Bone Regeneration Using a Three-Dimensional Hexahedron Channeled BCP Block Combined with Bone Morphogenic Protein-2 in Rat Calvarial Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D Hexahedron Channeled Synthetic Block Bone
- Boneplant group: Untreated 3D hexahedron channeled BCP block;
- Boneplant/CMC group: 3D Hexahedron channeled BCP block coated with CMC;
- Boneplant/CMC/BMP group: 3D Hexahedron channeled BCP block coated with CMC containing rhBMP-2.
2.2. Scanning Electron Microscope Surface Analysis
2.3. In Vivo Animal Study
2.3.1. Experimental Animals
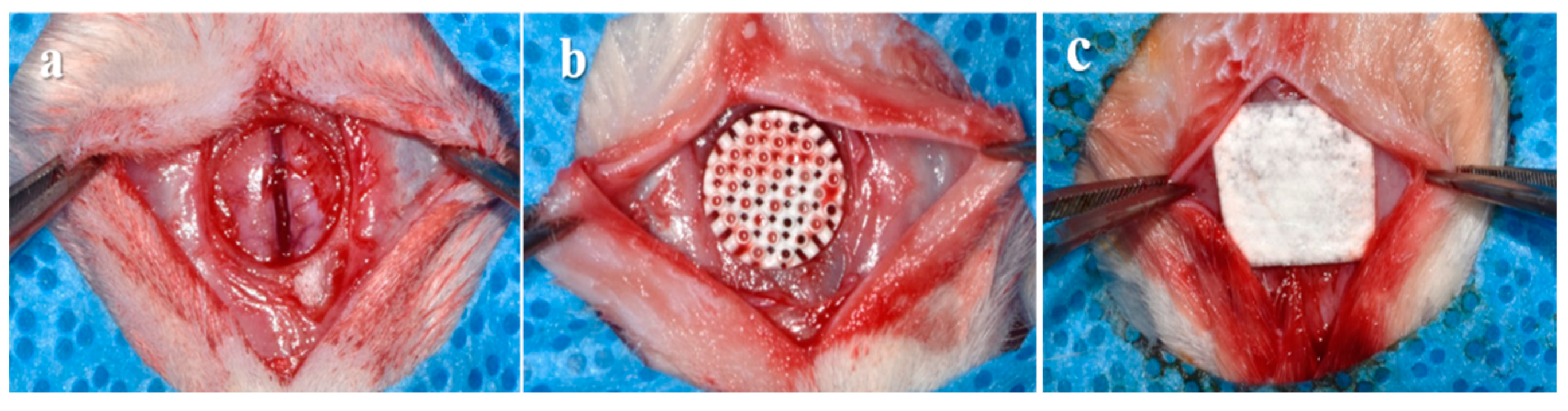
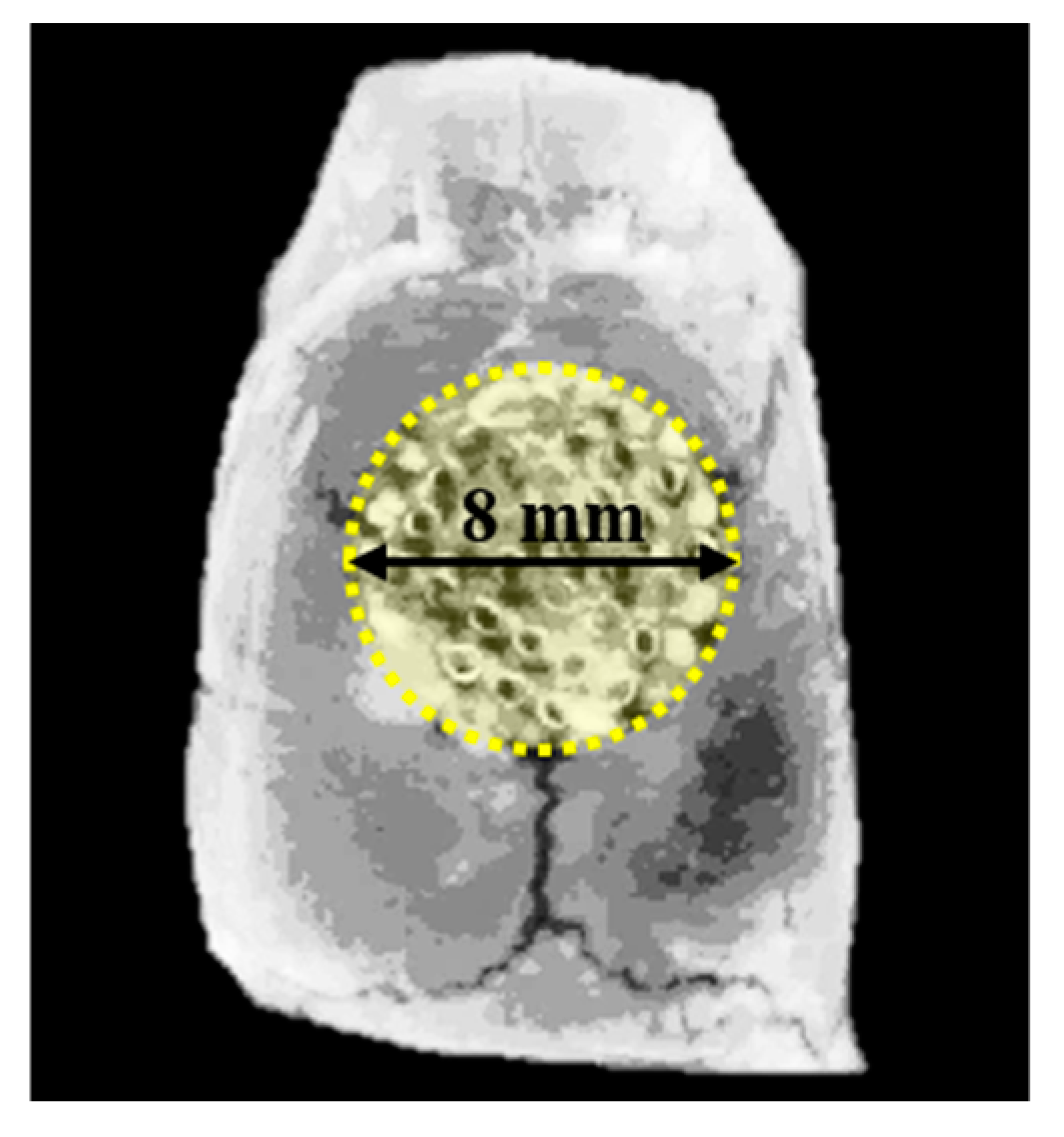
2.3.2. Surgical Procedures
2.3.3. Micro-Computed Tomography (μCT) Analysis
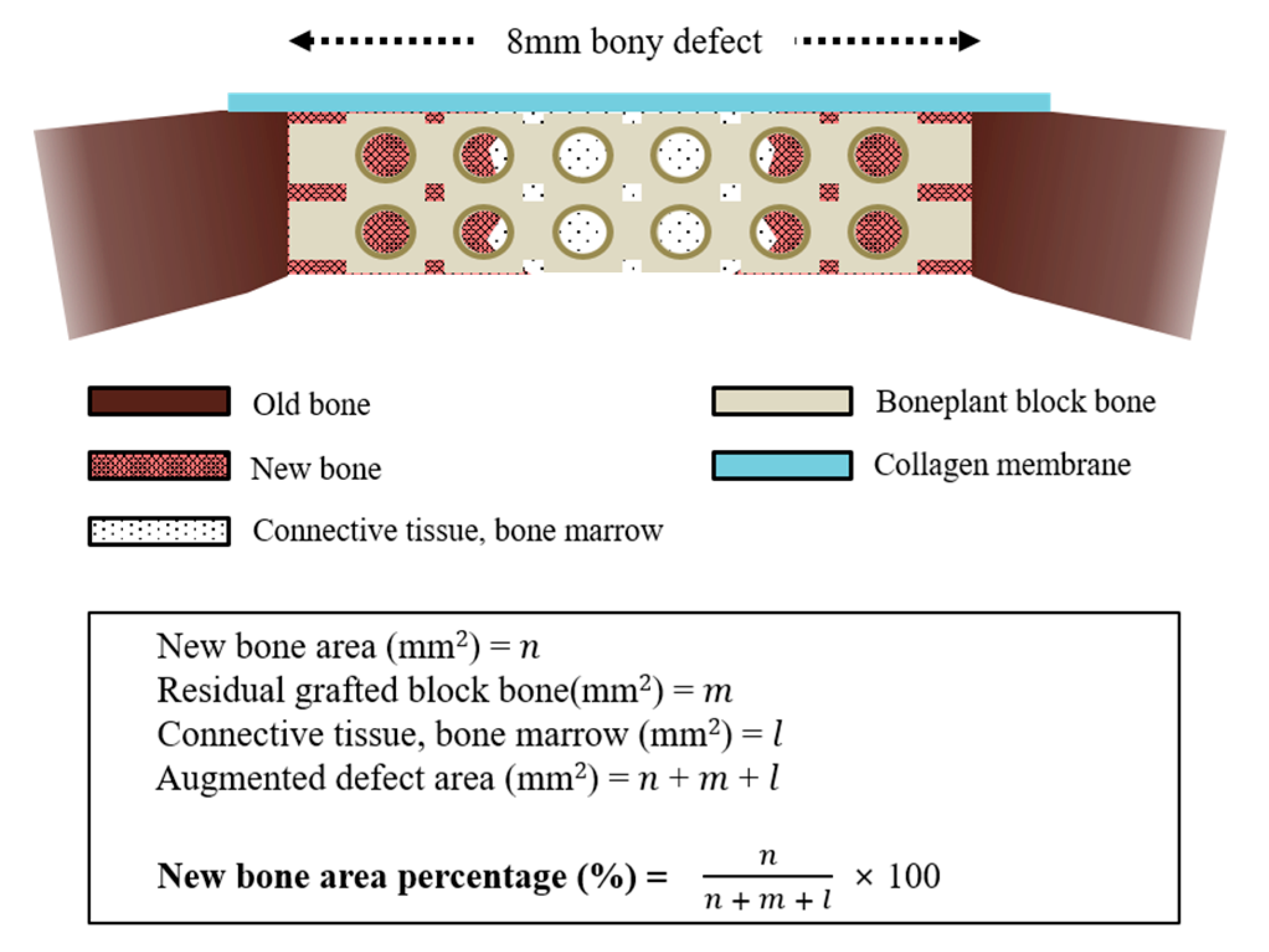
2.3.4. Histologic and Histometric Procedures
2.4. Statistical Analysis
3. Results
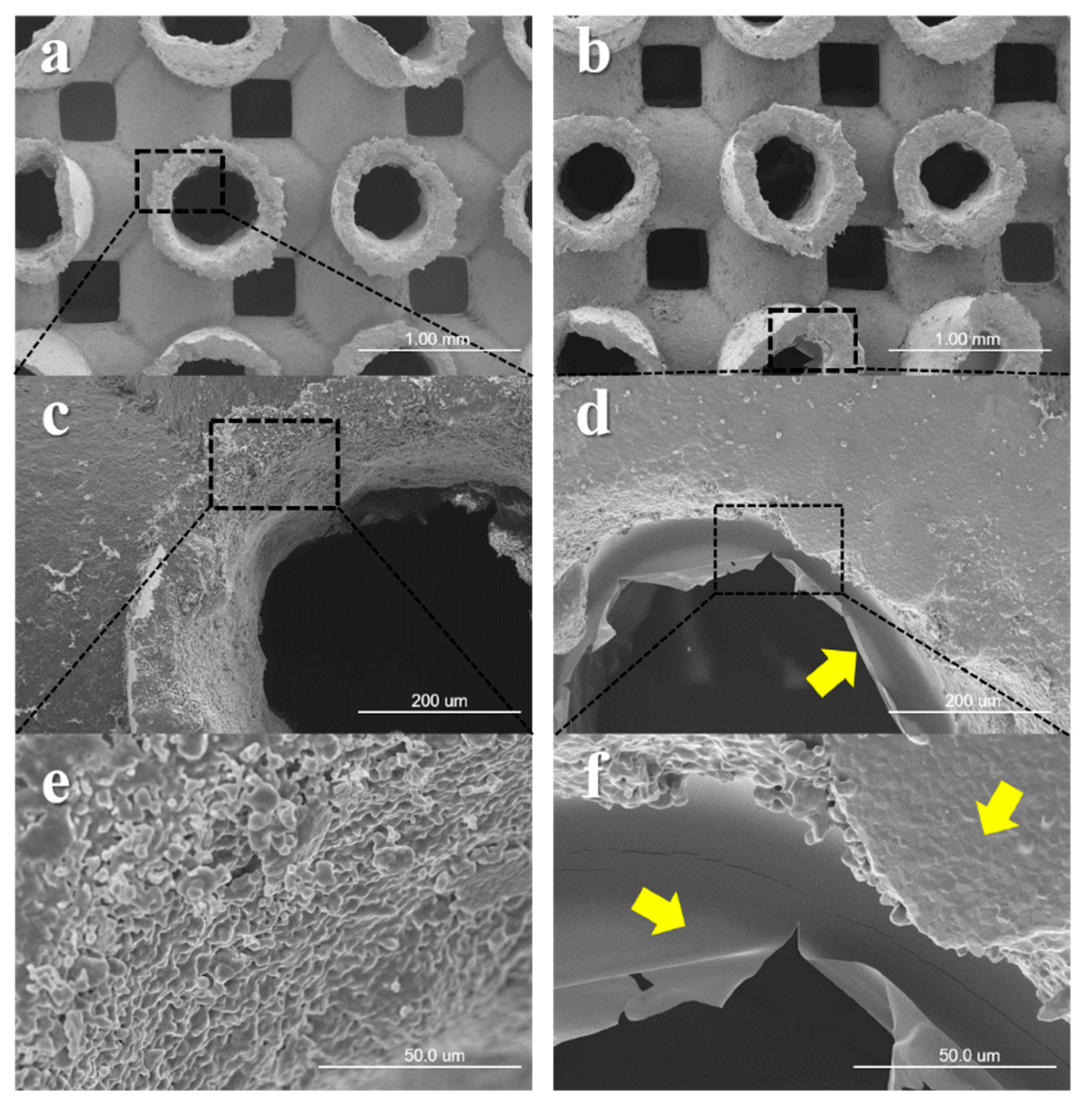
3.1. Observations of Surface Morphology
3.2. In Vivo Animal Study
3.2.1. Clinical Findings
3.2.2. Volumetric Findings
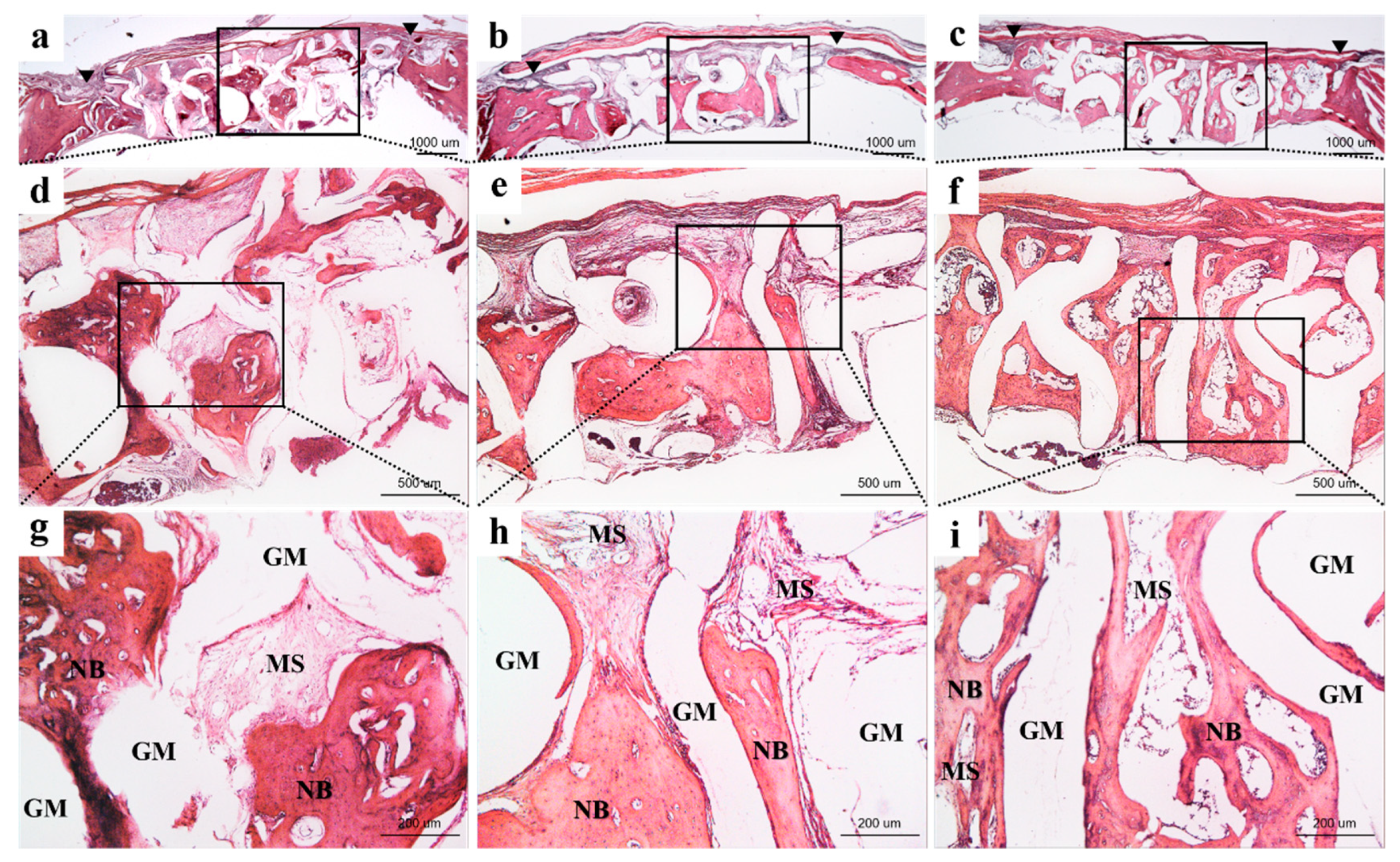
3.2.3. Histological Findings
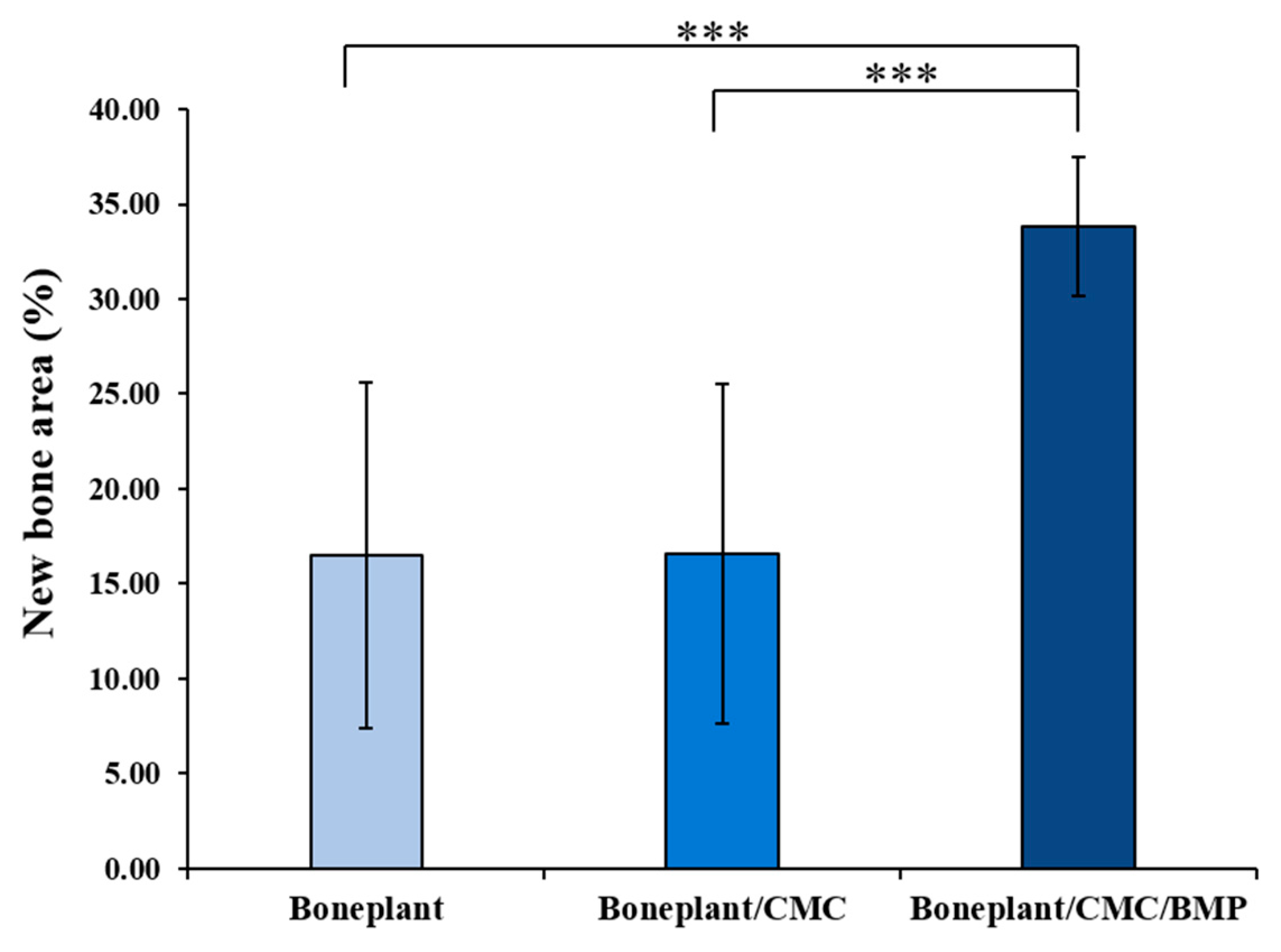
3.2.4. Histometric Findings
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, M.; Rosano, G.; Taschieri, S. Implant survival rates after maxillary sinus augmentation. Eur. J. Oral Sci. 2008, 116, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Guarino, V.; Causa, F.; Ambrosio, L. Bioactive scaffolds for bone and ligament tissue. Expert Rev. Med Devices 2007, 4, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. BioMed Res. Int. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krasny, M.; Krasny, K.; Fiedor, P.; Zadurska, M.; Kamiński, A. Long-term outcomes of the use of allogeneic, radiation-sterilised bone blocks in reconstruction of the atrophied alveolar ridge in the maxilla and mandible. Cell Tissue Bank. 2015, 16, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Borzabadi-Farahani, A.; Nielsen, B. Treatment of labial soft tissue recession around dental implants in the esthetic zone using guided bone regeneration with mineralized allograft: A retrospective clinical case series. J. Oral Maxillofac. Surg. 2016, 74, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Vecchio, K.S. Conversion of natural marine skeletons as scaffolds for bone tissue engineering. Front. Mater. Sci. 2013, 7, 103–117. [Google Scholar] [CrossRef]
