Evaluation of a Ti–Base Alloy as Steam Cracking Reactor Material
Abstract
:1. Introduction
2. Experimental Section
2.1. ElectroBalance Unit
2.2. Experimental Procedures and Conditions
2.3. Surface Characterization
2.4. Coke Formation Mechanisms
3. Experimental Results
3.1. Product Yields and Coking Tendency of Ti–Base Alloys
3.1.1. Fe–Ni–Cr Alloy versus Ti–Base Alloy
3.1.2. Pretreatment Effect
3.2. Evaluation of Ti–Base Alloy Using SEM and EDX Analysis
3.2.1. Decoked Samples
3.2.2. Coked Samples
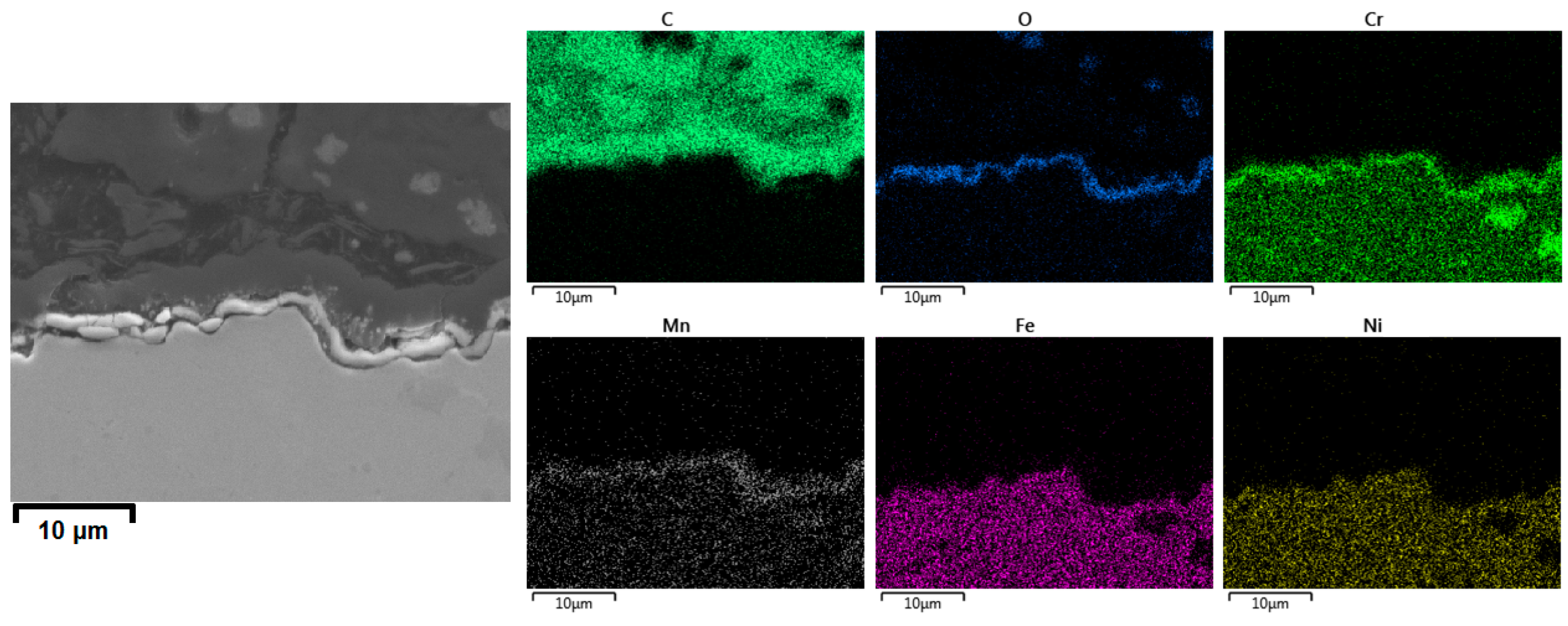
3.2.3. Cross-Sectional Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | atomic mass of element [g·mol−1] |
| EDM | wire-cut electrical discharge machining |
| EDX | energy dispersive X-ray analysis |
| FID | flame ionization detector |
| F | flow rate [10−6 kg s−1] |
| H | penetration depth [µm] |
| cc | Coking/decoking cycle number i |
| JSR | jet-stirred reactor |
| L | nominal thickness [μm] |
| mtj | mass of coke at time j [kg] |
| Mc | amount of coke deposited on the sample [g] |
| Ptot | reactor pressure [MPa] |
| P/E | ratio of propylene [wt.%] to ethylene [wt.%] |
| rc | coking rate [kg·s−1·m−2] |
| rc, initial | initial catalytic coking rate [kg·s−1·m−2] |
| rc, asymptotic | asymptotic coking rate [kg·s−1·m−2] |
| rx-y | average coking rate in between the given time intervals [kg·s−1·m−2] |
| RGA | refinery gas analyzer |
| S | surface area of the coupon [m2] |
| SEM | scanning electron microscopy |
| TCD | thermal conductivity detector |
| T | temperature [K] |
| V | accelerating voltage [kV] |
| z | atomic number |
| δ | dilution [kg·kg−1] |
| ρ | density of the material [g·cm-3] |
| τ | mean residence time [s] |
References
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Rao, M.R.; Plehiers, P.M.; Froment, G.F. The coupled simulation of heat transfer and reaction in a pyrolysis furnace. Chem. Eng. Sci. 1988, 43, 1223–1229. [Google Scholar]
- Stefanidis, G.; Merci, B.; Heynderickx, G.J.; Marin, G.B. CFD simulations of steam cracking furnaces using detailed combustion mechanisms. Comput. Chem. Eng. 2006, 30, 635–649. [Google Scholar] [CrossRef]
- Heynderickx, G.; Schools, E.; Marin, G. Optimization of the decoking procedure of an ethane cracker with a steam/air mixture. Ind. Eng. Chem. Res. 2006, 45, 7520–7529. [Google Scholar] [CrossRef]
- Brown, D.J. Internally finned radiant coils: A valuable tool for improving ethylene plant economics. In Proceedings of the 6th EMEA Petrochemicals Technology Conference, London, UK, 2004. [Google Scholar]
