Utilization of Carbon Nanospheres in Photocatalyst Production: From Composites to Highly Active Hollow Structures
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Carbon Sphere Templates
2.3. Preparation of the TiO2–CS Composites and TiO2 Hollow Structures
2.4. Characterization Methods and Instrumentation
2.5. Evaluation of the Photocatalytic Efficiencies
3. Results and Discussion
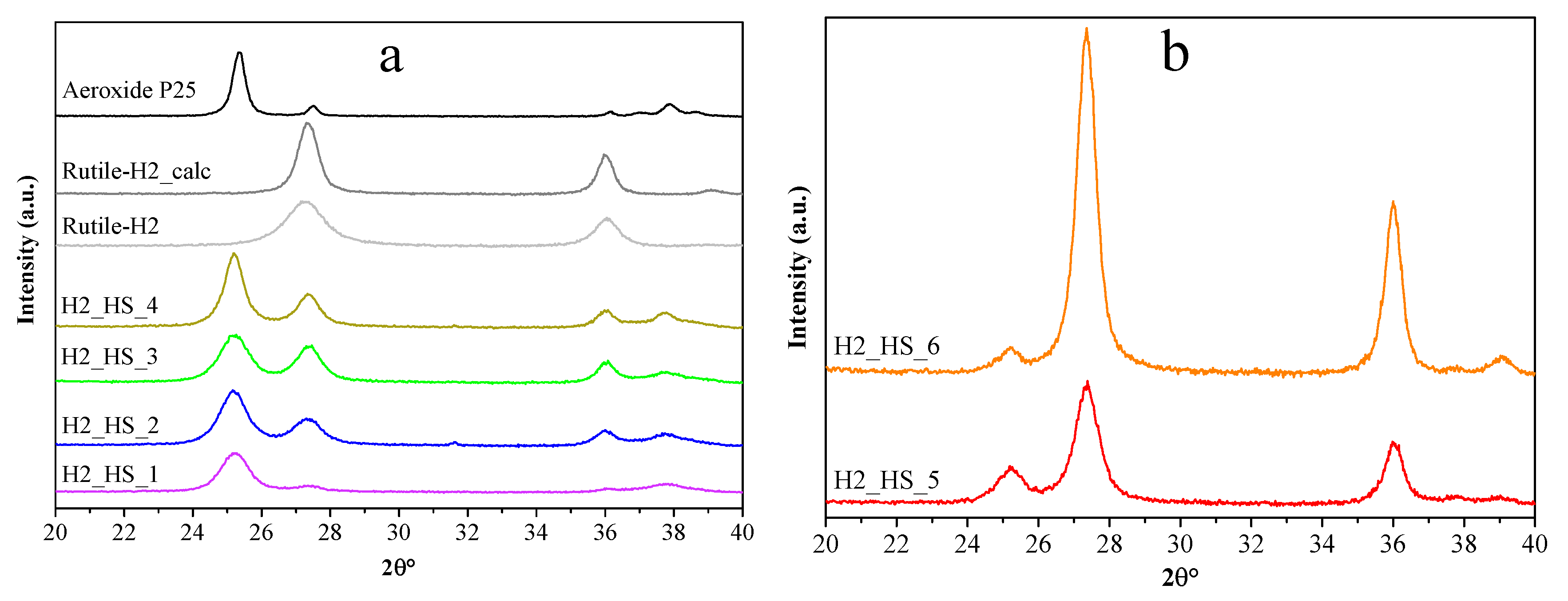
