Preparation of Carbon-Silicon Doping Composite Adsorbent Material for Removal of VOCs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
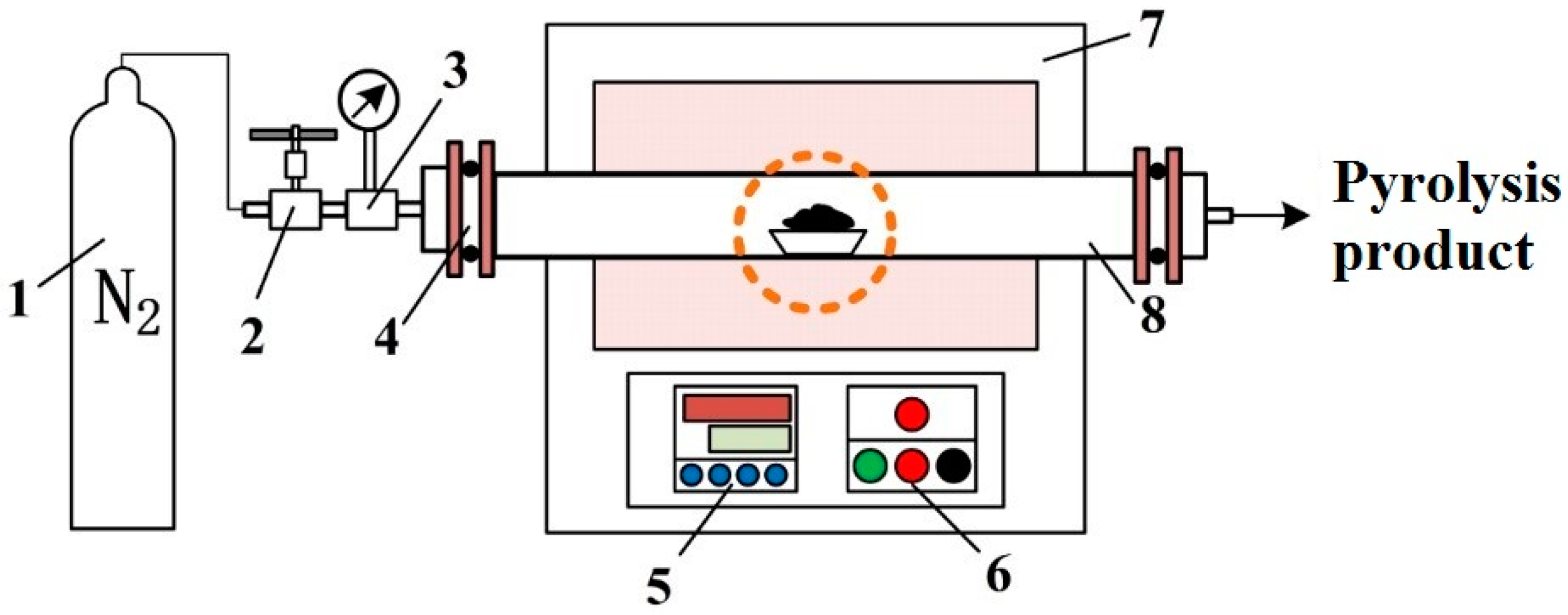
2.2. Technological Process
2.3. Optimization of Operational Conditions
2.4. Properties Characterization
2.5. Dynamic Adsorption Test
2.5.1. Adsorption and Desorption of p-xylene
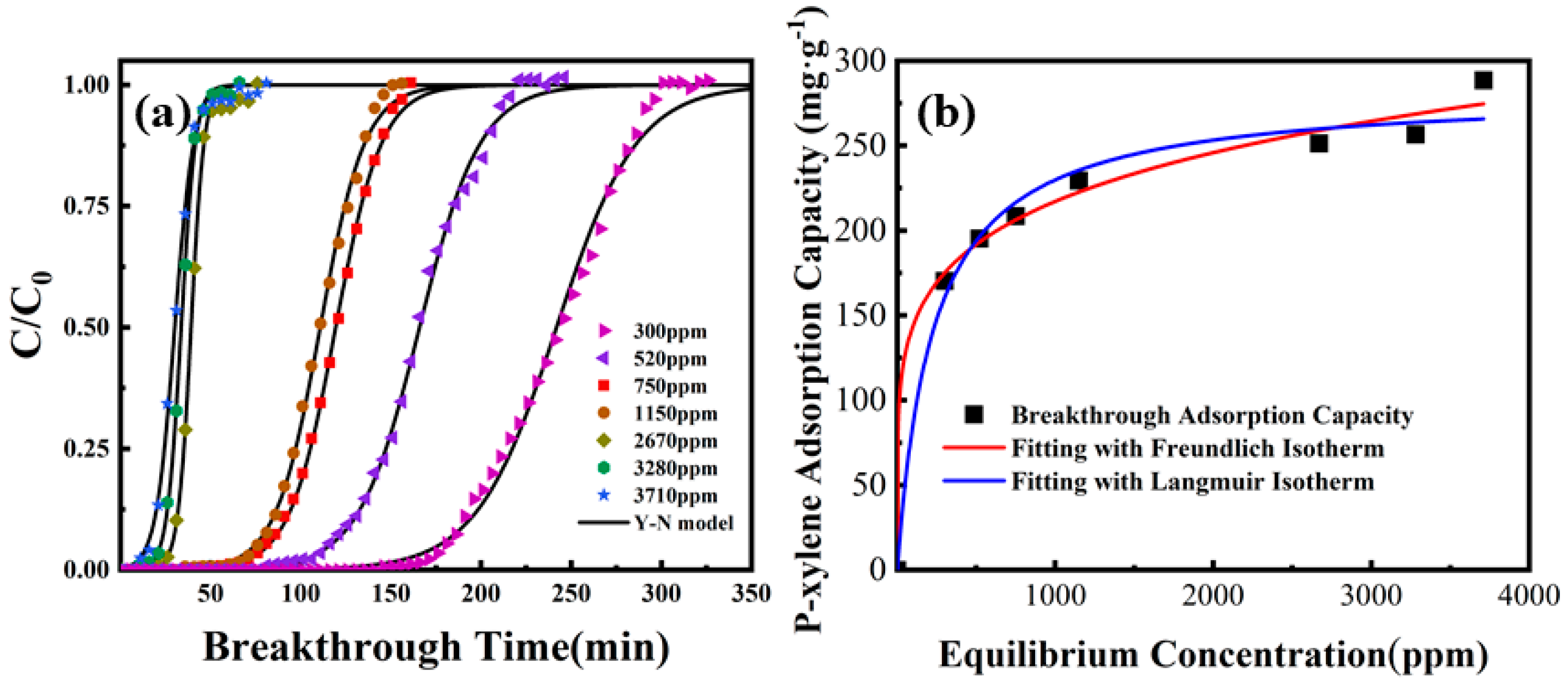
2.5.2. Adsorption Isotherm
2.5.3. Adsorption Kinetics
3. Results and Discussion
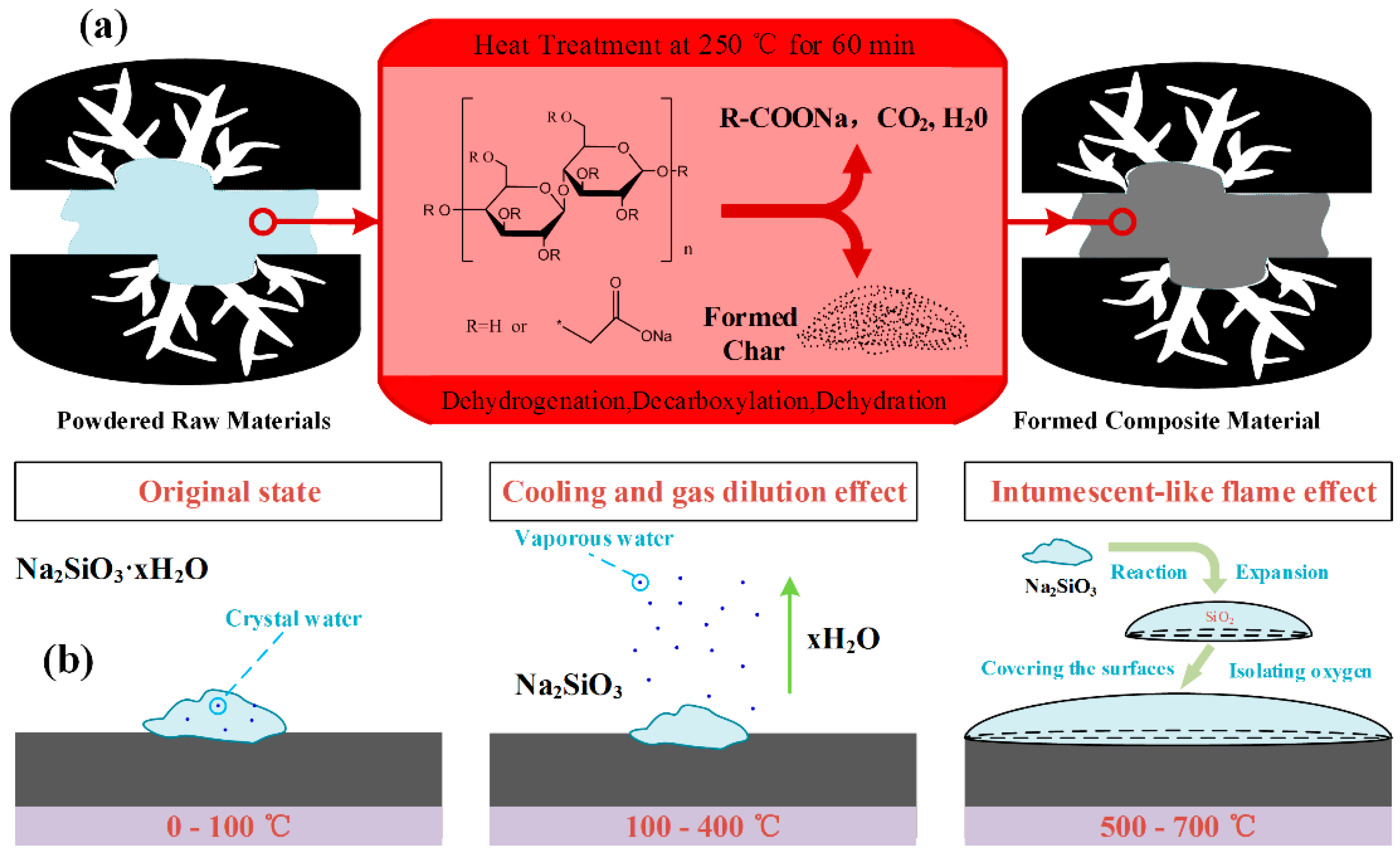
3.1. Silicon Doping on Activated Carbon
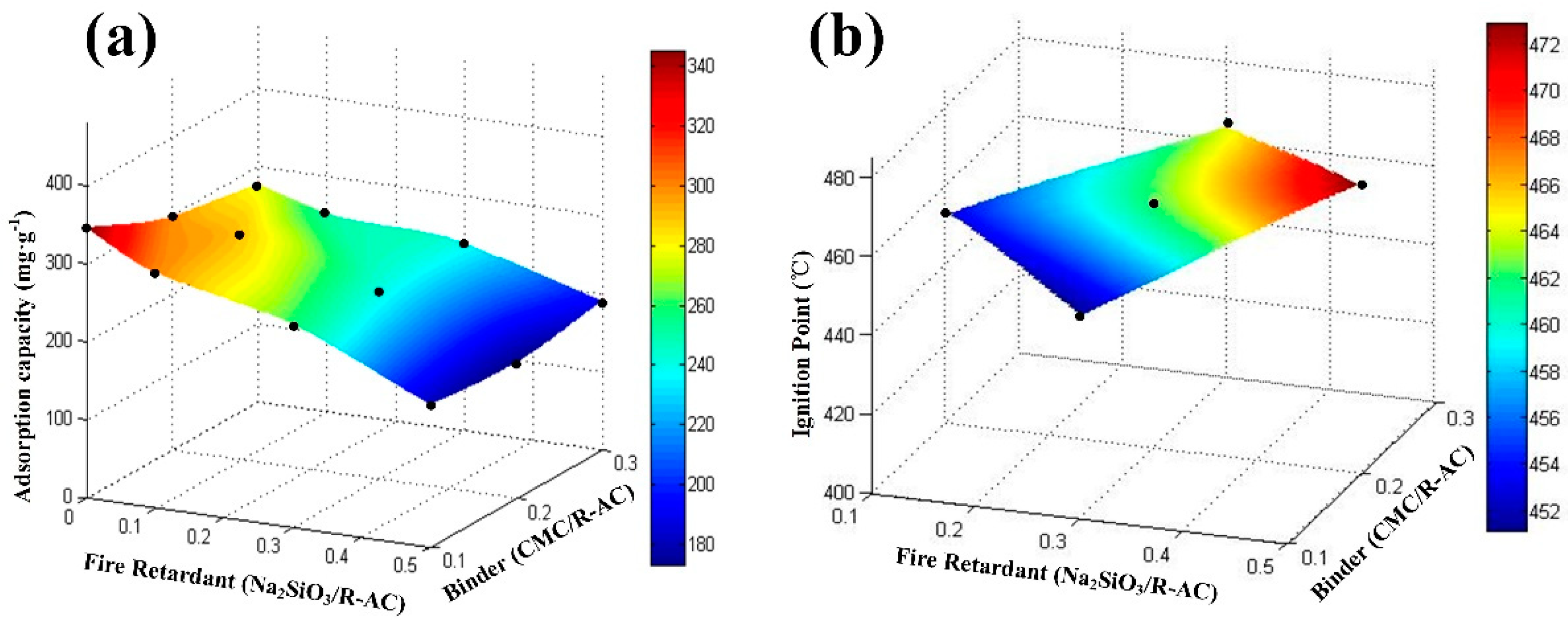
3.2. Optimizing Conditions of Preparation
3.3. Characteristic of Adsorbing Material
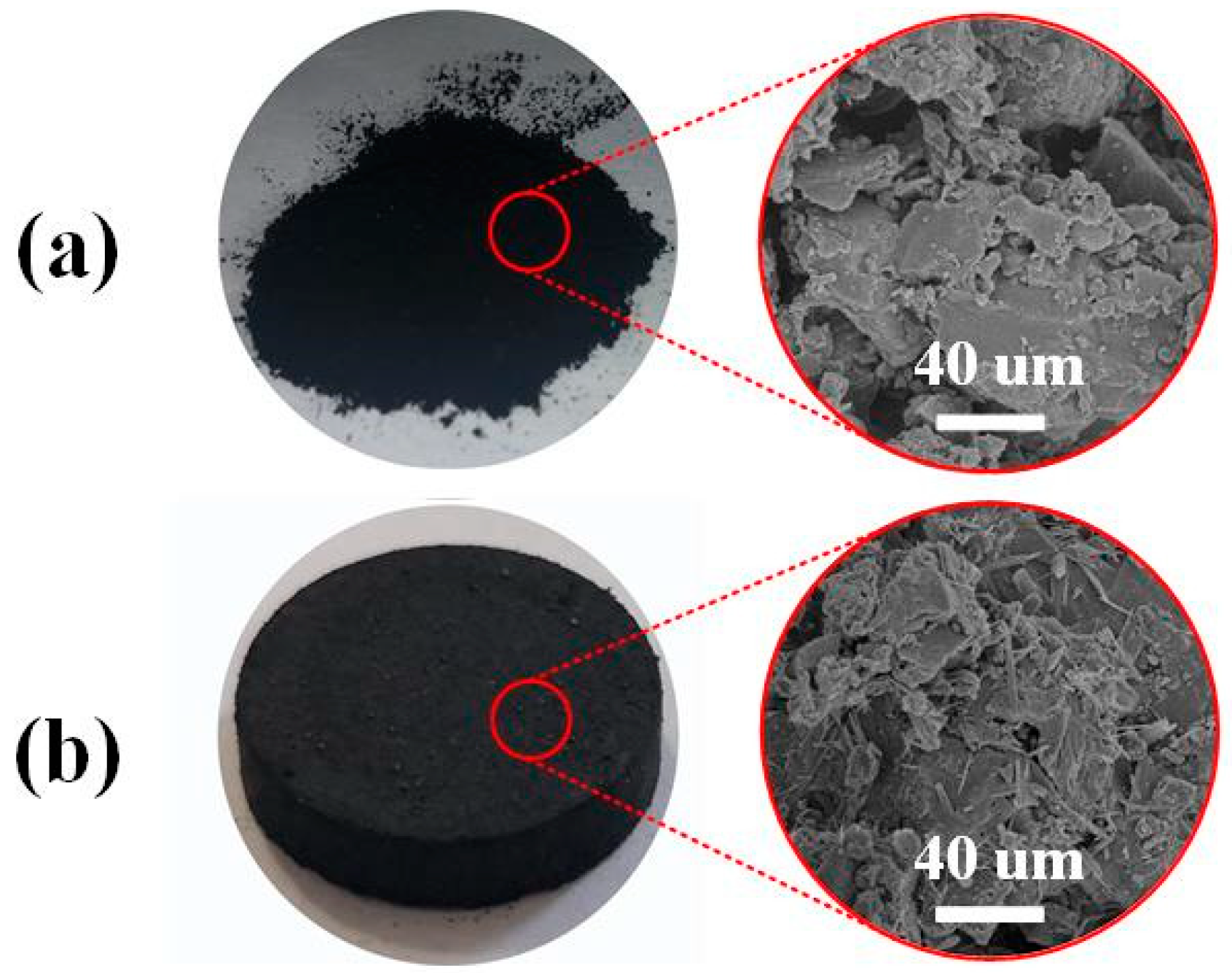
3.3.1. Scanning Electron Microscope (SEM)
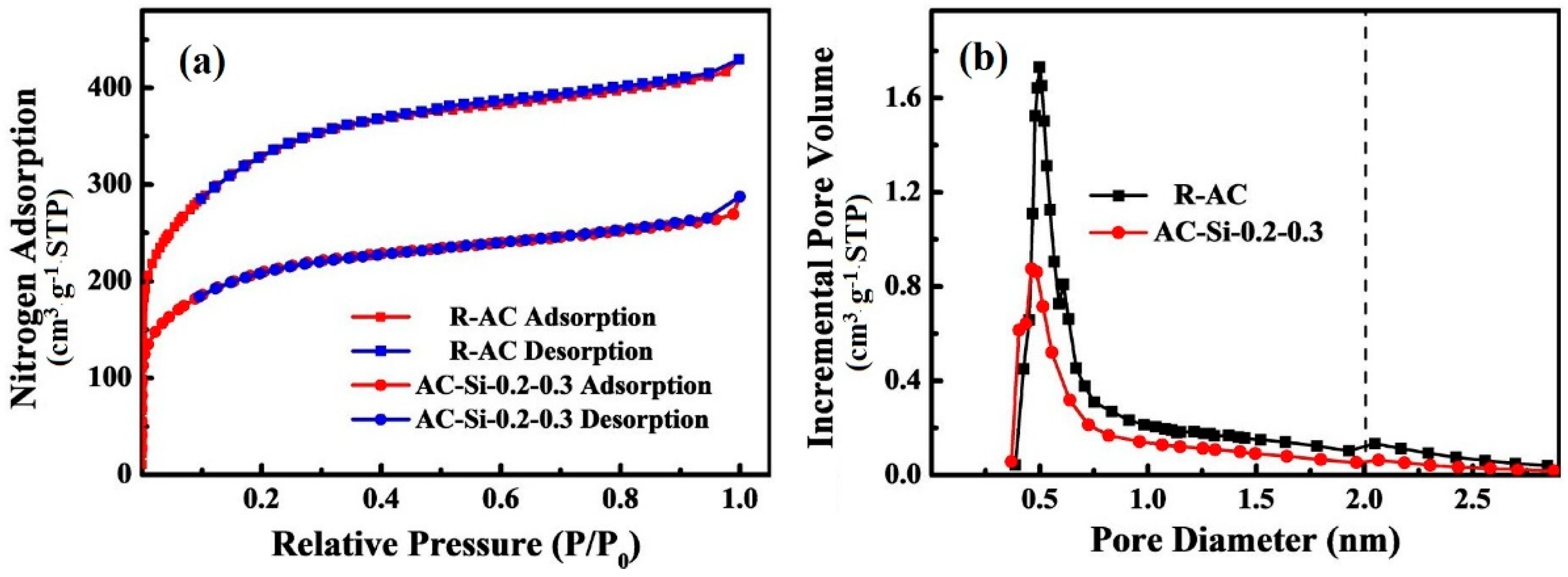
3.3.2. Nitrogen Adsorption and Desorption (BET)
