Synthesis, X-ray Crystal Structure, and Photochromism of a Sandwich-Type Mono-Aluminum Complex Composed of Two Tri-Lacunary α-Dawson-Type Polyoxotungstates
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [(n-C4H9)4N]7[H14Al(B-α-P2W15O56)2] (TBA-1)
2.3. X-ray Crystallography
2.4. Crystal Data of TBA-1
3. Results and Discussion
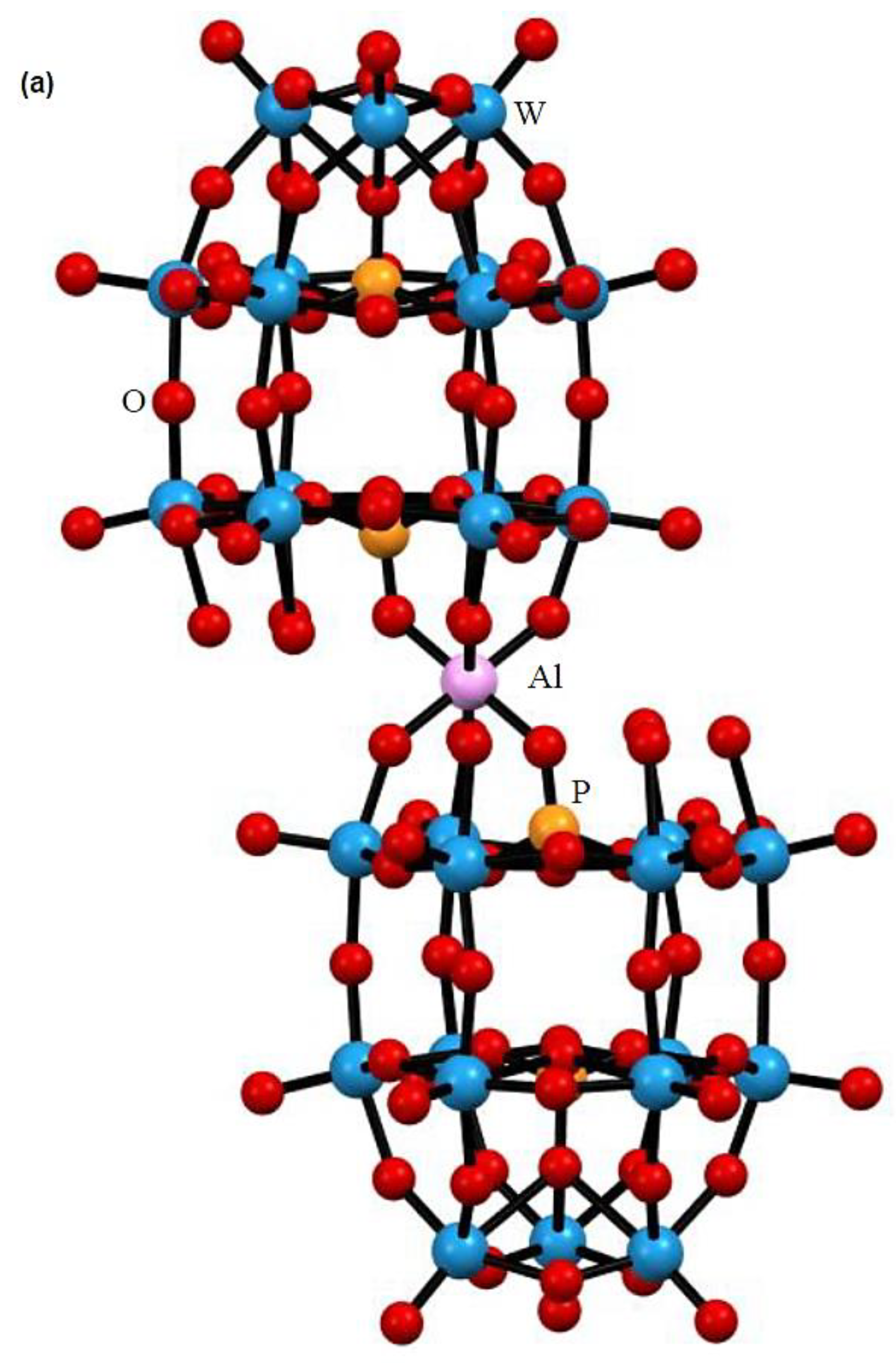
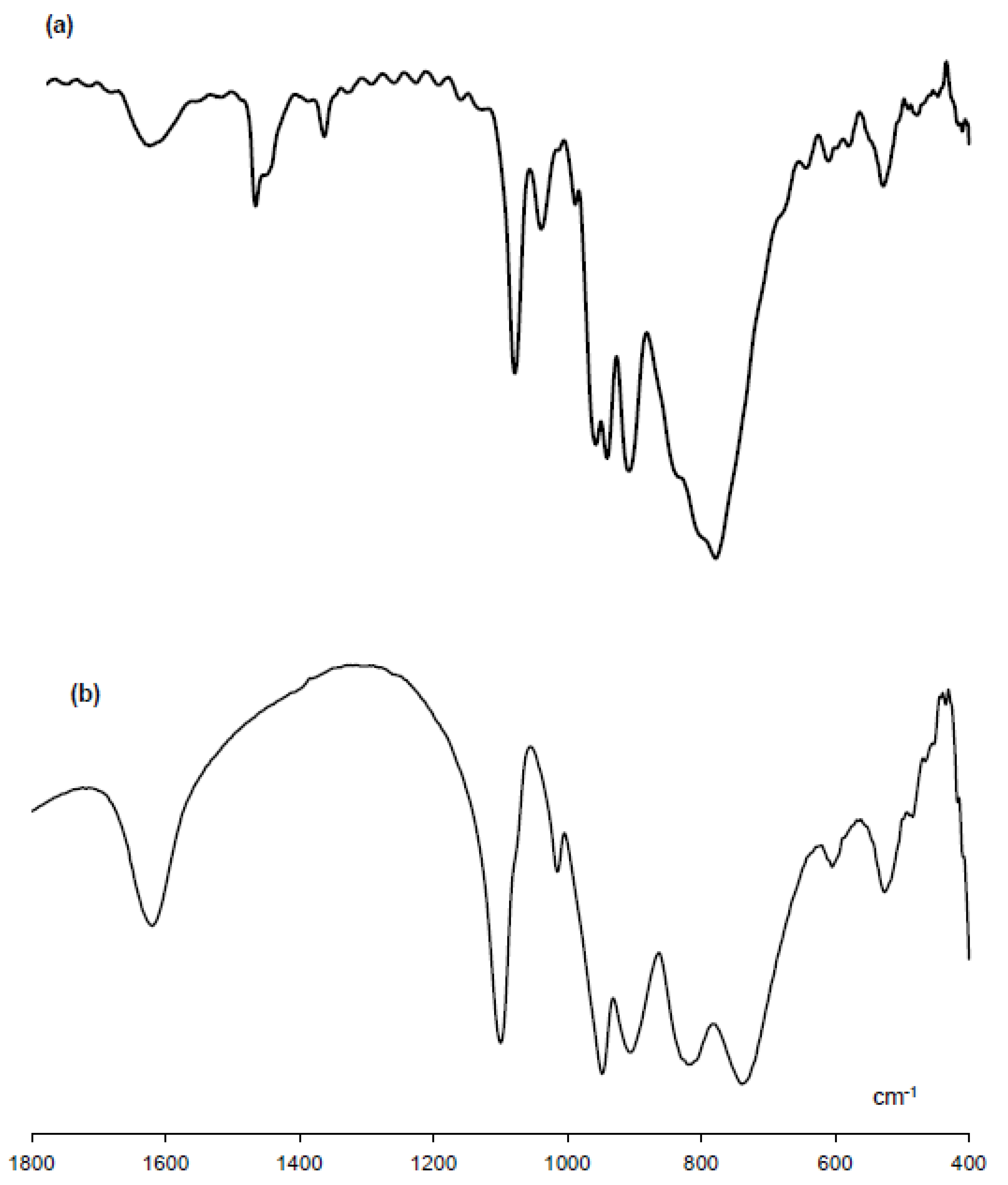
3.1. Synthesis and Characterization of [(n-C4H9)4N]7[H14Al(B-α-P2W15O56)2] (TBA-1)
3.2. Photochromism of TBA-1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cotton, F.A.; Wilkinson, G. Advanced Inorganic Chemistry, 5th ed.; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Ono, Y.; Hattori, H. Solid Base Catalysis; Springer: Berlin, Germany; Tokyo Institute of Technology Press: Tokyo, Japan, 2011. [Google Scholar]
- Djurdjevic, P.; Jelic, R.; Dzajevic, D. The effect of surface active substances on hydrolysis of aluminum (III) ion. Main Group Met. Chem. 2000, 23, 409–422. [Google Scholar] [CrossRef]
- Baes, C.F., Jr.; Mesmer, R.E. The Hydrolysis of Cations; John Wiley: New York, NY, USA, 1976. [Google Scholar]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin, Germany, 1983. [Google Scholar]
- Pope, M.T.; Müller, A. Polyoxometalate chemistry: An old field with new dimensions in several disciplines. Angew. Chem. Int. Ed. Engl. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Alipour, M.; Frontera, A. Recent developments in the crystal engineering of diverse coordination modes (0–12) for Keggin-type polyoxometalates in hybrid inorganic–organic architectures. Coord. Chem. Rev. 2014, 275, 1–18. [Google Scholar] [CrossRef]
- Taleghani, S.; Mirzaei, M.; Eshtiagh-Hosseini, H.; Frontera, A. Tuning the topology of hybrid inorganic–organic materials based on the study of flexible ligands and negative charge of polyoxometalates: A crystal engineering perspective. Coord. Chem. Rev. 2016, 309, 84–106. [Google Scholar] [CrossRef]
