iPSC Bioprinting: Where are We at?
Abstract
:1. Introduction
2. iPSC Generation
2.1. Virus-Based Methods
2.2. Proteins
2.3. Somatic Cell Nuclear Transfer (SCNT)
2.4. Other Methods
2.5. iPSC Cell Source
3. 3D Bioprinting Techniques
3.1. Biomaterials
3.2. 3D Bioprinting Strategies
4. Bioprinting Undifferentiated iPSC
4.1. Bioprinting Techniques and Nozzle Diameters
4.2. Bioinks and Crosslinkers
5. Bioprinting iPSC Differentiated iPSC
5.1. Cartilage and Bone
5.2. Heart
5.3. Hepatic Tissue
5.4. Neural Tissue
5.5. Skin
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three Dimensional |
| bFGF | Basic fibroblast growth factor |
| BM-MSC | Bone marrow derived mesenchymal stem cells |
| CAD | Computer-aided design |
| CM | Cardiomyocytes |
| EB | Embryoid bodies |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| eGFP | Enhanced green fluorescent protein |
| EHM | Engineered heart muscle |
| ES | Embryonic stem cells |
| GelMA | Gelatin methacrylate |
| HA | Hyaluronic acid |
| hiPSC | Human induced pluripotent stem cells |
| hiPSC-NPC | Human induced pluripotent stem cell-derived neural progenitor cells |
| HPCH | Hydroxypropyl chitin |
| HUVEC | Human umbilical vein endothelial cells |
| iEC | Induced pluripotent stem cell-derived endothelial cells |
| iPSC | Induced pluripotent stem cells |
| iPSC-CM | Induced pluripotent stem cell-derived cardiomyocytes |
| iPSC-MSC | Induced pluripotent stem cell-derived MSC |
| KFG | Keratinocyte growth factor |
| MPH | Microfluidic printing head |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| MSC | Mesenchymal stem cells |
| NFC | Nanofibrillated cellulose |
| NHDF | Normal human dermal fibroblasts |
| OPCs | Oligodendrocyte progenitor cells |
| PRP | Platelet-rich plasma |
| RGD | Arg-Gly-Asp |
| SCNT | Somatic cell nuclear transfer |
| SLA | Stereolithography |
| SMC | Smooth muscle cell |
| sNPCs | Spinal neuronal progenitor cells |
| UV | Ultraviolet |
References
- Shafiee, A.; Atala, A. Tissue engineering: Toward a new era of medicine. Annu. Rev. Med. 2017, 68, 29–40. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar]
- Gepstein, L. Derivation and potential applications of human embryonic stem cells. Circ. Res. 2002, 91, 866–876. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.E.; Wingert, R.A. Renal stem cell reprogramming: Prospects in regenerative medicine. World J. Stem Cells 2014, 6, 458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Li, W.; Zhou, H.; Wei, W.; Ambasudhan, R.; Lin, T.; Kim, J.; Zhang, K.; Ding, S. Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell 2010, 7, 1–9. [Google Scholar] [CrossRef]
- Bilic, J.; Belmonte, J.C.I. Concise Review: Induced. Stem Cells 2012, 30, 33–41. [Google Scholar] [CrossRef]
- Guhr, A.; Kobold, S.; Seltmann, S.; Wulczyn, A.E.S.; Kurtz, A.; Löser, P. Recent trends in research with human pluripotent stem cells: impact of research and use of cell lines in experimental research and clinical trials. Stem cell Rep. 2018, 11, 485–496. [Google Scholar] [CrossRef]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915–946. [Google Scholar] [CrossRef]