- Albano, J.; Sundaram, K.; Maddock, M. Application of Extended Surfaces in Pyrolysis Coils; American Institute of Chemical Engineers: New York, NY, USA, 1988. [Google Scholar]
- Schietekat, C.M.; van Goethem, M.W.M.; Van Geem, K.M.; Marin, G.B. Swirl flow tube reactor technology: An experimental and computational fluid dynamics study. Chem. Eng. J. 2014, 238, 56–65. [Google Scholar] [CrossRef]
- Schietekat, C.M.; Van Cauwenberge, D.J.; Van Geem, K.M.; Marin, G.B. Computational fluid dynamics-based design of finned steam cracking reactors. AIChE J. 2014, 60, 794–808. [Google Scholar] [CrossRef]
- Wang, J.; Reyniers, M.-F.; Marin, G.B. The influence of phosphorus containing compounds on steam cracking of n-hexane. J. Anal. Appl. Pyrolysis 2006, 77, 133–148. [Google Scholar] [CrossRef]
- Wang, J.; Reyniers, M.-F.; Van Geem, K.M.; Marin, G.B. Influence of Silicon and Silicon/Sulfur-Containing Additives on Coke Formation during Steam Cracking of Hydrocarbons. Ind. Eng. Chem. Res. 2008, 47, 1468–1482. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Reyniers, M.-F.; Marin, G.B. Influence of Dimethyl Disulfide on Coke Formation during Steam Cracking of Hydrocarbons. Ind. Eng. Chem. Res. 2007, 46, 4134–4148. [Google Scholar] [CrossRef]
- Reyniers, M.-F.; Froment, G.F. Influence of metal-surface and sulfur addition on coke deposition in the thermal-cracking of hydrocarbons. Ind. Eng. Chem. Res. 1995, 34, 773–785. [Google Scholar] [CrossRef]
- Dhuyvetter, I.; Reyniers, M.-F.; Froment, G.F.; Marin, G.B.; Viennet, D. The influence of dimethyl disulfide on naphtha steam cracking. Ind. Eng. Chem. Res. 2001, 40, 4353–4362. [Google Scholar] [CrossRef]
- Bajus, M.; Vesely, V.; Baxa, J.; Leclercq, P.A.; Rijks, J.A. Steam cracking of hydrocarbons: 5. Effect of thiophene on reaction-kinetics and coking. Ind. Eng. Chem. Prod. Res. Dev. 1981, 20, 741–745. [Google Scholar] [CrossRef]
- Bajus, M.; Vesely, V. Pyrolysis of hydrocarbons in the presence of elemental sulfur. Collect. Czechoslov. Chem. Commun. 1980, 45, 238–254. [Google Scholar] [CrossRef]
- Bajus, M.; Baxa, J.; Leclercq, P.A.; Rijks, J.A. Steam cracking of hydrocarbons: 6. Effect of dibenzyl sulfide and dibenzyl disulfide on reaction-kinetics and coking. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 335–343. [Google Scholar] [CrossRef]