- Kikukawa, Y.; Yamaguchi, S.; Nakagawa, Y.; Uehara, K.; Uchida, S.; Yamaguchi, K.; Mizuno, N. Synthesis of a dialuminum-substituted silicotungstate and the diasteroselective cyclization of citronellal derivatives. J. Am. Chem. Soc. 2008, 130, 15872–15878. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Nagami, M.; Ukai, N. Strong influence of structures around aluminum centers constructed in polyoxotungstates for catalytic oxidation of alcohols with dioxygen in water. Appl. Catal. A Gen. 2013, 452, 69–74. [Google Scholar] [CrossRef]
- Carraro, M.; Bassil, B.S.; Sorarù, A.; Berardi, S.; Suchopar, A.; Kortz, U.; Bonchio, M. A Lewis acid catalytic core sandwiched by inorganic polyoxoanion caps: Selective H2O2-based oxidations with [AlIII4(H2O)10(α-XW9O33H)2]6− (X = AsIII, SbIII). Chem. Commun. 2013, 49, 7914–7916. [Google Scholar] [CrossRef] [PubMed]
- Kato, C.N.; Kashiwagi, T.; Unno, W.; Nakagawa, M.; Uno, H. Syntheses and molecular structures of monomeric and hydrogen-bonded dimeric Dawson-type tri-aluminum-substituted polyoxotungstates derived under acidic and basic conditions. Inorg. Chem. 2014, 53, 4824–4832. [Google Scholar] [CrossRef]
- Knoth, W.H.; Domaille, P.J.; Roe, D.C. Halometal derivatives of W12PO403− and related 183W NMR studies. Inorg. Chem. 1983, 22, 198–201. [Google Scholar] [CrossRef]
- Kato, C.N.; Katayama, Y.; Nagami, M.; Kato, M.; Yamasaki, M. A sandwich-type aluminium complex composed of tri-lacunary Keggin-type polyoxotungstate: Synthesis and X-ray crystal structure of [(A-PW9O34)2{W(OH)(OH2)}{Al(OH)(OH2)}{Al(α-OH)(OH2)2}2]7−. Dalton Trans. 2010, 39, 11469–11474. [Google Scholar] [CrossRef]
- Zonnevijlle, F.; Tourné, C.M.; Tourné, G.F. Preparation and characterization of heteropolytungstates containing group 3a elements. Inorg. Chem. 1982, 21, 2742–2750. [Google Scholar] [CrossRef]
- Yang, Q.H.; Zhou, D.F.; Dai, H.C.; Liu, J.F.; Xing, Y.; Lin, Y.H.; Jia, H.Q. Synthesis, structure and properties of undecatungstozincate containing 3A elements. Polyhedron 1997, 16, 3985–3989. [Google Scholar] [CrossRef]
- He, T.; Yao, J. Photochromism in composite and hybrid materials based on transition-metal oxides and polyoxometalates. Prog. Mater. Sci. 2006, 51, 810–879. [Google Scholar]