- Herford, A.S.; Tandon, R.; Stevens, T.W.; Stoffella, E.; Cicciu, M. Immediate distraction osteogenesis: The sandwich technique in combination with rhBMP-2 for anterior maxillary and mandibular defects. J. Craniofacial Surg. 2013, 24, 1383–1387. [Google Scholar] [CrossRef]
- Cicciù, M.; Herford, A.; Stoffella, E.; Cervino, G.; Cicciù, D. Protein-Signaled Guided Bone Regeneration Using Titanium Mesh and Rh-BMP2 in Oral Surgery: A Case Report Involving Left Mandibular Reconstruction after Tumor Resection. Open Dent. J. 2012, 6, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Athanasiou, V.T.; Papachristou, D.J.; Panagopoulos, A.; Saridis, A.; Scopa, C.D.; Megas, P. Histological comparison of autograft, allograft-DBM, xenograft, and synthetic grafts in a trabecular bone defect: An experimental study in rabbits. Med. Sci. Monit. 2009, 16, BR24–BR31. [Google Scholar]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Asa’Ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-printed scaffolds and biomaterials: Review of alveolar bone augmentation and periodontal regeneration applications. Int. J. Dent. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Boyd, D.; Beyea, S.D.; Bezuhly, M. Enhancement of bone consolidation in mandibular distraction osteogenesis: A contemporary review of experimental studies involving adjuvant therapies. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Moon, T.S.; Yun, M.J.; Jeon, Y.C.; Jeong, C.M.; Cho, D.W.; Huh, J.B. Stimulation of healing within a rabbit calvarial defect by a PCL/PLGA scaffold blended with TCP using solid freeform fabrication technology. J. Mater. Sci. Mater. Med. 2012, 23, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; You, C.; Kim, K.H. Combined effect of a microporous layer and type I collagen coating on a biphasic calcium phosphate scaffold for bone tissue engineering. Materials 2015, 8, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Maeda, H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci. World