- Omole, A.E.; Fakoya, A.O.J. Ten years of progress and promise of induced pluripotent stem cells: historical origins, characteristics, mechanisms, limitations, and potential applications. PeerJ. 2018, 6, e4370. [Google Scholar] [CrossRef] [Green Version]
- Malik, N.; Rao, M.S. A review of the methods for human ipsc derivation. In Pluripotent Stem Cells; Springer: Berlin, Germany, 2013; pp. 23–33. [Google Scholar]
- Wernig, M.; Gotz, M.; Eto, K. Overcoming ipsc obstacles. Cell Stem Cell 2016, 19, 291–292. [Google Scholar]
- Kim, S.K.; Baldwin, K. Voices iPSCs: 10 years and counting foster our scientific roots reprogramming for all. Cell. 2016, 165, 1041–1042. [Google Scholar]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, S.P.; Shevchenko, A.I.; Zakian, S.M. Induced Pluripotent stem cells: problems and advantages when applying them in regenerative medicine. Acta Nat. 2010, 2, 18–28. [Google Scholar]
- Seo, B.; Hong, Y.; Do, J. Cellular reprogramming using protein and cell-penetrating peptides. Int. journal Mol. Sci. 2017, 18, 552. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Yoo, J.; Jang, Y.; Han, J.; Yu, S.J.; Park, J.; Jung, S.Y.; Ahn, K.H.; Im, S.G.; Char, K. Nanothin coculture membranes with tunable pore architecture and thermoresponsive functionality for transfer-printable stem cell-derived cardiac sheets. ACS nano 2015, 9, 10186–10202. [Google Scholar] [CrossRef]
- Kim, D.; Kim, C.-H.; Moon, J.-I.; Chung, Y.-G.; Chang, M.-Y.; Han, B.-S.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell stem cell 2009, 4, 472. [Google Scholar] [CrossRef]
- Telpalo-Carpio, S.; Aguilar-Yañez, J.; Gonzalez-Garza, M.; Cruz-Vega, D.; Moreno-Cuevas, J. Ips cells generation: An overview of techniques and methods. J. stem cells Regen. Med. 2013, 9, 2. [Google Scholar]
- Raab, S.; Klingenstein, M.; Liebau, S.; Linta, L. A comparative view on human somatic cell sources for ipsc generation. Stem cells Int. 2014, 2014, 768391. [Google Scholar] [CrossRef]
- Ebrahimi, B. Reprogramming of adult stem/progenitor cells into ipscs without reprogramming factors. J. Med.l Hypotheses Ideas 2015, 9, 99–103. [Google Scholar] [CrossRef]
- Liebau, S.; Mahaddalkar, P.U.; Kestler, H.A.; Illing, A.; Seufferlein, T.; Kleger, A. A hierarchy in reprogramming capacity in different tissue microenvironments: What we know and what we need to know. Stem cells Dev. 2012, 22, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3d bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R. 3d bioprinting for tissue and organ fabrication. Annals Biomed. engineering 2017, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Ozbolat, I.T. Scaffold-based or scaffold-free bioprinting: Competing or complementing approaches? J. Nanotechnol Eng. Med. 2015, 6, 024701. [Google Scholar] [CrossRef]