3.3.3. Infrared Spectroscopy (IR)
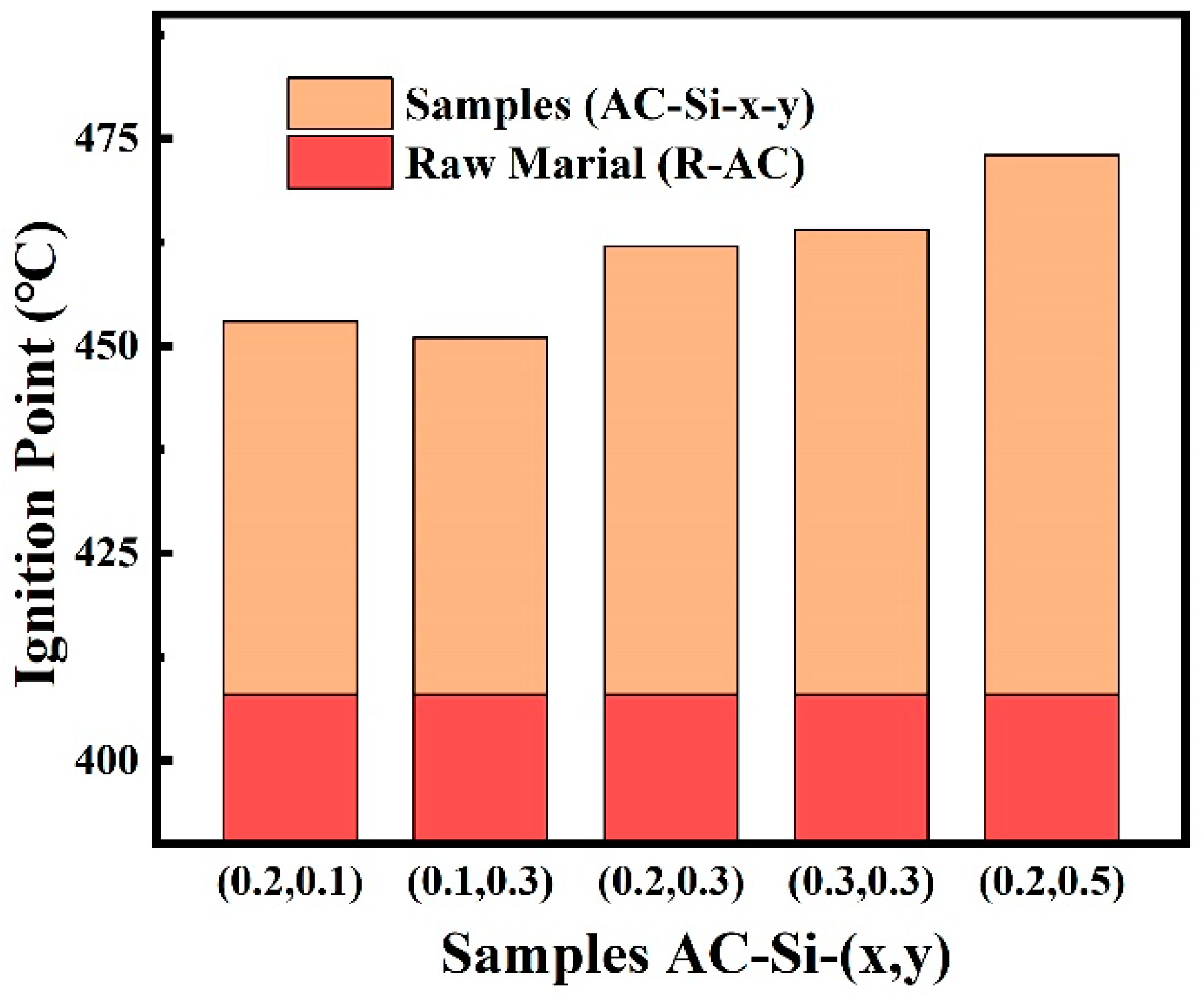
3.3.4. Ignition Point
3.4. Adsorption Behaviors to p-xylene
3.4.1. Effects of Inlet Concentrations
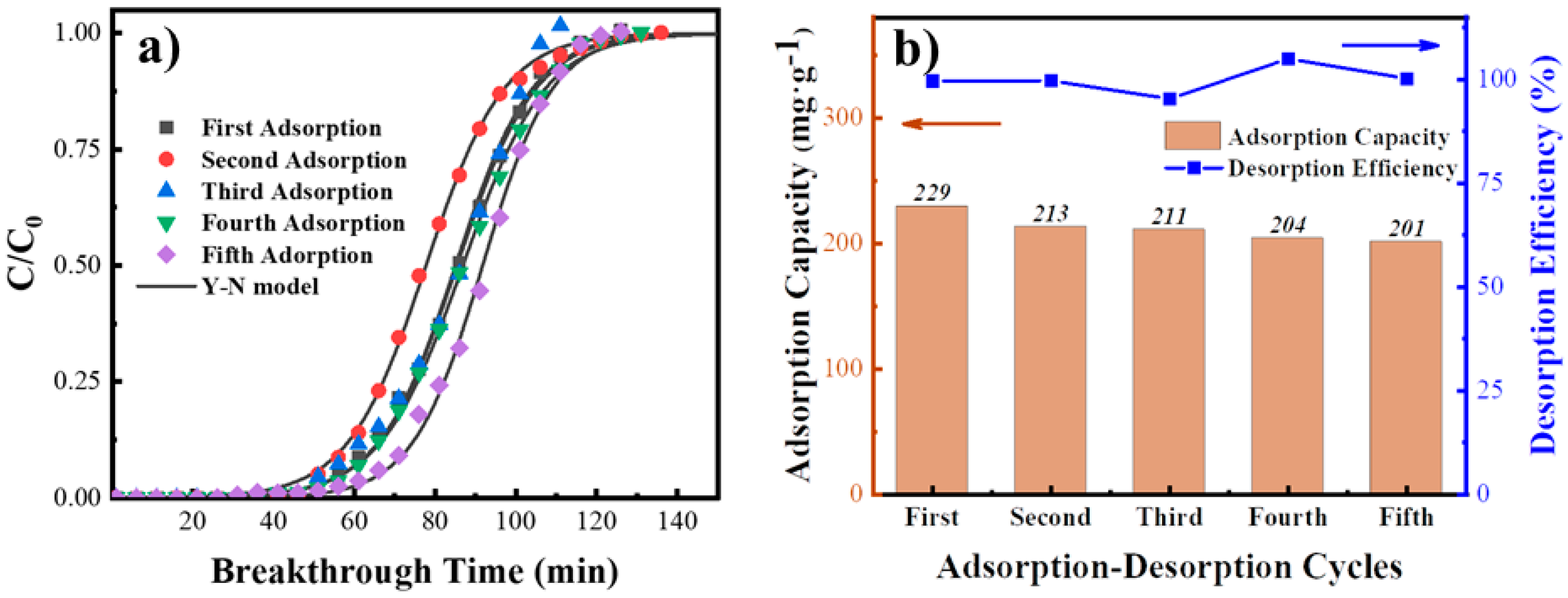
3.4.2. Cycle Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Atkinson, R.; Arey, J. Atmospheric degradation of volatile organic compounds. Chem. Rev. 2003, 103, 4605–4638. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Mo, Z.; Shao, M.; Lu, S.; Xie, S. Screening the emission sources of volatile organic compounds (VOCs) in China by multi-effects evaluation. Front. Environ. Sci. Eng. 2016, 10, 1. [Google Scholar] [CrossRef]
- Zhou, J.; You, Y.; Bai, Z.; Hu, Y.; Zhang, J.; Zhang, N. Health risk assessment of personal inhalation exposure to volatile organic compounds in Tianjin, China. Sci. Total Environ. 