- Bajus, M.; Baxa, J. Coke formation during the pyrolysis of hydrocarbons in the presence of sulfur-compounds. Collect. Czechoslov. Chem. Commun. 1985, 50, 2903–2909. [Google Scholar] [CrossRef]
- Depeyre, D.; Filcoteaux, C.; Blouri, B.; Ossebi, J.G. Pure norma-nonane steam cracking and the influence of sulfur-compounds. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 920–924. [Google Scholar] [CrossRef]
- Vaish, S.; Kunzru, D. Triphenyl phosphite as a coke inhibitor during naphtha pyrolysis. Ind. Eng. Chem. Res. 1989, 28, 1293–1299. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Kunzru, D. Reduction of coke formation during naphtha pyrolysis using triethyl phosphite. Ind. Eng. Chem. Res. 1988, 27, 559–565. [Google Scholar] [CrossRef]
- Olahová, N.; Sarris, S.A.; Reyniers, M.-F.; Marin, G.B.; Van Geem, K.M. Coking Tendency of 25Cr-35Ni Alloys: Influence of Temperature, Sulfur Addition, and Cyclic Aging. Ind. Eng. Chem. Res. 2018, 57, 3138–3148. [Google Scholar] [CrossRef]
- Muñoz Gandarillas, A.E.; Van Geem, K.M.; Reyniers, M.-F.; Marin, G.B. Influence of the Reactor Material Composition on Coke Formation during Ethane Steam Cracking. Ind. Eng. Chem. Res. 2014, 53, 6358–6371. [Google Scholar] [CrossRef]
- Muñoz Gandarillas, A.E.; Van Geem, K.M.; Reyniers, M.-F.; Marin, G.B. Coking Resistance of Specialized Coil Materials during Steam Cracking of Sulfur-Free Naphtha. Ind. Eng. Chem. Res. 2014, 53, 13644–13655. [Google Scholar] [CrossRef]
- Verdier, G.; Carpentier, F. Consider new materials for ethylene furnace applications: An innovative metallurgy solves maintenance issues. Hydrocarb. Process. 2011, 90, 61–62. [Google Scholar]
- Wolpert, P.; Ganser, B.; Jakobi, D.; Kirchheiner, R. Using Steam; Tube with Uniform Cross-Section; Pyrolysis; Antideposit Agents; Interior Fins; Swirling Gas Flow. Google Patents US20050131263A1, 16 June 2005. [Google Scholar]
- Jakobi, D.; van Moesdijk, C.; Karduck, P.; von Richthofen, A. Tailor-made materials for high temperature applications: New strategies for radiant coil material development. In Proceedings of the CORROSION 2009, Atlanta, GA, USA, 22–26 March 2009. [Google Scholar]
- Jakobi, D.; Karduck, P.; von Richthofen, A.F. The High-Temperature Corrosion Resistance of Spun-Cast Materials for Steam-Cracker Furnaces-A Comparative Study of Alumina-and Chromia-Forming Alloys. In Proceedings of the CORROSION 2013, Orlando, FL, USA, 17–21 March 2013. [Google Scholar]