- Hospodiuk, M.; Dey, M.; Sosnoski, D.; Ozbolat, I.T. The bioink: A comprehensive review on bioprintable materials. Biotechnol. Adv. 2017, 35, 217–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, E.S.; Mostafa, S.; Pakvasa, M.; Luu, H.H.; Lee, M.J.; Wolf, J.M.; Ameer, G.A.; He, T.-C.; Reid, R.R. 3-d bioprinting technologies in tissue engineering and regenerative medicine: Current and future trends. Genes Dis. 2017, 4, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio. 2019, 1, 100008. [Google Scholar] [CrossRef]
- Faramarzi, N.; Yazdi, I.K.; Nabavinia, M.; Gemma, A.; Fanelli, A.; Caizzone, A.; Ptaszek, L.M.; Sinha, I.; Khademhosseini, A.; Ruskin, J.N. Patient-specific bioinks for 3d bioprinting of tissue engineering scaffolds. Adv. Healthc. Mater. 2018, 7, 1701347. [Google Scholar] [CrossRef] [PubMed]
- Saunders, R.E.; Derby, B. Inkjet printing biomaterials for tissue engineering: Bioprinting. Int. Mater. Rev. 2014, 59, 430–448. [Google Scholar] [CrossRef]
- Foyt, D.A.; Norman, M.D.; Tracy, T.; Gentleman, E. Exploiting advanced hydrogel technologies to address key challenges in regenerative medicine. Adv. Healthc. Mater. 2018, 7, 1700939. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, B.; Ali, M.; Ducom, A.; Catros, S.; Keriquel, V.; Souquet, A.; Remy, M.; Fricain, J.-C.; Guillemot, F. Laser-assisted bioprinting for tissue engineering. In Biofabrication; Elsevier: Amsterdam, The Netherlands, 2013; pp. 95–118. [Google Scholar]
- Feinberg, A.W.; Miller, J.S. Progress in three-dimensional bioprinting. MRS Bull. 2017, 8, 557–562. [Google Scholar] [CrossRef]
- Francis, S.L.; di Bella, C.; Wallace, G.G.; Choong, P.F.M. Cartilage Tissue Engineering Using Stem Cells and Bioprinting Technology-Barriers to Clinical Translation. Front. Surg. 2018, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Pusch, K.; Hinton, T.J.; Feinberg, A.W. Large volume syringe pump extruder for desktop 3D printers. HardwareX 2018, 3, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Thayer, P.S.; Orrhult, L.S.; Martínez, H. Bioprinting of Cartilage and Skin Tissue Analogs Utilizing a Novel Passive Mixing Unit Technique for Bioink Precellularization. J. Vis. Exp. 2018, 131, e56372. [Google Scholar] [CrossRef]
- Reid, J.A.; Mollica, P.M.; Bruno, R.D.; Sachs, P.C. Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res. 2018, 20, 122. [Google Scholar]
- Barron, J.A.; Krizman, D.B.; Ringeisen, B.R. Laser Printing of Single Cells: Statistical Analysis, Cell Viability, and Stress. Ann. Biomed. Eng. 2005, 33, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Pinckney, J.; Lee, V.; Lee, J.-H.; Fischer, K.; Polio, S.; Park, J.-K.; Yoo, S.-S. Three-dimensional bioprinting of rat embryonic neural cells. Neuroreport 2009, 20, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Yeong, W.Y. Design and printing strategies in 3d bioprinting of cell-hydrogels: A review. Adv. Healthc. Mater. 2016, 5, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Dubbin, K.; Tabet, A.; Heilshorn, S. Quantitative criteria to benchmark new and existing bio-inks for cell compatibility. Biofabrication 2017, 9, 044102. [Google Scholar] [CrossRef]