3.1. Characterization of the Carbon Sphere Templates
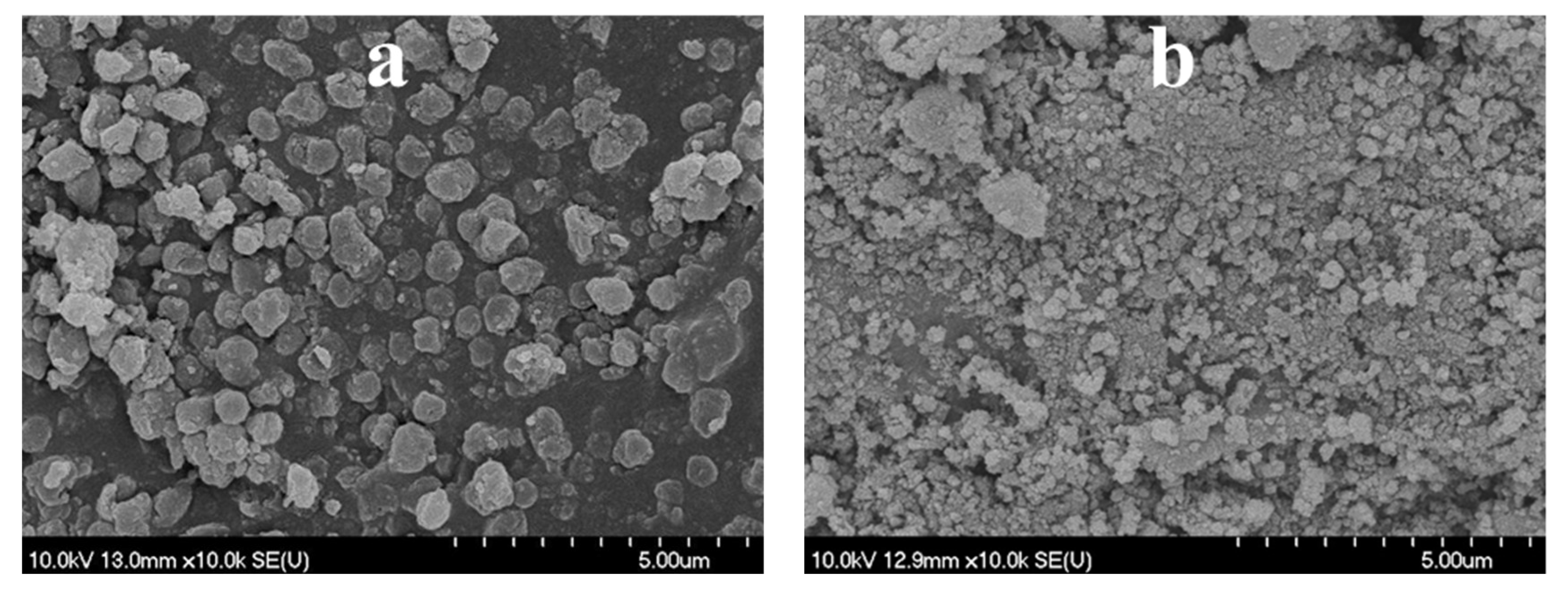
3.2. Comparison of the TiO2–CS Composite and TiO2 Hollow Structure
3.3. Evaluation of Photocatalytic Activity
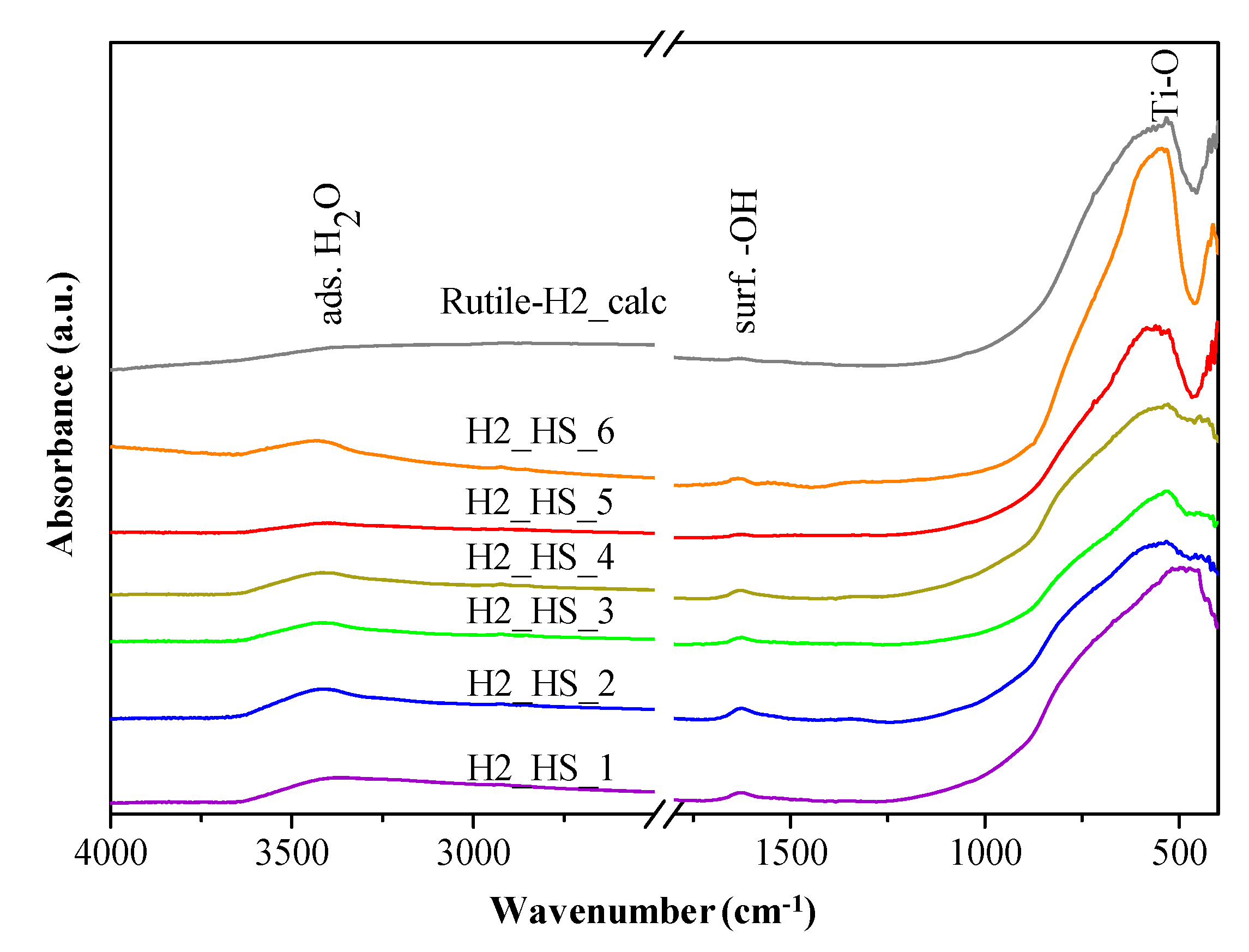
3.4. XPS Measurements
- The total amount of carbon in sample H2_HS_6 (30.72 at%) was determined to be higher than that of sample H2_HS_5 (8.91 at%).