- Asteman, H.; Hartnagel, W.; Jakobi, D. The Influence of Al Content on the High Temperature Oxidation Properties of State-of-the-Art Cast Ni-base Alloys. Oxid. Met. 2013, 80, 3–12. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, W.; Gao, X.; Zhou, D.; Lai, Y. Role of titanium carbides on microstructural evolution of Ti-35V-15Cr-0.3Si-0.1C alloy during hot working. J. Alloys Compd. 2016, 684, 201–210. [Google Scholar] [CrossRef]
- Rawlings, M.J.S.; Liebscher, C.H.; Asta, M.; Dunand, D.C. Effect of titanium additions upon microstructure and properties of precipitation-strengthened Fe-Ni-Al-Cr ferritic alloys. Acta Mater. 2017, 128, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Polmear, I.; StJohn, D.; Nie, J.-F.; Qian, M. 7-Titanium Alloys. In Light Alloys, 5th ed.; Butterworth-Heinemann: Boston, MA, USA, 2017; pp. 369–460. [Google Scholar]
- Petrone, S.S.A.; Mandyam, R.C.; Wysiekierski, A.G. Surface Alloyed High Temperature Alloys. Google Patents WO2001090441A2, 25 May 2000. [Google Scholar]
- Li, Y.; Zhang, Z.; Jia, P.; Dong, D.; Wang, Y.; Hu, S.; Xiang, J.; Liu, Q.; Hu, X. Ethanol steam reforming over cobalt catalysts: Effect of a range of additives on the catalytic behaviors. J. Energy Inst. 2019. [Google Scholar] [CrossRef]
- Zimmermann, H.; Walzl, R. “Ethylene” in Ullmann’s Encyclopedia of Industrial Chemistry; Wiley–VCH: Weinheim, Germany, 15 April 2009; Volume 12. [Google Scholar]
- Tang, S.; Shi, N.; Wang, J.; Tang, A. Comparison of the anti-coking performance of CVD TiN, TiO2 and TiC coatings for hydrocarbon fuel pyrolysis. Ceram. Int. 2017, 43, 3818–3823. [Google Scholar] [CrossRef]
- Sarris, S.A.; Olahova, N.; Verbeken, K.; Reyniers, M.-F.; Marin, G.B.; Van Geem, K.M. Optimization of the in-situ pretreatment of high temperature Ni-Cr Alloys for Ethane steam cracking. Ind. Eng. Chem. Res. 2017, 56, 1424–1438. [Google Scholar] [CrossRef]
- Sarris, S.A.; Patil, M.; Verbeken, K.; Reyniers, M.-F.; Van Geem, K.M. Effect of Long-Term High Temperature Oxidation on the Coking Behavior of Ni-Cr Superalloys. Materials 2018, 11, 1899. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Zimmermann, G.; Nowak, S. On the mechanism of coke formation in steam cracking—Conclusions from results obtained by tracer experiments. Carbon 1988, 26, 117–124. [Google Scholar] [CrossRef]
- Cai, H.; Krzywicki, A.; Oballa, M.C. Coke formation in steam crackers for ethylene production. Chem. Eng. Process. Process Intensif. 2002, 41, 199–214. [Google Scholar] [CrossRef]