- Mozetic, P.; Giannitelli, S.M.; Gori, M.; Trombetta, M.; Rainer, A. Engineering muscle cell alignment through 3D bioprinting, J. J. Biomed. Mater. Res. Part A 2017, 105, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for organ regeneration. Adv. Healthc. Mater. 2017, 6, 1601118. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, N.I. Progress in scaffold-free bioprinting for cardiovascular medicine. J. Cell. Mol. Med. 2018, 22, 2964–2969. [Google Scholar] [CrossRef] [PubMed]
- Verissimo, A.R.; Nakayama, K. Scaffold-free biofabrication. 3D print. Biofabrication 2017, 17, 1–20. [Google Scholar]
- Bakirci, E.; Toprakhisar, B.; Zeybek, M.C.; Ince, G.O.; Koc, B. Cell sheet based bioink for 3D bioprinting applications. Biofabrication 2017, 9, 024105. [Google Scholar] [CrossRef]
- Konagaya, S.; Ando, T.; Yamauchi, T.; Suemori, H.; Iwata, H. Long-term maintenance of human induced pluripotent stem cells by automated cell culture system. Sci. Rep. 2015, 5, 16647. [Google Scholar] [CrossRef] [PubMed]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.-i.; Muguruma, K. A rock inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681. [Google Scholar] [CrossRef]
- Sheridan, S.D.; Surampudi, V.; Rao, R.R. Analysis of embryoid bodies derived from human induced pluripotent stem cells as a means to assess pluripotency. Stem cells Int. 2012, 2012, 738910. [Google Scholar] [CrossRef]
- Faulkner-Jones, A.; Fyfe, C.; Cornelissen, D.-J.; Gardner, J.; King, J.; Courtney, A.; Shu, W. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3d. Biofabrication 2015, 7, 044102. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D bioprinting human induced pluripotent stem cell constructs for in situ cell proliferation and successive multilineage differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, X.; Li, L.; Chen, Z.-N.; Gao, G.; Yao, R.; Sun, W. 3d printing human induced pluripotent stem cells with novel hydroxypropyl chitin bioink: Scalable expansion and uniform aggregation. Biofabrication 2018, 10, 044101. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Franke, A.; Schwanke, K.; Haverich, A.; Zweigerdt, R.; Chichkov, B. Laser bioprinting of human induced pluripotent stem cells—the effect of printing and biomaterials on cell survival, pluripotency, and differentiation. Biofabrication 2018, 10, 035005. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M. Cartilage tissue engineering by the 3d bioprinting of ips cells in a nanocellulose/alginate bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Reid, J.A.; Mollica, P.A.; Johnson, G.D.; Ogle, R.C.; Bruno, R.D.; Sachs, P.C. Accessible bioprinting: Adaptation of a low-cost 3d-printer for precise cell placement and stem cell differentiation. Biofabrication 2016, 8, 025017. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M. Applications of alginate-based bioinks in 3d bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3d bioprinting using stem cells. Pediatric Res. 2018, 83, 223. [Google Scholar] [CrossRef]