- Moreover, in sample H2_HS_5, the ratio of O–C=O bonds was higher (15.18% from the total amount of carbon species) than in the case of H2_HS_6 (12.38% from the total amount of carbon species), pointing out a more efficient carbon removal (oxidation) process in case of the latter sample.
- The fact that CS templates could be eliminated to a greater degree without additional air supply (muffle furnace) compared to their removability by providing additional air (tube furnace with constant air supply) was very surprising. The whole synthesis procedure and comparison was repeated three times, and the result was consistent in all cases. A plausible explanation for this could be that during the introduction of air, the partial pressure of oxygen increases within the system (tube furnace, in the case of sample H2_HS_6), facilitating its adsorption to the titania surface leading to the formation of an oxygen monolayer. This monolayer could act as a trap for the exiting CO2 hindering its transport through the titania shell. Since a TiO2 coating with a sensitized TiO2 layer containing peroxo groups (Ti–O–O–Ti) was used and the degradation of these peroxo groups might proceed more easily in the absence of additional oxygen (muffle furnace, in the case of sample H2_HS_5), the necessary oxygen for the elimination of CS templates (partly) could still be provided locally by the degradation of peroxo groups during the calcination process.
- The above listed observations emphasize the fact that, as the carbon was more efficiently eliminated from the samples (shown by the lower carbon content and higher amount of oxidized carbon species), the photocatalytic activity was higher (sample H2_HS_6 possessed only 52.6% of the photocatalytic activity of sample H2_HS_5), because in the case of the samples with lower photocatalytic activity, the surface available for the photocatalytic process could be partially blocked and therefore more hydrophobic as well. Simultaneously, if the ratio of the O–C=O in the surface deposited carbon was higher, again supports the observed activity increase, since polar functional groups facilitate the direct contact between water (the matrix of the photocatalytic process) and the surface of the photocatalyst.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Moreira, N.F.F.; Narciso-da-Rocha, C.; Polo-Lopez, M.I.; Pastrana-Martinez, L.M.; Faria, J.L.; Manaia, C.M.; Fernandez-Ibanez, P.; Nunes, O.C.; Silva, A.M.T. Solar treatment (H2O2, TiO2-P25 and GO-TiO2 photocatalysis, photo-Fenton) of organic micropollutants, human pathogen indicators, antibiotic resistant bacteria and related genes in urban wastewater. Water Res. 2018, 135, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Pablos, C.; Marugan, J.; van Grieken, R.; Dunlop, P.S.M.; Hamilton, J.W.J.; Dionysiou, D.D.; Byrne, J.A. Electrochemical Enhancement of Photocatalytic Disinfection on Aligned TiO2 and Nitrogen Doped TiO2 Nanotubes. Molecules 2017, 22, 704. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.; Pulgarin, C.; Giannakis, S.; Bensimon, M.; Kiwi, J. New evidence for disinfection, self-cleaning and pollutant degradation mediated by GF-TiO2-Cu mats under solar/visible light in mild oxidative conditions. J. Photochem. Photobiol. A Chem. 2017, 346, 351–363. [Google Scholar] [CrossRef]
