3. [Google Scholar]
- Horčic, M.; Kozmík, V.; Svoboda, J.; Novotná, V.; Pociecha, D. Transformation from a rod-like to a hockey-stick-like and bent-shaped molecule in 3,4′-disubstituted azobenzene-based mesogens. J. Mater. Chem. C 2013, 1, 7560–7567. [Google Scholar] [CrossRef]
- Kajitani, T.; Kohmoto, S.; Yamamoto, M.; Kishikawa, K. Liquid crystalline amides: Linear arrangement of rod-like molecules by lateral intermolecular hydrogen bonding and molecular shape effect. J. Mater. Chem. 2004, 14, 3449–3456. [Google Scholar] [CrossRef]
- Pradhan, B.; Vaisakh, V.M.; Nair, G.G.; Rao, D.S.S.; Prasad, S.K.; Sudhakar, A.A. Effect of Atomic-Scale Differences on the Self-Assembly of Thiophene-based Polycatenars in Liquid Crystalline and Organogel States. Chem.–Eur. J. 2016, 22, 17843–17856. [Google Scholar] [CrossRef]
- Han, J.; Zhang, F.-Y.; Wang, J.-Y.; Wang, Y.-M.; Pang, M.-L.; Meng, J.-B. Synthesis and comparative study of the heterocyclic rings on liquid crystalline properties of 2,5-aryl-1,3,4-oxa(thia)diazole derivatives containing furan and thiophene units. Liq. Cryst. 2009, 36, 825–833. [Google Scholar] [CrossRef]
- Han, J.; Chang, X.-Y.; Cao, B.-N.; Wang, Q.-C. Synthesis, Single Crystal Structures, and Liquid Crystal Property of 2,5-Diphenyl-1,3,4-Oxadiazoles/1,3,4-Thiadiazoles. Soft Mater. 2009, 7, 342–354. [Google Scholar] [CrossRef]
- Han, J.; Wang, J.-Y.; Zhang, F.-Y.; Zhu, L.-R.; Pang, M.-L.; Meng, J.-B. Synthesis and mesomorphic behaviour of heterocycle-based liquid crystals containing 1,3,4-oxadiazole/thiadiazole and thiophene units. Liq. Cryst. 2008, 35, 1205–1214. [Google Scholar] [CrossRef]
- Lavigueur, C.; Foster, E.J.; Williams, V.E. Self-Assembly of Discotic Mesogens in Solution and in Liquid Crystalline Phases: Effects of Substituent Position and Hydrogen Bonding. J. Am. Chem. Soc. 2008, 130, 11791–11800. [Google Scholar] [CrossRef]
- Foster, E.J.; Jones, R.B.; Lavigueur, C.; Williams, V.E. Structural Factors Controlling the Self-Assembly of Columnar Liquid Crystals. J. Am. Chem. Soc. 2006, 128, 8569–8574. [Google Scholar] [CrossRef]
- Lercher, C.; Röthel, C.; Roscioni, O.M.; Geerts, Y.H.; Shen, Q.; Teichert, C.; Fischer, R.; Leising, G.; Sferrazza, M.; Gbabode, G.; et al. Polymorphism of dioctyl-terthiophene within thin films: The role of the first monolayer. Chem. Phys. Lett. 2015, 630, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Van Bolhuis, F.; Wynberg, H.; Havinga, E.E.; Meijer, E.W.; Staring, E.G.J. The X-ray structure and MNDO calculations of α-terthienyl: A model for polythiophenes. Synth. Met. 1989, 30, 381–389. [Google Scholar] [CrossRef]
- Dhar, J.; Karothu, D.P.; Patil, S. Herringbone to cofacial solid state packing via H-bonding in diketopyrrolopyrrole (DPP) based molecular crystals: Influence on charge transport. Chem. Commun. 2014, 51, 97–100. [Google Scholar] [CrossRef]
- McGarry, K.A.; Xie, W.; Sutton, C.; Risko, C.; Wu, Y.; Young, V.G.; Brédas, J.-L.; Frisbie, C.D.; Douglas, C.J. Rubrene-Based Single-Crystal Organic Semiconductors: Synthesis, Electronic Structure, and Charge-Transport Properties. Chem. Mater. 2013, 25, 2254–2263. [Google Scholar] [CrossRef]