- Dhawan, A.; Kennedy, P.M.; Rizk, E.B.; Ozbolat, I.T. Three-dimensional bioprinting for bone and cartilage restoration in orthopaedic surgery. JAAOS-J. Am. Acad. Orthop. Surg. 2019, 27, e215–e226. [Google Scholar] [CrossRef]
- Roseti, L.; Cavallo, C.; Desando, G.; Parisi, V.; Petretta, M.; Bartolotti, I.; Grigolo, B. Three-dimensional bioprinting of cartilage by the use of stem cells: A strategy to improve regeneration. Materials 2018, 11, 1749. [Google Scholar] [CrossRef]
- Scotti, C.; Hirschmann, M.; Antinolfi, P.; Martin, I.; Peretti, G. Meniscus repair and regeneration: review on current methods and research potential. Eur Cell Mater 2013, 26, 150–170. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Cao, Y. Recent progress in cartilage tissue engineering—our experience and future directions. Engineering 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Leberfinger, A.N.; Ravnic, D.J.; Dhawan, A.; Ozbolat, I.T. Concise Review: Bioprinting of Stem Cells for Transplantable Tissue Fabrication. Stem Cells Transl. Med. 2017, 6, 1940–1948. [Google Scholar] [CrossRef] [Green Version]
- Daly, A.C.; Critchley, S.E.; Rencsok, E.M.; Kelly, D.J. A comparison of different bioinks for 3d bioprinting of fibrocartilage and hyaline cartilage. Biofabrication 2016, 8, 045002. [Google Scholar] [CrossRef]
- Di Bella, C.; Duchi, S.; O'Connell, C.D.; Blanchard, R.; Augustine, C.; Yue, Z.; Thompson, F.; Richards, C.; Beirne, S.; Onofrillo, C. In situ handheld three-dimensional bioprinting for cartilage regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 611–621. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martiínez Ávila, H.C.; Hägg, D.; Gatenholm, P. 3d bioprinting human chondrocytes with nanocellulose–alginate bioink for cartilage tissue engineering applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Critchley, S.E.; Kelly, D.J. Bioinks for bioprinting functional meniscus and articular cartilage. J. 3D Print. Med. 2017, 1, 269–290. [Google Scholar] [CrossRef]
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3d printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials. 2018, 162, 34–46. [Google Scholar] [CrossRef]
- Tsumaki, N.; Okada, M.; Yamashita, A. Ips cell technologies and cartilage regeneration. Bone 2015, 70, 48–54. [Google Scholar] [CrossRef]
- Guzzo, R.M.; O’Sullivan, M.B. Human pluripotent stem cells: Advances in chondrogenic differentiation and articular cartilage regeneration. Curr. Mol. Biol. Rep. 2016, 2, 113–122. [Google Scholar] [CrossRef]
- Bigdeli, N.; Karlsson, C.; Strehl, R.; Concaro, S.; Hyllner, J.; Lindahl, A. Coculture of human embryonic stem cells and human articular chondrocytes results in significantly altered phenotype and improved chondrogenic differentiation. Stem cells 2009, 27, 1812–1821. [Google Scholar] [CrossRef]