2011, 409, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-S.; Yi, J. Simulation and optimization on the regenerative thermal oxidation of volatile organic compounds. Chem. Eng. J. 2000, 76, 103–114. [Google Scholar] [CrossRef]
- Dunn, R.F.; El-Halwagi, M.M. Selection of optimal VOC-condensation systems. Waste Manag. 1994, 14, 103–113. [Google Scholar] [CrossRef]
- Lou, J.-C.; Huang, S.-W. Treating isopropyl alcohol by a regenerative catalytic oxidizer. Sep. Purif. Technol. 2008, 62, 71–78. [Google Scholar] [CrossRef]
- Zabiegaja, D.; Cacciab, M.; Cascob, M.E.; Raveraa, F.; Narcisob, J. Synthesis of carbon monoliths with a tailored hierarchical pore structure for selective CO2 capture. J. CO2 Util. 2018, 26, 36–44. [Google Scholar] [CrossRef]
- Sang, P.-L.; Wang, Y.-Y.; Zhang, L.-Y.; Chai, L.-Y.; Wang, H.-Y. Effective adsorption of sulfate ions with poly (m-phenylenediamine) in aqueous solution and its adsorption mechanism. Trans. Nonferrous Met. Soc. China 2013, 23, 243–252. [Google Scholar] [CrossRef]