- Wang, C.; Dong, H.; Hu, W.; Liu, Y.; Zhu, D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. [Google Scholar] [CrossRef]
- Locklin, J.; Roberts, M.E.; Mannsfeld, S.C.B.; Bao, Z. Optimizing the Thin Film Morphology of Organic Field-Effect Transistors: The Influence of Molecular Structure and Vacuum Deposition Parameters on Device Performance. J. Macromol. Sci. Part C 2006, 46, 79–101. [Google Scholar] [CrossRef]
- Hutchison, G.R.; Ratner, M.A.; Marks, T.J. Intermolecular Charge Transfer between Heterocyclic Oligomers. Effects of Heteroatom and Molecular Packing on Hopping Transport in Organic Semiconductors. J. Am. Chem. Soc. 2005, 127, 16866–16881. [Google Scholar] [CrossRef]
- Oliva, M.M.; Pappenfus, T.M.; Melby, J.H.; Schwaderer, K.M.; Johnson, J.C.; McGee, K.A.; da Silva Filho, D.A.; Bredas, J.-L.; Casado, J.; López Navarrete, J.T. Comparison of Thiophene–Pyrrole Oligomers with Oligothiophenes: A Joint Experimental and Theoretical Investigation of Their Structural and Spectroscopic Properties. Chem.–A Eur. J. 2010, 16, 6866–6876. [Google Scholar] [CrossRef]
- Facchetti, A.; Yoon, M.-H.; Stern, C.L.; Hutchison, G.R.; Ratner, M.A.; Marks, T.J. Building Blocks for N-Type Molecular and Polymeric Electronics. Perfluoroalkyl- versus Alkyl-Functionalized Oligothiophenes (nTs; n = 2−6). Systematic Synthesis, Spectroscopy, Electrochemistry, and Solid-State Organization. J. Am. Chem. Soc. 2004, 126, 13480–13501. [Google Scholar] [CrossRef]
- Nepomnyashchii, A.B.; Ono, R.J.; Lyons, D.M.; Bielawski, C.W.; Sessler, J.L.; Bard, A.J. Electrochemistry and electrogenerated chemiluminescence of thiophene and fluorene oligomers. Benzoyl peroxide as a coreactant for oligomerization of thiophene dimers. Chem. Sci. 2012, 3, 2628–2638. [Google Scholar] [CrossRef] [Green Version]
- Bredas, J.L. Mind the gap! Mater. Horiz. 2013, 1, 17–19. [Google Scholar] [CrossRef]
- Seixas de Melo, J.; Elisei, F.; Becker, R.S. Photophysical studies of mixed furan, pyrrole, and thiophene-containing oligomers with three and five rings. J. Chem. Phys. 2002, 117, 4428–4435. [Google Scholar] [CrossRef]
- Jursic, B.S. Suitability of furan, pyrrole and thiophene as dienes for Diels–Alder reactions viewed through their stability and reaction barriers for reactions with acetylene, ethylene and cyclopropene. An AM1 semiempirical and B3LYP hybrid density functional theory study. J. Mol. Struct. THEOCHEM 1998, 454, 105–116. [Google Scholar]
- Pineda, R.F.; Troughton, J.; Planells, M.; Santos, I.S.-M.; Muhith, F.; Nichol, G.S.; Haque, S.; Watson, T.; Robertson, N. Effect of alkyl chain length on the properties of triphenylamine-based hole transport materials and their performance in perovskite solar cells. Phys. Chem. Chem. Phys. 2018, 20, 1252–1260. [Google Scholar] [CrossRef]
- Granadino-Roldán, J.M.; Garzón, A.; García, G.; Moral, M.; Navarro, A.; Fernández-Liencres, M.P.; Peña-Ruiz, T.; Fernández-Gómez, M. Theoretical Study of the Effect of Alkyl and Alkoxy Lateral Chains on the Structural and Electronic Properties of π-Conjugated Polymers Consisting of Phenylethynyl-1,3,4-thiadiazole. J. Phys. Chem. C 2011, 115, 2865–2873. [Google Scholar] [CrossRef]