- Dogaki, Y.; Lee, S.Y.; Niikura, T.; Iwakura, T.; Okumachi, E.; Waki, T.; Kakutani, K.; Nishida, K.; Kuroda, R.; Kurosaka, M. Efficient derivation of osteoprogenitor cells from induced pluripotent stem cells for bone regeneration. Int. Orthop. 2014, 38, 1779–1785. [Google Scholar] [CrossRef]
- De Peppo, G.M.; Marcos-Campos, I.; Kahler, D.J.; Alsalman, D.; Shang, L.; Vunjak-Novakovic, G.; Marolt, D. Engineering bone tissue substitutes from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. USA 2013, 110, 8680–8685. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Schilling, A.F.; Hubbell, K.; Yonezawa, T.; Truong, D.; Hong, Y.; Dai, G.; Cui, X. Improved properties of bone and cartilage tissue from 3d inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in peg-gelma. Biotechnol. Lett. 2015, 37, 2349–2355. [Google Scholar] [CrossRef]
- Daly, A.C.; Cunniffe, G.M.; Sathy, B.N.; Jeon, O.; Alsberg, E.; Kelly, D.J. 3d bioprinting of developmentally inspired templates for whole bone organ engineering. Adv. Healthc. Mater. 2016, 5, 2353–2362. [Google Scholar] [CrossRef]
- Qasim, M.; Haq, F.; Kang, M.-H.; Kim, J.-H. 3d printing approaches for cardiac tissue engineering and role of immune modulation in tissue regeneration. Int. J. Nanomed. 2019, 14, 1311. [Google Scholar] [CrossRef]
- Liu, J.; Chen, W.; Zhao, Z.; Xu, H.H. Reprogramming of mesenchymal stem cells derived from ipscs seeded on biofunctionalized calcium phosphate scaffold for bone engineering. Biomaterials 2013, 34, 7862–7872. [Google Scholar] [CrossRef]
- Fujita, B.; Zimmermann, W.-H. Myocardial tissue engineering strategies for heart repair: Current state of the art. Interact. Cardiovasc. Thorac. Surg. 2018, 27, 916–920. [Google Scholar] [CrossRef]
- Shiba, Y.; Gomibuchi, T.; Seto, T.; Wada, Y.; Ichimura, H.; Tanaka, Y.; Ogasawara, T.; Okada, K.; Shiba, N.; Sakamoto, K. Allogeneic transplantation of ips cell-derived cardiomyocytes regenerates primate hearts. Nature 2016, 538, 388. [Google Scholar] [CrossRef]
- Arai, K.; Murata, D.; Verissimo, A.R.; Mukae, Y.; Itoh, M.; Nakamura, A.; Morita, S.; Nakayama, K. Fabrication of scaffold-free tubular cardiac constructs using a bio-3d printer. PLoS ONE 2018, 13, e0209162. [Google Scholar] [CrossRef]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Tran, Q.; Ajeti, V.; Freeman, B.T.; Fast, V.G. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Chang Liao, M.-L.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E. Defined engineered human myocardium with advanced maturation for applications in heart failure modeling and repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef]
- Gu, Q.; Hao, J.; Lu, Y.; Wang, L.; Wallace, G.G.; Zhou, Q. Three-dimensional bio-printing. Sci. China Life Sci. 2015, 58, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 2017, 8, 1825. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.-S.; Seliktar, D. A multi-cellular 3d bioprinting approach for vascularized heart tissue engineering based on huvecs and ipsc-derived cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3d printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Mazza, G.; Al-Akkad, W.; Rombouts, K.; Pinzani, M. Liver tissue engineering: From implantable tissue to whole organ engineering. Hepatol. Commun. 2018, 2, 131–141. [Google Scholar] [CrossRef]