J. 2013, 2013, 1–21. [Google Scholar] [CrossRef]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes-different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Seong, Y.J.; Kang, I.G.; Song, E.H.; Kim, H.E.; Jeong, S.H.; Seong, Y.; Kang, I.; Song, E.; Kim, H.; Jeong, S. Calcium phosphate-collagen scaffold with aligned pore channels for enhanced osteochondral regeneration. Adv. Heal. Mater. 2017, 6, 1700966. [Google Scholar] [CrossRef]
- Lobo, S.E.; Arinzeh, T.L. Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.C.; Zhang, M.L.; Lee, J.S.; Jung, U.W.; Choi, S.H. Effect of different hydroxyapatite: β–Tricalcium phosphate ratios on the osteoconductivity of biphasic calcium phosphate in the rabbit sinus model. Int. J. Oral Maxillofac. Implant. 2015, 30, 56–72. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Electron. 2003, 14, 201–209. [Google Scholar] [CrossRef]
- Herford, A.S.; Lü, M.; Akin, L.; Cicciù, M. Evaluation of a porcine matrix with and without platelet-derived growth factor for bone graft coverage in pigs. Int. J. Oral Maxillofac. Implant. 2012, 27, 1351–1358. [Google Scholar]
- Chan, O.; Coathup, M.; Nesbitt, A.; Ho, C.Y.; Hing, K.; Buckland, T.; Campion, C.; Blunn, G. The effects of microporosity on osteoinduction of calcium phosphate bone graft substitute biomaterials. Acta Biomater. 2012, 8, 2788–2794. [Google Scholar] [CrossRef] [PubMed]
- Solofomalala, G.D.; Guery, M.; Lesiourd, A.; Le Huec, J.C.; Chauveaux, D.; Laffenetre, O. Bone morphogenetic proteins: From their discoveries till their clinical applications. Eur. J. Orthop. Surg. Traumatol. 2007, 17, 609–615. [Google Scholar] [CrossRef]
- Kim, H.C.; Kim, S.N.; Lee, J.Y.; Kim, U.C. Successful strategy of treatment using rhBMP-2 for maxillary sinus graft. J. Korean Dent. Assoc. 2015, 53, 14–27. [Google Scholar]
- Liu, S.; Liu, Y.; Jiang, L.; Li, Z.; Lee, S.; Liu, C.; Wang, J.; Zhang, J. Recombinant human BMP-2 accelerates the migration of bone marrow mesenchymal stem cells via the CDC42/PAK1/LIMK1 pathway in vitro and in vivo. Biomater. Sci. 2018, 7, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Dohi, Y.; Horiuchi, K.; Ohgushi, H.; Noshi, T.; Yoshikawa, T.; Yamamoto, K.; Sugimura, M. Recombinant