- Veréb, G.; Manczinger, L.; Bozsó, G.; Sienkiewicz, A.; Forró, L.; Mogyorósi, K.; Hernádi, K.; Dombi, A. Comparison of the photocatalytic efficiencies of bare and doped rutile and anatase TiO2 photocatalysts under visible light for phenol degradation and E. coli inactivation. Appl. Catal. B Environ. 2013, 129, 566–574. [Google Scholar] [CrossRef]
- Pezzi, L.; Pane, A.; Annesi, F.; Losso, M.A.; Guglielmelli, A.; Umeton, C.; De Sio, L. Antimicrobial Effects of Chemically Functionalized and/or Photo-Heated Nanoparticles. Materials 2019, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; García, A.M.; Fernández, M.; Vicente, M.A.; González-Rodríguez, B.; Rives, V.; Korili, S.A. Effect of dopants on the structure of titanium oxide used as a photocatalyst for the removal of emergent contaminants. J. Ind. Eng. Chem. 2017, 53, 183–191. [Google Scholar] [CrossRef]
- Matos, J.; Miralles-Cuevas, S.; Ruíz-Delgado, A.; Oller, I.; Malato, S. Development of TiO2-C photocatalysts for solar treatment of polluted water. Carbon 2017, 122, 361–373. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic degradation of organic pollutants with simultaneous production of hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Simon, G.; Gyulavári, T.; Hernádi, K.; Molnár, M.; Pap, Z.; Veréb, G.; Schrantz, K.; Náfrádi, M.; Alapi, T. Photocatalytic ozonation of monuron over suspended and immobilized TiO2–study of transformation, mineralization and economic feasibility. J. Photochem. Photobiol. A Chem. 2018, 356, 512–520. [Google Scholar] [CrossRef]
- Samokhvalov, A. Hydrogen by photocatalysis with nitrogen codoped titanium dioxide. Renew. Sustain. Energy Rev. 2017, 72, 981–1000. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.-W.; Ma, D.; Fan, Z.; Lu, L.; Niu, C. In situ synthesis of C-doped TiO2@g-C3N4 core-shell hollow nanospheres with enhanced visible-light photocatalytic activity for H2 evolution. Chem. Eng. J. 2017, 322, 435–444. [Google Scholar] [CrossRef]
- Mogyorósi, K.; Kmetykó, Á.; Czirbus, N.; Veréb, G.; Sipos, P.; Dombi, A. Comparison of the substrate dependent performance of Pt-, Au- and Ag-doped TiO2 photocatalysts in H2-production and in decomposition of various organics. React. Kinet. Catal. Lett. 2009, 98, 215–225. [Google Scholar] [CrossRef]
- Banerjee, S.; Gopal, J.; Muraleedharan, P.; Tyagi, A.K.; Rai, B. Physics and chemistry of photocatalytic titanium dioxide: Visualization of bactericidal activity using atomic force microscopy. Curr. Sci. 2006, 90, 1378–1383. [Google Scholar]
- Wu, M.C.; Hiltunen, J.; Sapi, A.; Avila, A.; Larsson, W.; Liao, H.C.; Huuhtanen, M.; Toth, G.; Shchukarev, A.; Laufer, N.; et al. Nitrogen-doped anatase nanofibers decorated with noble metal nanoparticles for photocatalytic production of hydrogen. ACS Nano 2011, 5, 5025–5030. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.; Balčytis, A.; Nitta, A.; Méndez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Kmetykó, Á.; Szániel, Á.; Tsakiroglou, C.; Dombi, A.; Hernádi, K. Enhanced photocatalytic H2 generation on noble metal modified TiO2 catalysts excited with visible light irradiation. React. Kinet. Mech. Catal. 2015, 117, 379–390. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Trykowski, G.; Zaleska-Medynska, A. Noble metal modified TiO2 microspheres: Surface properties and photocatalytic activity under UV–vis and visible light. J. Mol. Catal. A Chem. 2016, 423, 191–206. [Google Scholar] [CrossRef]
- Gratzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef]
- Youssef, Z.; Colombeau, L.; Yesmurzayeva, N.; Baros, F.; Vanderesse, R.; Hamieh, T.; Toufaily, J.; Frochot, C.; Roques-Carmes, T.; Acherar, S. Dye-sensitized nanoparticles for heterogeneous photocatalysis: Cases studies with TiO2, ZnO, fullerene and graphene for water purification. Dyes Pigments 2018, 159, 49–71. [Google Scholar] [CrossRef]
- Xiang, L.; Zhao, X. Wet-Chemical Preparation of TiO2-Based Composites with Different Morphologies and Photocatalytic Properties. Nanomaterials 2017, 7, 310. [Google Scholar] [CrossRef]
- Lu, Y.; Hao, L.; Matsuzaka, K.; Yoshida, H.; Asanuma, H.; Chen, J.; Pan, F. Titanium dioxide–nickel oxide composite coatings: Preparation by mechanical coating/thermal oxidation and photocatalytic activity. Mater. Sci. Semicond. Process. 2014, 24, 138–145. [Google Scholar] [CrossRef]
- Karácsonyi, É.; Baia, L.; Dombi, A.; Danciu, V.; Mogyorósi, K.; Pop, L.C.; Kovács, G.; Coşoveanu, V.; Vulpoi, A.; Simon, S.; et al. The photocatalytic activity of TiO2/WO3/noble metal (Au or Pt) nanoarchitectures obtained by selective photodeposition. Catal. Today 2013, 208, 19–27. [Google Scholar] [CrossRef]
- Siwinska-Stefanska, K.; Kubiaka, A.; Piasecki, A.; Goscianska, J.; Nowaczyk, G.; Jurga, S.; Jesionowski, T. TiO2-ZnO Binary Oxide Systems: Comprehensive Characterization and Tests of Photocatalytic Activity. Materials 2018, 11, 841. [Google Scholar] [CrossRef] [PubMed]
- Kamil, A.M.; Mohammed, H.T.; Balakit, A.A.; Hussein, F.H.; Bahnemann, D.W.; El-Hiti, G.A. Synthesis, Characterization and Photocatalytic Activity of Carbon Nanotube/Titanium Dioxide Nanocomposites. Arab. J. Sci. Eng. 2017, 43, 199–210. [Google Scholar] [CrossRef]
- Réti, B.; Mogyorósi, K.; Dombi, A.; Hernádi, K. Substrate dependent photocatalytic performance of TiO2/MWCNT photocatalysts. Appl. Catal. A Gen. 2014, 469, 153–158. [Google Scholar] [CrossRef]
- Daram, P.; Banjongprasert, C.; Thongsuwan, W.; Jiansirisomboon, S. Microstructure and photocatalytic activities of thermal sprayed titanium dioxide/carbon nanotubes composite coatings. Surf. Coat. Technol. 2016, 306, 290–294. [Google Scholar] [CrossRef]
- Majrik, K.; Turcsanyi, A.; Paszti, Z.; Szabo, T.; Domjan, A.; Mihaly, J.; Tompos, A.; Dekany, I.; Talas, E. Graphite Oxide-TiO2 Nanocomposite Type Photocatalyst for Methanol Photocatalytic Reforming Reaction. Top. Catal. 2018, 61, 1323–1334. [Google Scholar] [CrossRef]
- Janković, A.; Eraković, S.; Vukašinović-Sekulić, M.; Mišković-Stanković, V.; Park, S.J.; Rhee, K.Y. Graphene-based antibacterial composite coatings electrodeposited on titanium for biomedical applications. Prog. Org. Coat. 2015, 83, 1–10. [Google Scholar] [CrossRef]
- Faraldos, M.; Bahamonde, A. Environmental applications of titania-graphene photocatalysts. Catal. Today 2017, 285, 13–28. [Google Scholar] [CrossRef]
- Chen, D.; Zou, L.; Li, S.; Zheng, F. Nanospherical like reduced graphene oxide decorated TiO2 nanoparticles: An advanced catalyst for the hydrogen evolution reaction. Sci. Rep. 2016, 6, 20335. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, X.-L.; Wang, Z.-M.; Aoki, H.; Kutsuna, S.; Jimura, K.; Hayashi, S. Anchoring titanium dioxide on carbon spheres for high-performance visible light photocatalysis. Appl. Catal. B Environ. 2017, 207, 255–266. [Google Scholar] [CrossRef]