- Ma, X.; Qu, X.; Zhu, W.; Li, Y.-S.; Yuan, S.; Zhang, H.; Liu, J.; Wang, P.; Lai, C.S.E.; Zanella, F. Deterministically patterned biomimetic human ipsc-derived hepatic model via rapid 3d bioprinting. Proc. Natl. Acad. Sci. USA 2016, 113, 2206–2211. [Google Scholar] [CrossRef]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Wu, S.; Xu, R.; Duan, B.; Jiang, P. Three-dimensional hyaluronic acid hydrogel-based models for in vitro human iPSC-derived NPC culture and differentiation. J. Mater. Chem. B. 2017, 5, 3870–3878. [Google Scholar] [CrossRef]
- Cairns, D.M.; Chwalek, K.; Moore, Y.E.; Kelley, M.R.; Abbott, R.D.; Moss, S.; Kaplan, D.L. Expandable and Rapidly Differentiating Human Induced Neural Stem Cell Lines for Multiple Tissue Engineering Applications. Stem Cell Rep. 2016, 7, 557–570. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S.-H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef]
- Lin, W.; Chen, M.; Hu, C.; Qin, S.; Chu, C.; Xiang, L.; Man, Y.; Qu, Y. Endowing ipsc-derived mscs with angiogenic and keratinogenic differentiation potential: A promising cell source for skin tissue engineering. BioMed Res. Int. 2018, 2018, 8459503. [Google Scholar] [CrossRef]
- Abaci, H.E.; Guo, Z.; Coffman, A.; Gillette, B.; Lee, W.h.; Sia, S.K.; Christiano, A.M. Human Skin Constructs with Spatially Controlled Vasculature Using Primary and iPSC-Derived Endothelial Cells. Adv. Healthc. Mater. 2016, 5, 1800–1807. [Google Scholar] [CrossRef]
- Kim, Y.; Park, N.; Rim, Y.A.; Nam, Y.; Jung, H.; Lee, K.; Ju, J.H. Establishment of a complex skin structure via layered co-culture of keratinocytes and fibroblasts derived from induced pluripotent stem cells. Stem Cell Res. Ther. 2018, 9, 217. [Google Scholar] [CrossRef]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef]


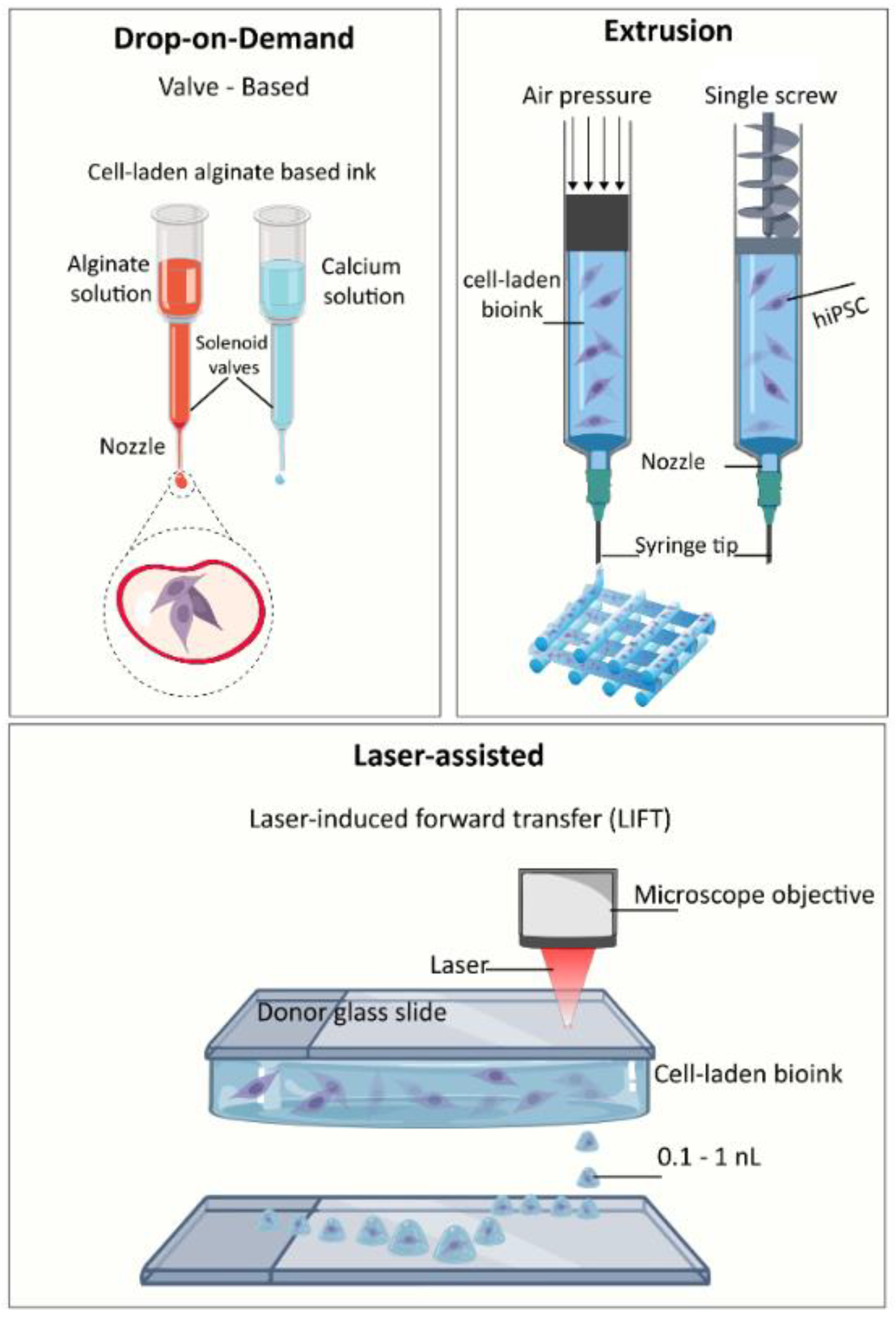
| Printing Technique | Printer | Nozzle Diameter | Bioink | Crosslinker | Cell Source | Function | Reference |
|---|---|---|---|---|---|---|---|
| Drop-on-Demand | Custom 3-axis stage | 101.6 µm | 1.5% w/v alginate | 6% CaCl2 | hiPSC cell lines RCi-22, RCi-50 | N/A | Faulkner- Jones et al. 2015 |
| Extrusion | Felix 3.0 | 40 µm | Geltrex | None | Custom made BJ fibroblasts derived hiPSC | 3 germ-layers | Reid et al. 2016 |
| 3D Bioplotter Envision TEC | 200 µm | 5% w/v alginate, 5% w/v carboxymethyl-chitosan, 1.5% w/v agarose | CaCl2 | hiPSCs (source not specified) | 3 germ-layers, neural tissues | Gu et al. 2017 | |
| 3D Discovery regenHu | 300 µm | Nanofibrillated cellulose (NFC) alginate (60:40) NFC with HA | CaCl2 (for alginate) H2O2 (for HA) | Custom made A2B iPSC line, iPSC derived chondrocytes | Pluripotency, chondrocytes | Nguyen et al. 2017 | |