human bone morphogenetic protein-2 promotes osteogenesis within atelopeptide type I collagen solution by combination with rat cultured marrow cells. J. Biomed. Mater. Res. 2002, 60, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Vehof, J.; Ruhe, P.; Kroeze-Deutman, H.; Kuboki, Y.; Takita, H.; Hedberg, E.; Mikos, A. Growth factor-loaded scaffolds for bone engineering. J. Controll. Release 2005, 101, 127–136. [Google Scholar] [CrossRef]
- Babensee, J.E.; McIntire, L.V.; Mikos, A.G. Growth Factor Delivery for Tissue Engineering. Pharm. Res. 2000, 17, 497–504. [Google Scholar] [CrossRef]
- Epstein, N.E. Pros, cons, and costs of INFUSE in spinal surgery. Surg. Neurol. Int. 2011, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S.; Cicciù, M.; Eftimie, L.F.; Miller, M.; Signorino, F.; Famà, F.; Cervino, G.; Lo Giudice, G.; Bramanti, E.; Lauritano, F. rhBMP-2 applied as support of distraction osteogenesis: A split–mouth histological study over nonhuman primates mandibles. Int. J. Clin. Exp. Med. 2016, 9, 17187–17194. [Google Scholar]
- Blokhuis, T.J. Formulations and delivery vehicles for bone morphogenetic proteins: Latest advances and future directions. Injury 2009, 40, S8–S11. [Google Scholar] [CrossRef]
- Salama, A.; El-Sakhawy, M. Preparation of polyelectrolyte/calcium phosphate hybrids for drug delivery application. Carbohydr. Polym. 2014, 113, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Liuyun, J.; Yubao, L.; Chengdong, X. A novel composite membrane of chitosan–carboxymethyl cellulose polyelectrolyte complex membrane filled with nano–hydroxyapatite I. Preparation and properties. J. Mater. Sci. Mater. Med. 2009, 20, 1645–1652. [Google Scholar] [CrossRef]
- Stelzer, G.I.; Klug, E. Carboxymethylcellulose. In Handbook of Watersoluble Gums and Resins; McGraw-Hill: New York, NY, USA, 1980; pp. 1–4. [Google Scholar]
- Barbucci, R.; Magnani, A.; Consumi, M. swelling behavior of carboxymethylcellulose hydrogels in relation to cross-linking, pH, and charge density. Macromolecules 2000, 33, 7475–7480. [Google Scholar] [CrossRef]