- Maletić, M.; Vukčević, M.; Kalijadis, A.; Janković-Častvan, I.; Dapčević, A.; Laušević, Z.; Laušević, M. Hydrothermal synthesis of TiO2/carbon composites and their application for removal of organic pollutants. Arab. J. Chem. 2016. [Google Scholar] [CrossRef]
- Li, Y.; Ma, M.; Sun, S.; Yan, W.; Ouyang, Y. Preparation of TiO2–carbon surface composites with high photoactivity by supercritical pretreatment and sol–gel processing. Appl. Surf. Sci. 2008, 254, 4154–4158. [Google Scholar] [CrossRef]
- Bakos, L.P.; Justh, N.; Hernádi, K.; Kiss, G.; Réti, B.; Erdélyi, Z.; Parditka, B.; Szilágyi, I.M. Core-shell carbon nanosphere-TiO2 composite and hollow TiO2 nanospheres prepared by atomic layer deposition. J. Phys. Conf. Ser. 2016, 764, 012005. [Google Scholar] [CrossRef]
- Raza, W.; Haque, M.M.; Muneer, M.; Bahnemann, D. Synthesis of visible light driven TiO2 coated carbon nanospheres for degradation of dyes. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Justh, N.; Bakos, L.P.; Hernadi, K.; Kiss, G.; Reti, B.; Erdelyi, Z.; Parditka, B.; Szilagyi, I.M. Photocatalytic hollow TiO2 and ZnO nanospheres prepared by atomic layer deposition. Sci. Rep. 2017, 7, 4337. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L. The Size Effect of TiO2 Hollow Microspheres on Photovoltaic Performance of ZnS/CdS Quantum Dots Sensitized Solar Cell. Materials 2019, 12, 1583. [Google Scholar] [CrossRef]
- Ao, Y.; Xu, J.; Fu, D.; Yuan, C. A simple method for the preparation of titania hollow sphere. Catal. Commun. 2008, 9, 2574–2577. [Google Scholar] [CrossRef]
- Zheng, M.; Cao, J.; Chang, X.; Wang, J.; Liu, J.; Ma, X. Preparation of oxide hollow spheres by colloidal carbon spheres. Mater. Lett. 2006, 60, 2991–2993. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Mhlanga, S.D.; Coville, N.J. Carbon spheres. Mater. Sci. Eng. R Rep. 2010, 70, 1–28. [Google Scholar] [CrossRef]
- Réti, B.; Kiss, G.I.; Gyulavári, T.; Baan, K.; Magyari, K.; Hernadi, K. Carbon sphere templates for TiO2 hollow structures: Preparation, characterization and photocatalytic activity. Catal. Today 2017, 284, 160–168. [Google Scholar] [CrossRef]
- Bao, Y.; Kang, Q.L.; Liu, C.; Ma, J.Z. Sol-gel-controlled synthesis of hollow TiO2 spheres and their photocatalytic activities and lithium storage properties. Mater. Lett. 2018, 214, 272–275. [Google Scholar] [CrossRef]
- Gyulavári, T.; Pap, Z.; Kovács, G.; Baia, L.; Todea, M.; Hernádi, K.; Veréb, G. Peroxo group enhanced nanorutile as visible light active photocatalyst. Catal. Today 2017, 284, 129–136. [Google Scholar] [CrossRef]
- Spurr, R.A.; Myers, H. Quantitative Analysis of Anatase-Rutile Mixtures with an X-Ray Diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Vardar, G.; Wood, T.K. Protein engineering of toluene-o-xylene monooxygenase from Pseudomonas stutzeri OX1 for synthesizing 4-methylresorcinol, methylhydroquinone, and pyrogallol. Appl. Environ. Microbiol. 2004, 70, 3253–3262. [Google Scholar] [CrossRef] [PubMed]
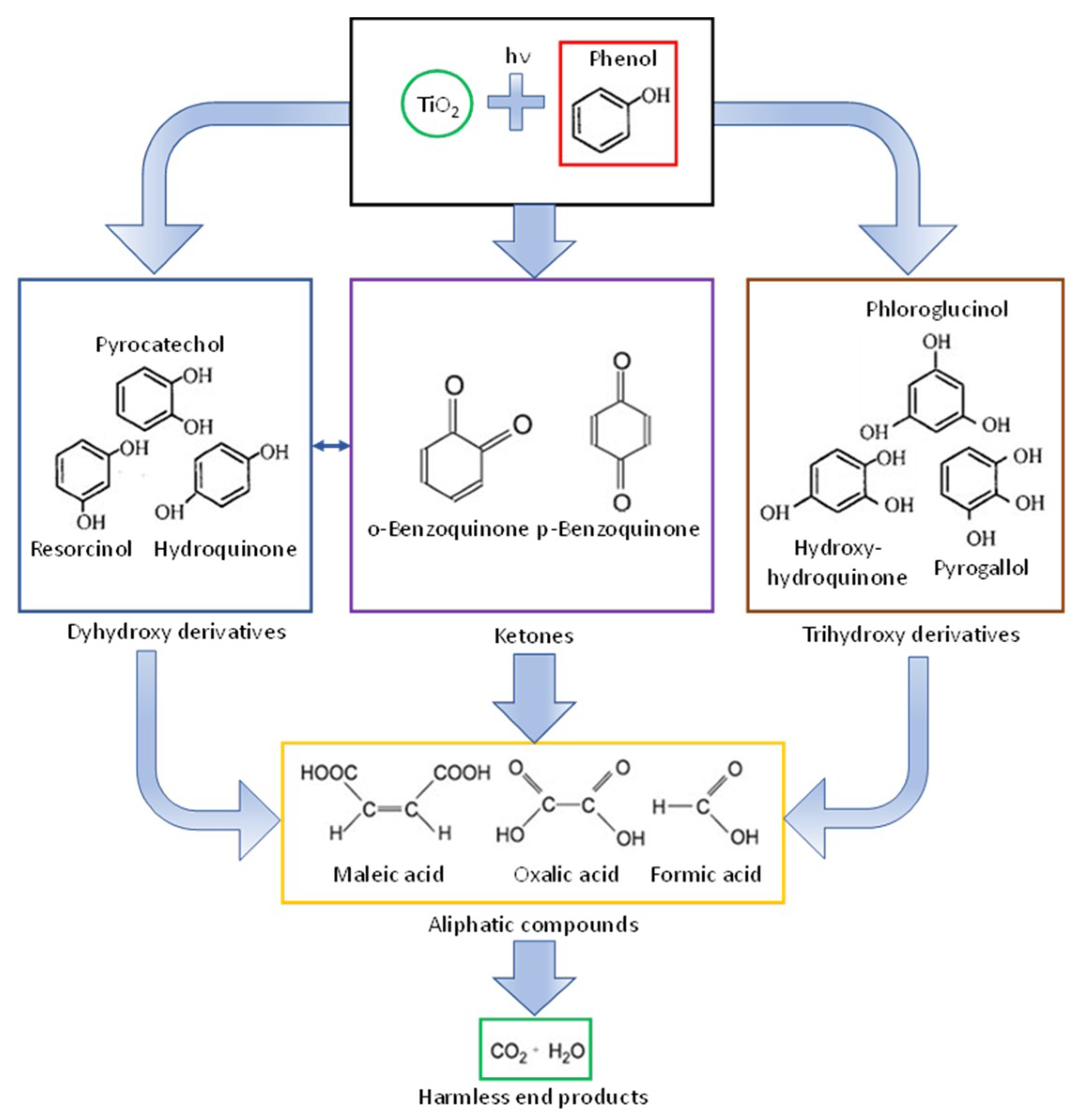
- Grabowska, E.; Reszczynska, J.; Zaleska, A. Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: A review. Water Res. 2012, 46, 5453–5471. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, W.; Hong, X.; Zhao, X.; Xu, F.; Cai, C. Photocatalytic degradation of phenol in aqueous nitrogen-doped TiO2 suspensions with various light sources. Appl. Catal. B Environ. 2005, 57, 223–231. [Google Scholar] [CrossRef]