- Albright, L.F.; Marek, J.C. Mechanistic model for formation of coke in pyrolysis units producing ethylene. Ind. Eng. Chem. Res. 1988, 27, 755–759. [Google Scholar] [CrossRef]
- Lahaye, J.; Badie, P.; Ducret, J. Mechanism of carbon formation during steamcracking of hydrocarbons. Carbon 1977, 15, 87–93. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Yates, D.J.C.; Dumesic, J.A. Filamentous Carbon Formation over Iron Surfaces. In Coke Formation on Metal Surfaces; American Chemical Society: Washington, WA, USA, 1983; Volume 202, pp. 1–21. [Google Scholar]
- Lyle, F.A.; Baker, R.T.K. Coke Formation on Metal Surfaces; American Chemical Society: Washington, WA, USA, 1983; Volume 202, p. 332. [Google Scholar]
- Figueiredo, J.L. Reactivity of coke deposited on metal surfaces. Mater. Corros. 1999, 50, 696–699. [Google Scholar] [CrossRef]
- Snoeck, J.-W.; Froment, G.; Fowles, M. Filamentous carbon formation and gasification: Thermodynamics, driving force, nucleation, and steady-state growth. J. Catal. 1997, 169, 240–249. [Google Scholar] [CrossRef]
- Bonnet, F.; Ropital, F.; Berthier, Y.; Marcus, P. Filamentous carbon formation caused by catalytic metal particles from iron oxide. Mater. Corros. 2003, 54, 870–880. [Google Scholar] [CrossRef]
- Ranzi, E.; Dente, M.; Goldaniga, A.; Bozzano, G.; Faravelli, T. Lumping procedures in detailed kinetic modeling of gasification, pyrolysis, partial oxidation and combustion of hydrocarbon mixtures. Prog. Energy Combust. Sci. 2001, 27, 99–139. [Google Scholar] [CrossRef]
- Kopinke, F.D.; Zimmermann, G.; Reyniers, G.C.; Froment, G.F. Relative rates of coke formation from hydrocarbons in steam cracking of naphtha. 2. Paraffins, naphthenes, mono-, di-, and cycloolefins, and acetylenes. Ind. Eng. Chem. Res. 1993, 32, 56–61. [Google Scholar] [CrossRef]
- Wauters, S.; Marin, G.B. Computer generation of a network of elementary steps for coke formation during the thermal cracking of hydrocarbons. Chem. Eng. J. 2001, 82, 267–279. [Google Scholar] [CrossRef]
- Reyniers, G.C.; Froment, G.F.; Kopinke, F.-D.; Zimmermann, G. Coke Formation in the Thermal Cracking of Hydrocarbons. 4. Modeling of Coke Formation in Naphtha Cracking. Ind. Eng. Chem. Res. 1994, 33, 2584–2590. [Google Scholar] [CrossRef]
- Bach, G.; Zimmermann, G.; Kopinke, F.-D.; Barendregt, S.; van den Oosterkamp, P.; Woerde, H. Transfer-Line Heat Exchanger Fouling during Pyrolysis of Hydrocarbons. 1. Deposits from Dry Cracked Gases. Ind. Eng. Chem. Res. 1995, 34, 1132–1139. [Google Scholar] [CrossRef]