- Schweizer, S.; Taubert, A. Polymer-controlled, bio-inspired calcium phosphate mineralization from aqueous solution. Macromol. Biosci. 2007, 7, 1085–1099. [Google Scholar] [CrossRef]
- Salama, A.; Abou-Zeid, R.E.; El-Sakhawy, M.; El-Gendy, A.A. Carboxymethyl cellulose/silica hybrids as templates for calcium phosphate biomimetic mineralization. Int. J. Boil. Macromol. 2015, 74, 155–161. [Google Scholar] [CrossRef]
- Santa-Comba, A.; Pereira, A.; Lemos, R.; Santos, D.; Amarante, J.; Pinto, M.; Tavares, P.; Bahia, F. Evaluation of carboxymethylcellulose, hydroxypropylmethylcellulose, and aluminum hydroxide as potential carriers for rhBMP-2. J. Biomed. Mater. Res. 2001, 55, 396–400. [Google Scholar] [CrossRef]
- Cook, S.D.; Salkeld, S.L.; Patron, L.P. Bone defect healing with an osteogenic protein-1 device combined with carboxymethylcellulose. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 137–145. [Google Scholar] [CrossRef]
- Yoo, H.S.; Bae, J.H.; Kim, S.E.; Bae, E.B.; Kim, S.Y.; Choi, K.H.; Moon, K.O.; Jeong, C.M.; Huh, J.B. The effect of bisphasic calcium phosphate block bone graft materials with polysaccharides on bone regeneration. Materials 2017, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Hollister, S.J. Porous scaffold design for tissue engineering. Nat. Mater. 2005, 4, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.C.; Kang, J.H.; Cha, J.K.; Lee, J.S.; Paik, J.W.; Jung, U.W.; Choi, S.H. Bone regeneration using three-dimensional hexahedron channel structured BCP block in rabbit calvarial defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019. [Google Scholar] [CrossRef] [PubMed]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jeong, B. The effect of hyaluronate-carboxymethyl cellulose on bone graft substitute healing in a rat spinal fusion model. J. Korean Neurosurg. Soc. 2011, 50, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Cicciù, M.; Cervino, G.; Herford, A.S.; Famà, F.; Bramanti, E.; Fiorillo, L.; Lauritano, F.; Sambataro, S.; Troiano, G.; Laino, L. Facial bone reconstruction using both marine or non-marine bone substitutes: Evaluation of current outcomes in a systematic literature review. Mar. Drugs 2018, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomater. 