- Mer, V.K.L. Nucleation in Phase Transitions. Ind. Eng. Chem. 1952, 44, 1270–1277. [Google Scholar] [CrossRef]
- Sun, X.; Li, Y. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. Engl. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Szabo, T.; Berkesi, O.; Forgo, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dekany, I. Evolution of surface functional groups in a series of progressively oxidized graphite oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Jiang, X.; Niu, D.; Yu, A.; Liu, Z.; Li, J.G. A Two-Step Hydrothermal Synthesis Approach to Monodispersed Colloidal Carbon Spheres. Nanoscale Res. Lett. 2009, 4, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Vajda, K.; Kasa, Z.; Dombi, A.; Nemeth, Z.; Kovacs, G.; Danciu, V.; Radu, T.; Ghica, C.; Baia, L.; Hernadi, K.; et al. “Crystallographic” holes: New insights for a beneficial structural feature for photocatalytic applications. Nanoscale 2015, 7, 5776–5786. [Google Scholar] [CrossRef] [PubMed]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Investigation of stretching vibrations of glycosidic linkages in disaccharides and polysaccarides with use of IR spectra deconvolution. Biopolymers 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Karimi, S.; Feizy, J.; Mehrjo, F.; Farrokhnia, M. Detection and quantification of food colorant adulteration in saffron sample using chemometric analysis of FT-IR spectra. RSC Adv. 2016, 6, 23085–23093. [Google Scholar] [CrossRef]
- Busani, T.; Devine, R.A.B. Dielectric and infrared properties of TiO2 films containing anatase and rutile. Semicond. Sci. Technol. 2005, 20, 870–875. [Google Scholar] [CrossRef]
- Mahyar, A.; Amani-Ghadim, A.R. Influence of solvent type on the characteristics and photocatalytic activity of TiO2 nanoparticles prepared by the sol–gel method. Micro Nano Lett. 2011, 6, 244. [Google Scholar] [CrossRef]
- Orlikowski, J.; Tryba, B.; Ziebro, J.; Morawski, A.W.; Przepiórski, J. A new method for preparation of rutile phase titania photoactive under visible light. Catal. Commun. 2012, 24, 5–10. [Google Scholar] [CrossRef]
- Ye, X.; Zheng, C.; Ma, L.; Huang, X. Microemulsion-assisted hydrothermal preparation and infrared radiation property of TiO2 nanomaterials with tunable morphologies and crystal form. Mater. Sci. Semicond. Process. 2015, 31, 295–301. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic Investigation of the Anatase-to-Rutile Transformation of Sol−Gel-Synthesized TiO2 Photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Xiong, L.-B.; Li, J.-L.; Yang, B.; Yu, Y. Ti3+ in the Surface of Titanium Dioxide: Generation, Properties and Photocatalytic Application. J. Nanomater. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Pap, Z.; Karácsonyi, É.; Cegléd, Z.; Dombi, A.; Danciu, V.; Popescu, I.C.; Baia, L.; Oszkó, A.; Mogyorósi, K. Dynamic changes on the surface during the calcination of rapid heat treated TiO2 photocatalysts. Appl. Catal. B Environ. 2012, 111, 595–604. [Google Scholar] [CrossRef]
- Pap, Z.; Danciu, V.; Cegléd, Z.; Kukovecz, Á.; Oszkó, A.; Dombi, A.; Mogyorósi, K. The influence of rapid heat treatment in still air on the photocatalytic activity of titania photocatalysts for phenol and monuron degradation. Appl. Catal. B Environ. 2011, 101, 461–470. [Google Scholar] [CrossRef]