- Yamase, T. Photo- and electrochromism of polyoxometalates and related materials. Chem. Rev. 1998, 98, 307–325. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Nomiya, K.; Takahashi, M.; Ohsawa, K.; Widegren, J.A. Synthesis and characterization of tri-titanium(IV)-1,2,3-substituted α-Keggin polyoxotungstates with heteroatoms P and Si. Crystal structure of the dimeric, Ti-O-Ti bridged anhydride form K10H2[α,α-P2W18Ti6O77]·17H2O and confirmation of dimeric forms in aqueous solution by ultracentrifugation molecular weight measurements. J. Chem. Soc. Dalton Trans. 2001, 2872–2878. [Google Scholar]
- Weakley, T.J.R.; Finke, R.G. Single-crystal x-ray structures of the polyoxotungstate salts K8.3Na1.7[Cu4(H2O)2(PW9O34)2]·24H2O and Na14Cu[Cu4(H2O)2(P2W15O56)2]·53H2O. Inorg. Chem. 1990, 29, 1235–1241. [Google Scholar] [CrossRef]
- Lin, Y.; Weakley, T.J.R.; Rapko, B.; Finke, R.G. Polyoxoanions derived from tungstosilicate (A-β-SiW9O3410−): Synthesis, single-crystal structural determination, and solution structural characterization by tungsten-183 NMR and IR of titanotungstosilicate (A-β-Si2W18Ti6O7714−). Inorg. Chem. 1993, 32, 5095–5101. [Google Scholar] [CrossRef]
- Yamase, T.; Ozeki, T.; Sakamoto, H.; Nishiya, S.; Yamamoto, A. Structure of hexatitanooctadecatungstodigermanate. Bull. Chem. Soc. Jpn. 1993, 66, 103–108. [Google Scholar] [CrossRef]
- Nomiya, K.; Takahashi, M.; Widegren, J.A.; Aizawa, T.; Sakai, Y.; Kasuga, N.C. Synthesis and pH-variable ultracentrifugation molecular weight measurements of the dimeric, Ti-O-Ti bridged anhydride form of a novel di-TiIV-1,2-substituted α-Keggin polyoxotungstate. Molecular structure of the [(α-1,2-PW10Ti2O39)2]10− polyoxoanion. J. Chem. Soc. Dalton Trans. 2002, 19, 3679–3685. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H.; Hayashi, K.; Tsukada, I.; Hasegawa, T.; Yoshida, S.; Miyano, R.; Kato, C.N.; Nomiya, K. Novel solid-state 8H+-heteropolyacid. Synthesis and molecular structure of a free-acid form of Dawson-type sandwich complex, [Ti2{P2W15O54(OH2)2}2]8−. Bull. Chem. Soc. Jpn. 2007, 80, 2161–2169. [Google Scholar] [CrossRef]