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Bai, F.; Wang, Z.; Lu, J.; Liu, J.; Chen, G.; Lv, R.; Wang, J.; Lin, K.; Zhang, J.; Huang, X. The Correlation Between the Internal Structure and Vascularization of Controllable Porous Bioceramic Materials In Vivo: A Quantitative Study. Tissue Eng. Part A 2010, 16, 3791–3803. [Google Scholar] [CrossRef]
- Frame, J.W.; Rout, P.; Browne, R. Ridge augmentation using solid and porous hydroxylapatite particles with and without autogenous bone or plaster. J. Oral Maxillofac. Surg. 1987, 45, 771–777. [Google Scholar] [CrossRef]
- Wei, M.Z.; Da, P.W.; Jian, Y.X. Biological characteristics and clinical application of scaffold materials for bone tissue engineering. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 9781. [Google Scholar]
- Graziano, A.; d’Aquino, R.; Angelis, M.G.C.D.; De Francesco, F.; Giordano, A.; Laino, G.; Piattelli, A.; Traini, T.; De Rosa, A.; Papaccio, G. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J. Cell. Phys. 2008, 214, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials 1996, 17, 31–35. [Google Scholar] [CrossRef]
- Lundgren, A.; Sennerby, L.; Lundgren, D. Guided jaw-bone regeneration using an experimental rabbit model. Int. J. Oral Maxillofac. Surg. 1998, 27, 135–140. [Google Scholar] [CrossRef]
- Ripamonti, U.; Richter, P.W.; Thomas, M.E. Self-inducing shape memory geometric cues embedded within smart hydroxyapatite-based biomimetic matrices. Plast. Reconstr. Surg. 2007, 120, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.M.; Ko, L.Y.; Huang, T.J. Effect of pore size on ECM secretion and cell growth in gelatin scaffold for articular cartilage tissue engineering. Acta Biomater. 2009, 5, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Harley, B.A.; Kim, H.D.; Zaman, M.H.; Yannas, I.V.; Lauffenburger, D.A.; Gibson, L.J. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys. J. 2008, 95, 4013–4024. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Fang, H.Y.; Hsu, T.T.; Lin, C.Y.; Shie, M.Y. The characteristics of mineral trioxide aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef]
- Agrawal, V.; Sinha, M. A review on carrier systems for bone morphogenetic protein-2. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 904–925. [Google Scholar] [CrossRef]