- Pap, Z.; Mogyorósi, K.; Veréb, G.; Dombi, A.; Hernádi, K.; Danciu, V.; Baia, L. Commercial and home-made nitrogen modified titanias. A short reflection about the advantageous/disadvantageous properties of nitrogen doping in the frame of their applicability. J. Mol. Struct. 2014, 1073, 157–163. [Google Scholar] [CrossRef]











| CS Purification Method | Washing Cycles | TiO2 | TiO2:CS Weight Ratio | Furnace | Quality of HSs |
|---|---|---|---|---|---|
| Membrane filtration | 3 | H2_HS_1 | 0.84 | Muffle (without air supply) | * |
| Membrane filtration | 1 | H2_HS_2 | 0.26 | Muffle (without air supply) | ** |
| Membrane filtration | 3 | H2_HS_3 | 0.26 | Muffle (without air supply) | ** |
| Membrane filtration | 5 | H2_HS_4 | 0.26 | Muffle (without air supply) | ** |
| Centrifugation | - | H2_HS_5 | 0.26 | Muffle (without air supply) | *** |
| Centrifugation | - | H2_HS_6 | 0.26 | Tube (with constant air supply) | *** |
| Titanium-dioxide | Phase Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anatase | Rutile | ||||||||
| wt% | Particle Size (nm) | wt% | Particle Size (nm) | Average Diameter (nm) | Specific Surface Area (m−2 g−1) | Band Gap (eV) | r0,phenol (10−10 M s−1) | r0,phenol (10−12 M m−2 s−1) Surface Normalized | |
| H2_HS_1 | 87.72 | 9.7 | 12.28 | 10.7 | - | 89 | 3.14 | 8.1 | 9.1 |
| H2_HS_2 | 66.42 | 10.3 | 33.58 | 9.7 | 1202 | 70 | 3.14 | 6.03 | 8.6 |
| H2_HS_3 | 56.91 | 9.3 | 43.09 | 10.8 | 1137 | 65 | 3.15 | 5.26 | 8.1 |
| H2_HS_4 | 64.1 | 15 | 35.9 | 12.9 | 1237 | 46 | 3.13 | 7.02 | 15.3 |
| H2_HS_5 | 20.0 | 12.3 | 80.0 | 12.9 | 880 | 40 | 3.13 | 24.63 | 61.6 |
| H2_HS_6 | 4.8 | 18 | 95.2 | 15.5 | 943 | 46 | 3.13 | 13.65 | 29.7 |
| Rutile-H2 | <1 | - | >99 | 7.3 | - | 237 | 3.11 | 18.3 | 7.7 |
| Rutile-H2_calc | <1 | - | >99 | 14.1 | - | 39 | 3.09 | 3.4 | 8.8 |
| Aeroxide P25 | 90 | 25.4 | 10 | 40 | - | 49 | 3.11 | 12.3 | 25.1 |
| Species (at%) | Sample Name | ||
|---|---|---|---|
| H2_HS_2 | H2_HS_5 | H2_HS_6 | |
| OH_O* | 8.97 | 5.02 | 4.97 |
| Oxide_O* | 89.58 | 93.41 | 93.55 |
| Low_O* | 1.45 | 1.56 | 1.48 |
| C–O–C# | 13.09 | 12.07 | 11.11 |
| O-C=O (oxidized carbon)# | 7.72 | 15.19 | 12.38 |
| Total_C (at%) | 20.18 | 8.91 | 30.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyulavári, T.; Veréb, G.; Pap, Z.; Réti, B.; Baan, K.; Todea, M.; Magyari, K.; Szilágyi, I.M.; Hernadi, K. Utilization of Carbon Nanospheres in Photocatalyst Production: From Composites to Highly Active Hollow Structures. Materials 2019, 12, 2537. https://doi.org/10.3390/ma12162537
Gyulavári T, Veréb G, Pap Z, Réti B, Baan K, Todea M, Magyari K, Szilágyi IM, Hernadi K. Utilization of Carbon Nanospheres in Photocatalyst Production: From Composites to Highly Active Hollow Structures. Materials. 2019; 12(16):2537. https://doi.org/10.3390/ma12162537
Chicago/Turabian StyleGyulavári, Tamás, Gábor Veréb, Zsolt Pap, Balázs Réti, Kornelia Baan, Milica Todea, Klára Magyari, Imre Miklós Szilágyi, and Klara Hernadi. 2019. "Utilization of Carbon Nanospheres in Photocatalyst Production: From Composites to Highly Active Hollow Structures" Materials 12, no. 16: 2537. https://doi.org/10.3390/ma12162537
APA StyleGyulavári, T., Veréb, G., Pap, Z., Réti, B., Baan, K., Todea, M., Magyari, K., Szilágyi, I. M., & Hernadi, K. (2019). Utilization of Carbon Nanospheres in Photocatalyst Production: From Composites to Highly Active Hollow Structures. Materials, 12(16), 2537. https://doi.org/10.3390/ma12162537