| Custom-built | 260 µm | 2% w/v hydroxypropyl chitin (HPCH), 0-30% Matrigel | Temperature 37˚C | hiPSC from human peripheral blood mononuclear cells (hPBMCs) | Pluripotency | Li et al. 2018 | |
| Laser-assisted | Nd:YAG 1064 laser | N/A Droplet volume 0.01-1nL | 1 wt% HA Matrigel | - | hiPSC from cord blood or peripheral blood-derived hiPSC line | 3 germ layers | Koch et al. 2018 |
| Tissue | Cell | Bioink | Cross-Linker | Printer | Reference |
|---|---|---|---|---|---|
| Cartilage | hiPSC derived chondrocytes | NFC/A* NFC/HA* | CaCl2 | 3D Discovery (regenHu, Switzerland) | Nguyen et al. 2017 |
| iPSC source: chondrocytes | |||||
| Bone | Only BM-MSC | PEG-GelMA | UV polymerization | 3D inkjet printer, modified HP Deskjet 500 printer | Gao et al. 2015 |
| (no iPSC derived) | |||||
| Heart | hiPSC derived CM, SMC, EC | GelMA | † Multiphoton-excitation | Custom-built multiphoton laser-scanning 3D printer | Gao et al. 2017 |
| iPSC source: cardiac fibroblasts | |||||
| HUVEC and iPSC-CM | Alginate and PEG-fibrinogen hydrogel | CaCl2 and UV | Custom designed MPH for the simultaneous extrusion of multiple bioinks | Maiullari et al. 2018 | |
| iPSC source: mouse embryonic fibroblasts | |||||
| CM and EC derived from same iPSC | Decellularized omental tissue printed in supporting medium | 37 ºC for 45 min | 3D Discovery (RegenHU) | Noor et al. 2019 | |
| iPSC source: omental stromal cells | |||||
| Human skin fibroblasts | Scaffold free | -- | Novogen MMX (Organova) | Bakirci et al. 2017 | |
| iPSC-CM, HUVEC and NHDF | Scaffold free | -- | Regenova (Cyfuse Biomedical K.K.) | Arai et al. 2018 | |
| Hepatic tissue | iPSC-HPC iPSC source: human perinatal | GMHA*:GelMA | UV polymerization | Custom extrusion based 3D printer | Ma et al. 2016 |
| foreskin fibroblasts | |||||
| Neural tissue | SNPC and OPC | - Matrigel as cell laden bioink - AG/MC* as supporting ink | - Temperature - CaCl2 or BaCl2 | Custom microextrusion-based 3D printer | Joung et al. 2018 |
| iPSC source: †UMN-X7 and UMN-3F10 | |||||
| Neural stem cells | 2 thermoresponsive water-based biodegradable polyurethane dispersions (PU1 and PU2) | Pre-crosslinking at different set of temperatures and then at 37 ºC for 4h | Self- developed FDM equipment | Hsieh et al. 2015 | |
| SKIN | iPSC derived endothelial cells | Alginate molds | CaCl2 | † Objet24 3D-Printer (Stratasys) | Abaci et al. 2016 |
| iPSC source:human fibroblasts from foreskin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanazzo, S.; Nemec, S.; Roohani, I. iPSC Bioprinting: Where are We at? Materials 2019, 12, 2453. https://doi.org/10.3390/ma12152453
Romanazzo S, Nemec S, Roohani I. iPSC Bioprinting: Where are We at? Materials. 2019; 12(15):2453. https://doi.org/10.3390/ma12152453
Chicago/Turabian StyleRomanazzo, Sara, Stephanie Nemec, and Iman Roohani. 2019. "iPSC Bioprinting: Where are We at?" Materials 12, no. 15: 2453. https://doi.org/10.3390/ma12152453
APA StyleRomanazzo, S., Nemec, S., & Roohani, I. (2019). iPSC Bioprinting: Where are We at? Materials, 12(15), 2453. https://doi.org/10.3390/ma12152453