- Kruyt, M.C.; Wilson, C.E.; De Bruijn, J.D.; Van Blitterswijk, C.A.; Oner, C.F.; Verbout, A.J.; Dhert, W.J. The effect of cell-based bone tissue engineering in a goat transverse process model. Biomaterials. 2006, 27, 5099–5106. [Google Scholar] [CrossRef]
- Lim, H.P.; Mercado-Pagan, A.E.; Yun, K.D.; Kang, S.S.; Choi, T.H.; Bishop, J.; Koh, J.T.; Maloney, W.; Lee, K.M.; Yang, Y.P. The effect of rhBMP-2 and PRP delivery by biodegradable β-tricalcium phosphate scaffolds on new bone formation in a non-through rabbit cranial defect model. J. Mater. Sci. Mater. Med. 2013, 24, 1895–1903. [Google Scholar] [CrossRef]
- Magne, D.; Bluteau, G.; Lopez-Cazaux, S.; Weiss, P.; Pilet, P.; Ritchie, H.H.; Daculsi, G.; Guicheux, J. Development of an odontoblast in vitro model to study dentin mineralization. Connect. Tissue Res. 2004, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Choi, S.W.; Choi, J.W.; Paik, D.H.; Kang, S.S.; Kim, S.E.; Jeon, Y.C.; Huh, J.B. Effects of different rhBMP-2 release profiles in defect areas around dental implants on bone regeneration. Biomed. Mater. 2015, 10, 45007. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Arakawa, T.; Mano, H.; Kaneda, T.; Ogasawara, A.; Nakagawa, M.; Toyama, Y.; Yabe, Y.; Kumegawa, M.; Hakeda, Y. Direct stimulation of osteoclastic bone resorption by bone morphogenetic protein (BMP)-2 and expression of BMP receptors in mature osteoclasts. Bone 2000, 27, 479–486. [Google Scholar] [CrossRef]
- Takaoka, K.; Saito, N. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 2003, 24, 2287–2293. [Google Scholar]
- Fukui, T.; Ii, M.; Shoji, T.; Matsumoto, T.; Mifune, Y.; Kawakami, Y.; Akimaru, H.; Kawamoto, A.; Kuroda, T.; Saito, T.; et al. Therapeutic effect of local administration of low-dose simvastatin-conjugated gelatin hydrogel for fracture healing. J. Bone Miner. Res. 2012, 27, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Monjo, M.; Rubert, M.; Wohlfahrt, J.C.; Rønold, H.J.; Ellingsen, J.E.; Lyngstadaas, S.P. In vivo performance of absorbable collagen sponges with rosuvastatin in critical-size cortical bone defects. Acta Biomater. 2010, 6, 1405–1412. [Google Scholar] [CrossRef]