- Hong, S.; Liu, H.; Ping, A.; Lin, H.; Li, X.; Shan, C. Application of silica gel in removing high concentrations toluene vapor by adsorption and desorption process. J. Taiwan Inst. Chem. Eng. 2017, 74, 218–224. [Google Scholar]
- Sui, H.; An, P.; Li, X.; Cong, S.; He, L. Removal and recovery of o-xylene by silica gel using vacuum swing adsorption. Chem. Eng. J. 2017, 316, 232–242. [Google Scholar] [CrossRef]
- Oh, K.-J.; Park, D.-W.; Kim, S.-S.; Park, S.-W. Breakthrough data analysis of adsorption of volatile organic compounds on granular activated carbon. Korean J. Chem. Eng. 2010, 27, 632–638. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chung, T.-W.; Huang, C.-M.; Wu, H. Adsorption equilibria of acetate compounds on activated carbon, silica gel, and 13X zeolite. J. Chem. Eng. Data 2005, 50, 811–816. [Google Scholar] [CrossRef]
- Delage, F.; Pré, P.; Le Cloirec, P. Mass transfer and warming during adsorption of high concentrations of VOCs on an activated carbon bed: Experimental and theoretical analysis. Environ. Sci. Technol. 2000, 34, 4816–4821. [Google Scholar] [CrossRef]
- Sui, H.; Jiang, P.; Li, X.; Liu, J.; Li, X.; He, L. Binary Adsorption Equilibrium and Breakthrough of n-Butyl Acetate and p-Xylene on Granular Activated Carbon. Ind. Eng. Chem. Res. 2019, 58, 8279–8289. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Nelson, J.H. Application of gas adsorption kinetics. I. A theoretical model for respirator cartridge service life. Am. Ind. Hyg. Assoc. J. 1984, 45, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, M.; Zeng, Q. Adsorption of chromium (VI) by