- Yoshida, S.; Murakami, H.; Sakai, Y.; Nomiya, K. Syntheses, molecular structures and pH-dependent monomer-dimer equilibria of Dawson α2-monotitanium (IV)-substituted polyoxometalates. Dalton Trans. 2008, 34, 4630–4638. [Google Scholar] [CrossRef]
- Kortz, U.; Hamzeh, S.S.; Nasser, N.A. Supramolecular structures of titanium(IV)-substituted Wells-Dawson polyoxotungstates. Chem. Eur. J. 2003, 9, 2945–2952. [Google Scholar] [CrossRef]
- Fang, X.; Hill, C.L. Multiple reversible protonation of polyoxoanion surfaces: Direct observation of dynamic structural effects from proton transfer. Angew. Chem. Int. Ed. 2007, 46, 3877–3880. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. B 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Brown, I.D.; Shannon, R.D. Empirical bond-strength-bond-length curves for oxides. Acta Crystallogr. A 1973, 29, 266–282. [Google Scholar] [CrossRef]
- Brown, I.D. Chemical and steric constraints in inorganic solids. Acta Crystallogr. B 1992, 48, 553–572. [Google Scholar] [CrossRef]
- Brown, I.D. VALENCE: A program for calculating bond valences. J. Appl. Crystallogr. 1996, 29, 479–480. [Google Scholar] [CrossRef]
- Randall, W.J.; Droege, M.W.; Mizuno, N.; Nomiya, K.; Weakley, T.J.R.; Finke, R.G. Metal complexes of the lacunary heteropolytungstates [B-α-PW9O34]9− and [α-P2W15O56]12−. Inorg. Synth. 1997, 31, 167–185. [Google Scholar]
- Lyon, D.K.; Miller, W.K.; Novet, T.; Domaille, P.J.; Evitt, E.; Johnson, D.C.; Finke, R.G. Highly oxidation resistant inorganic-porphyrin analog polyoxometalate oxidation catalysts. 1. The synthesis and characterization of aqueous-soluble potassium salts of α2-P2W17O61(Mn+·OH2)(n−10) and organic solvent soluble tetra-n-butylammonium salts of α2-P2W17O61(Mn+·Br)(n−11) (M = Mn3+,Fe3+,Co2+,Ni2+,Cu2+). J. Am. Chem. Soc. 1991, 113, 7209–7221. [Google Scholar]
- Williamson, M.M.; Bouchard, D.A.; Hill, C.L. Characterization of a weak intermolecular photosensitive complex between an organic substrate and a polyoxometalate. Crystal and molecular structure of α-H3PMo12O40·6DMA·CH3CN·0.5H2O (DMA = N,N-dimethylacetamide). Inorg. Chem. 1987, 26, 1436–1441. [Google Scholar] [CrossRef]