- Walter, M.S.; Frank, M.J.; Rubert, M.; Monjo, M.; Lyngstadaas, S.P.; Haugen, H.J. Simvastatin-activated implant surface promotes osteoblast differentiation in vitro. J. Biomater. Appl. 2014, 28, 897–908. [Google Scholar] [CrossRef]
- Park, J.C.; Bae, E.B.; Kim, S.E.; Kim, S.Y.; Choi, K.H.; Choi, J.W.; Bae, J.H.; Ryu, J.J.; Huh, J.B. Effects of BMP-2 delivery in calcium phosphate bone graft materials with different compositions on bone regeneration. Materials 2016, 9, 954. [Google Scholar] [CrossRef]


| Groups | Mean | SD | P-Value | |
|---|---|---|---|---|
| New Bone Volume (%) | Boneplant | 10.77 | 4.87 | 0.013 * |
| Boneplant/CMC | 10.72 | 3.29 | ||
| Boneplant/CMC/BMP | 20.12 | 2.17 |
| Groups | Mean | SD | P-Value | |
|---|---|---|---|---|
| New Bone Area (%) | Boneplant | 16.48 | 9.11 | 0.000 *** |
| Boneplant/CMC | 16.57 | 8.94 | ||
| Boneplant/CMC/BMP | 33.79 | 3.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-Y.; Bae, E.-B.; Huh, J.-W.; Ahn, J.-J.; Bae, H.-Y.; Cho, W.-T.; Huh, J.-B. Bone Regeneration Using a Three-Dimensional Hexahedron Channeled BCP Block Combined with Bone Morphogenic Protein-2 in Rat Calvarial Defects. Materials 2019, 12, 2435. https://doi.org/10.3390/ma12152435
Kim S-Y, Bae E-B, Huh J-W, Ahn J-J, Bae H-Y, Cho W-T, Huh J-B. Bone Regeneration Using a Three-Dimensional Hexahedron Channeled BCP Block Combined with Bone Morphogenic Protein-2 in Rat Calvarial Defects. Materials. 2019; 12(15):2435. https://doi.org/10.3390/ma12152435
Chicago/Turabian StyleKim, So-Yeun, Eun-Bin Bae, Jae-Woong Huh, Jong-Ju Ahn, Hyun-Young Bae, Won-Tak Cho, and Jung-Bo Huh. 2019. "Bone Regeneration Using a Three-Dimensional Hexahedron Channeled BCP Block Combined with Bone Morphogenic Protein-2 in Rat Calvarial Defects" Materials 12, no. 15: 2435. https://doi.org/10.3390/ma12152435
APA StyleKim, S.-Y., Bae, E.-B., Huh, J.-W., Ahn, J.-J., Bae, H.-Y., Cho, W.-T., & Huh, J.-B. (2019). Bone Regeneration Using a Three-Dimensional Hexahedron Channeled BCP Block Combined with Bone Morphogenic Protein-2 in Rat Calvarial Defects. Materials, 12(15), 2435. https://doi.org/10.3390/ma12152435