strong alkaline anion exchange fiber in a fixed-bed column: Experiments and models fitting and evaluating. Sep. Purif. Technol. 2015, 149, 16–23. [Google Scholar] [CrossRef]
- Jakab, E.; Mészáros, E.; Borsa, J. Effect of slight chemical modification on the pyrolysis behavior of cellulose fibers. J. Anal. Appl. Pyrolysis 2010, 87, 117–123. [Google Scholar] [CrossRef]
- Tsuyumoto, I. High flame retardancy of amorphous sodium silicate on poly(ethylene-co-vinyl acetate) (EVA). Polym. Bull. 2018, 75, 4967–4976. [Google Scholar] [CrossRef]



| Samples | Adsorption Capacity | Y-N Model Fitting Parameters | ||
|---|---|---|---|---|
| (mg·g−1) | KYN (min−1) | τ (min) | *R2 (%) | |
| AC-Si-0.1-0 | 345.3 | 0.073 | 115.4 | 99.68 |
| AC-Si-0.2-0 | 298.6 | 0.109 | 67.4 | 99.83 |
| AC-Si-0.3-0 | 277.2 | 0.140 | 37.3 | 99.22 |
| AC-Si-0.1-0.1 | 300.2 | 0.078 | 130.7 | 99.90 |
| AC-Si-0.2-0.1 | 288.5 | 0.074 | 120.5 | 99.89 |
| AC-Si-0.3-0.1 | 253.3 | 0.060 | 115.6 | 99.50 |
| AC-Si-0.1-0.3 | 258.4 | 0.071 | 114.1 | 99.43 |
| AC-Si-0.2-0.3 | 240.9 | 0.081 | 110.0 | 99.88 |
| AC-Si-0.3-0.3 | 238.9 | 0.073 | 112.2 | 99.82 |
| AC-Si-0.1-0.5 | 190.3 | 0.077 | 99.1 | 99.95 |
| AC-Si-0.2-0.5 | 180.9 | 0.065 | 85.5 | 99.94 |
| AC-Si-0.3-0.5 | 173.4 | 0.074 | 83.0 | 99.87 |
| Yoon-Nelson Model | Langmuir | Freundlich | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C0 | 300 | 520 | 750 | 1150 | 2670 | 3280 | 3710 | - | - | - | - |
| KYN | 0.04 | 0.06 | 0.08 | 0.08 | 0.28 | 0.25 | 0.19 | qm | 293 | Kf | 68.63 |
| τ | 241.7 | 165.8 | 119.3 | 110.7 | 39.1 | 33.7 | 30.1 | b | 0.00317 | n | 5.94 |
| *R2 | 99.59 | 99.80 | 99.89% | 99.81% | 99.77% | 99.92% | 99.80% | R2 | 98.23% | R2 | 99.01% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Kong, S.; Sui, H.; Li, X.; Zhang, Z. Preparation of Carbon-Silicon Doping Composite Adsorbent Material for Removal of VOCs. Materials 2019, 12, 2438. https://doi.org/10.3390/ma12152438
Han Z, Kong S, Sui H, Li X, Zhang Z. Preparation of Carbon-Silicon Doping Composite Adsorbent Material for Removal of VOCs. Materials. 2019; 12(15):2438. https://doi.org/10.3390/ma12152438
Chicago/Turabian StyleHan, Zhenwei, Shunli Kong, Hong Sui, Xingang Li, and Zisheng Zhang. 2019. "Preparation of Carbon-Silicon Doping Composite Adsorbent Material for Removal of VOCs" Materials 12, no. 15: 2438. https://doi.org/10.3390/ma12152438
APA StyleHan, Z., Kong, S., Sui, H., Li, X., & Zhang, Z. (2019). Preparation of Carbon-Silicon Doping Composite Adsorbent Material for Removal of VOCs. Materials, 12(15), 2438. https://doi.org/10.3390/ma12152438