- Hill, C.L.; Bouchard, D.A.; Kadkhodayan, M.; Williamson, M.M.; Schmidt, J.A.; Hilinski, E.F. Catalytic photochemical oxidation of organic substrates by polyoxometalates. Picosecond spectroscopy, photochemistry, and structural properties of charge-transfer complexes between heteropolytungstic acids and dipolar organic compounds. J. Am. Chem. Soc. 1988, 110, 5471–5479. [Google Scholar] [CrossRef]
- Niu, J.; You, X.; Duan, C.; Fun, H.; Zhou, Z. A novel optical complex between an organic substrate and a polyoxometalate. Crystal and molecular structure of α-H4SiW12O40·4HMPA·2H2O (HMPA = Hexamethylphosphoramide). Inorg. Chem. 1996, 35, 4211–4217. [Google Scholar] [CrossRef]
- Papaconstantinou, E. Photochemistry of polyoxometalates of molybdenum and tungsten and/or vanadium. Chem. Soc. Rev. 1989, 18, 1–31. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, C.N.; Kato, D.; Kashiwagi, T.; Nagatani, S. Synthesis, X-ray Crystal Structure, and Photochromism of a Sandwich-Type Mono-Aluminum Complex Composed of Two Tri-Lacunary α-Dawson-Type Polyoxotungstates. Materials 2019, 12, 2383. https://doi.org/10.3390/ma12152383
Kato CN, Kato D, Kashiwagi T, Nagatani S. Synthesis, X-ray Crystal Structure, and Photochromism of a Sandwich-Type Mono-Aluminum Complex Composed of Two Tri-Lacunary α-Dawson-Type Polyoxotungstates. Materials. 2019; 12(15):2383. https://doi.org/10.3390/ma12152383
Chicago/Turabian StyleKato, Chika Nozaki, Daichi Kato, Toshifumi Kashiwagi, and Shunpei Nagatani. 2019. "Synthesis, X-ray Crystal Structure, and Photochromism of a Sandwich-Type Mono-Aluminum Complex Composed of Two Tri-Lacunary α-Dawson-Type Polyoxotungstates" Materials 12, no. 15: 2383. https://doi.org/10.3390/ma12152383
APA StyleKato, C. N., Kato, D., Kashiwagi, T., & Nagatani, S. (2019). Synthesis, X-ray Crystal Structure, and Photochromism of a Sandwich-Type Mono-Aluminum Complex Composed of Two Tri-Lacunary α-Dawson-Type Polyoxotungstates. Materials, 12(15), 2383. https://doi.org/10.3390/ma12152383