- Kumar, S.; Sankara Narayanan, T.S.N.; Ganesh Sundara Raman, S.; Seshadri, S.K. Thermal oxidation of Ti6Al4V alloy: Microstructural and electrochemical characterization. Mater. Chem. Phys. 2010, 119, 337–346. [Google Scholar] [CrossRef]
- Kofstad, P.; Anderson, P.B.; Krudtaa, O.J. Oxidation of titanium in the temperature range 800–1200 °C. J. Less Common Met. 1961, 3, 89–97. [Google Scholar] [CrossRef]
- Kofstad, P. High-temperature oxidation of titanium. J. Less Common Met. 1967, 12, 449–464. [Google Scholar] [CrossRef]
- Kumar, S.; Narayanan, T.S.N.S.; Raman, S.G.S.; Seshadri, S.K. Thermal oxidation of CP-Ti: Evaluation of characteristics and corrosion resistance as a function of treatment time. Mater. Sci. Eng. C 2009, 29, 1942–1949. [Google Scholar] [CrossRef]









| Pretreatment Id | Steps | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| A | 6.7 × 10−3 Nl s−1 of Air; 14 h; T = 1023 K | 1:1 8.15 × 10−3 Nl s−1 of Air:N2; 30 min; T = 1023–1173 K | 8.15 × 10−3 Nl s−1 of Air and 6.67 × 10−6 kg s−1 Steam; 15 min; T = 1173 K |
| B | 6.7 × 10−3 Nl s−1 of Air; 14 h; T = 1023 K | 1:1 8.15 × 10−3 Nl s−1 of Air:N2; 30 min; T = 1023–1273 K | 8.15 × 10−3 Nl s−1 of Air and 6.67 × 10−6 kg s−1 Steam; 15 min; T = 1273 K |
| Pretreatment A | |||||||||||
| Temperature | 1023 K | 1023–1173 K | 1173 K | 1173 K | 1023–1173 K | 1173 K | 1173 K | 1023–1173 K | 1173 K | 1173–273 K | |
| Feed | Air | N2 + Air | Air + H2O | C2H6 + H2O | N2 + Air | Air + H2O | C2H6 + H2O | N2 + Air | Air + H2O | He | |
| Step | In situ Preoxidation 14 h | mild Preoxidation 30 min | Steam Treatment 15 min | 1st coking cycle of 6 h | Decoking 30 min | Steam Treatment 15 min | 2nd–8th coking cycle of [2nd, 3rd, and 8th of 6 h, 4–7th of 1 h] | Decoking 30 min | Steam Treatment 15 min | Cooling down | time |
| Pretreatment B | |||||||||||
| Temperature | 1023 K | 1023–1273 K | 1273 K | 1173 °C | 1023–1273 K | 1273 K | 1173 K | 1023–1273 K | 1273 K | 1273–273 K | |
| Feed | Air | N2 + Air | Air + H2O | C2H6 + H2O | N2 + Air | Air + H2O | C2H6 + H2O | N2 + Air | Air + H2O | He | |
| Step | In situ Preoxidation 14 h | High T Preoxidation 50 min | Steam Treatment 15 min | 1st coking cycle of 6 h | Decoking at higher T 50 min | Steam Treatment 15 min | 2nd–8th coking cycle of [2nd, 3rd, and 8th of 6 h, 4–7th of 1 h] | Decoking at higher T 50 min | Steam Treatment 15 min | Cooling down | time |