- Lavigueur, C.; Foster, E.J.; Williams, V.E. A simple and inexpensive capillary furnace for variable-temperature X-ray diffraction. J. Appl. Crystallogr. 2008, 41, 214–216. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cancès, E. The IEF version of the PCM solvation method: An overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J. Chem. Phys. 1997, 107, 3210–3221. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Casida, M.E. Time-Dependent Density Functional Response Theory for Molecules. In Recent Advances in Density Functional Methods; Recent Advances in Computational Chemistry; World Scientific: Singapore, 1995; Volume 1, pp. 155–192. ISBN 978-981-02-2442-4. [Google Scholar]
- Huff, G.S.; Gallaher, J.K.; Hodgkiss, J.M.; Gordon, K.C. No single DFT method can predict Raman cross-sections, frequencies and electronic absorption maxima of oligothiophenes. Synth. Met. 2017, 231, 1–6. [Google Scholar] [CrossRef]
- Jacquemin, D.; Michaux, C.; Perpète, E.A.; Maurel, F.; Perrier, A. Photochromic molecular wires: Insights from theory. Chem. Phys. Lett. 2010, 488, 193–197. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Schäfer, A.; Horn, H.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets for atoms Li to Kr. J. Chem. Phys. 1992, 97, 2571–2577. [Google Scholar] [CrossRef]












| Compound | Phasea |  | Phasea |  | Phasea |  | Phasea |  | Phasea |
|---|---|---|---|---|---|---|---|---|---|
| Th3(10) | Cr’ |  | Cr |  | SmF |  | SmC |  | I |
| Th-Oxd-Th(10) | Cr” |  | Cr’ |  | Cr |  | I | ||
| Th-Thd-Th(4) | Cr” |  | Cr’ |  | Cr |  | I | ||
| Th-Thd-Th(6) | CrJ |  | SmC |  | I | ||||
| Th-Thd-Th(8) | Cr |  | CrJ |  | SmC |  | I | ||
| Th-Thd-Th(10) | Cr |  | CrJ |  | SmC |  | I | ||
| Th-Thd-Th(12) | Cr |  | CrJ |  | SmI |  | SmC |  | I |
| Compound | (nm) | ε (M−1·cm−1) | Egopt a (eV) [calc.] | (nm) | (nm) | Φf b |
|---|---|---|---|---|---|---|
| Th-Oxd-Th(10) | 328 | 28200 | 3.35 [3.26] | 340 | 387 | 0.48 |
| Th-Thd-Th(10) | 359 | 26800 | 3.04 [2.94] | 369 | 430 | 0.49 |
| Th3(10) | 367 | 25900 | 2.93 [2.67] | 380 | 443 | 0.56 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ester, D.F.; McKearney, D.; Herasymchuk, K.; Williams, V.E. Heterocycle Effects on the Liquid Crystallinity of Terthiophene Analogues. Materials 2019, 12, 2314. https://doi.org/10.3390/ma12142314
Ester DF, McKearney D, Herasymchuk K, Williams VE. Heterocycle Effects on the Liquid Crystallinity of Terthiophene Analogues. Materials. 2019; 12(14):2314. https://doi.org/10.3390/ma12142314
Chicago/Turabian StyleEster, David F., Declan McKearney, Khrystyna Herasymchuk, and Vance E. Williams. 2019. "Heterocycle Effects on the Liquid Crystallinity of Terthiophene Analogues" Materials 12, no. 14: 2314. https://doi.org/10.3390/ma12142314
APA StyleEster, D. F., McKearney, D., Herasymchuk, K., & Williams, V. E. (2019). Heterocycle Effects on the Liquid Crystallinity of Terthiophene Analogues. Materials, 12(14), 2314. https://doi.org/10.3390/ma12142314