| Components [wt.%] | Fe–Ni–Cr Alloy | Ti–Base Alloy | Ti–Base Alloy |
|---|---|---|---|
| Pretreatment | A | A | B |
| H2 [±0.07] | 4.24 | 4.39 | 4.45 |
| CO2 [±0.003] | 0.006 | 0.005 | 0.019 |
| CO | 0.05 1 | 0.56 2 | 1.87 3 |
| CH4 [±0.21] | 6.99 | 6.97 | 6.91 |
| C2H6 [±0.52] | 30.13 | 30.19 | 30.06 |
| C2H4 [±0.25] | 49.86 | 49.61 | 49.57 |
| C3H8 [±0.03] | 0.11 | 0.11 | 0.11 |
| C3H6 [±0.02] | 0.74 | 0.70 | 0.69 |
| C2H2 [±0.05] | 1.38 | 1.46 | 1.41 |
| 1,3-C4H6 [±0.07] | 2.00 | 1.93 | 1.89 |
| Benzene [±0.13] | 2.46 | 2.53 | 2.53 |
| Alloy | Fe–Ni–Cr Alloy | Ti–Base Alloy | Ti–Base Alloy |
|---|---|---|---|
| Conditions | Blank | Blank | Blank |
| Pretreatment | A | A | B |
| Cracking Temperature (K) | 1173 | 1173 | 1173 |
| dilution | 0.33 | 0.33 | 0.33 |
| cc | Coke Formed (mg) | ||
| 1 | 1.29 | 5.48 | 3.66 |
| 2 | 1.26 | 9.01 | 4.99 |
| 3 | 1.41 | 7.43 | 4.82 |
| 4 | 0.60 | 3.57 | 2.93 |
| 5 | 0.67 | 3.03 | 2.17 |
| 6 | 0.69 | 2.97 | 2.13 |
| 7 | 0.47 | 2.95 | 2.32 |
| 8 | 1.19 | 7.69 | 5.35 |
| cc | Initial Coking Rate [10−6 kg/s/m2] | ||
| 1 | 0.71 | 1.73 | 1.23 |
| 2 | 0.67 | 3.26 | 1.81 |
| 3 | 0.86 | 3.65 | 2.59 |
| 4 | 0.85 | 3.80 | 3.11 |
| 5 | 0.96 | 3.91 | 2.76 |
| 6 | 0.97 | 3.80 | 2.70 |
| 7 | 0.67 | 3.79 | 2.79 |
| 8 | 0.63 | 3.27 | 2.46 |
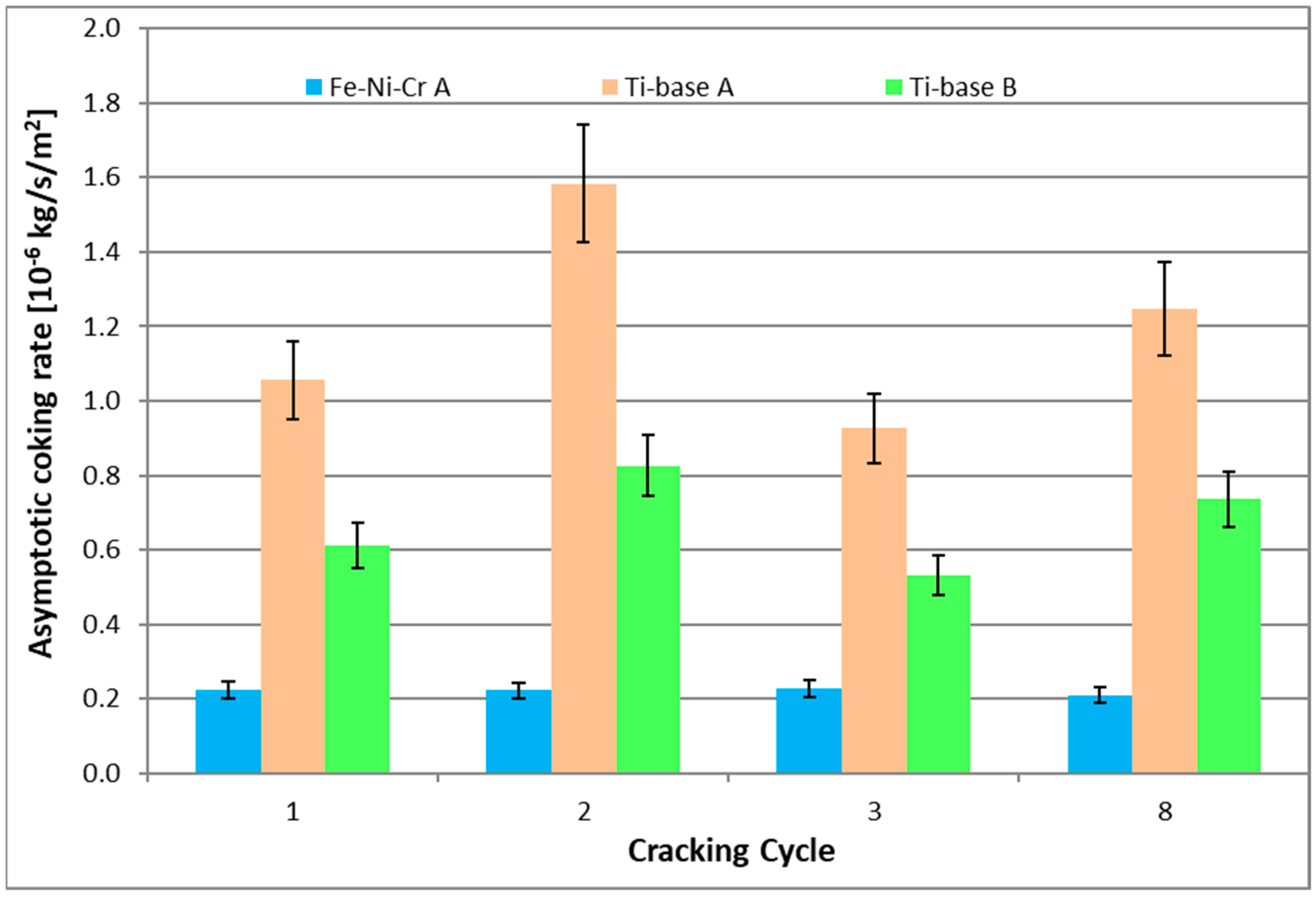
| cc | Asymptotic Coking Rate [10−6 kg/s/m2] | ||
| 1 | 0.22 | 1.06 | 0.61 |
| 2 | 0.22 | 1.58 | 0.83 |
| 3 | 0.23 | 0.93 | 0.53 |
| 8 | 0.21 | 1.25 | 0.74 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarris, S.A.; Verbeken, K.; Reyniers, M.-F.; Van Geem, K.M. Evaluation of a Ti–Base Alloy as Steam Cracking Reactor Material. Materials 2019, 12, 2550. https://doi.org/10.3390/ma12162550
Sarris SA, Verbeken K, Reyniers M-F, Van Geem KM. Evaluation of a Ti–Base Alloy as Steam Cracking Reactor Material. Materials. 2019; 12(16):2550. https://doi.org/10.3390/ma12162550
Chicago/Turabian StyleSarris, Stamatis A., Kim Verbeken, Marie-Françoise Reyniers, and Kevin M. Van Geem. 2019. "Evaluation of a Ti–Base Alloy as Steam Cracking Reactor Material" Materials 12, no. 16: 2550. https://doi.org/10.3390/ma12162550
APA StyleSarris, S. A., Verbeken, K., Reyniers, M.-F., & Van Geem, K. M. (2019). Evaluation of a Ti–Base Alloy as Steam Cracking Reactor Material. Materials, 12(16), 2550. https://doi.org/10.3390/ma12162550







