A Comparative Study of Localized Corrosion and Stress Corrosion Cracking of 13Cr Martensitic Stainless Steel Using Acoustic Emission and X-ray Computed Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Specimen Preparations
2.2. Microstructure Characterization and Precipitation Identification
2.3. SCC Testing and AE Measurements
2.4. X-ray CT and SEM-EBSD Measurements
3. Results
3.1. Microstructure and Precipitates
3.2. Corrosion Processes
3.3. Corrosion Morphologies
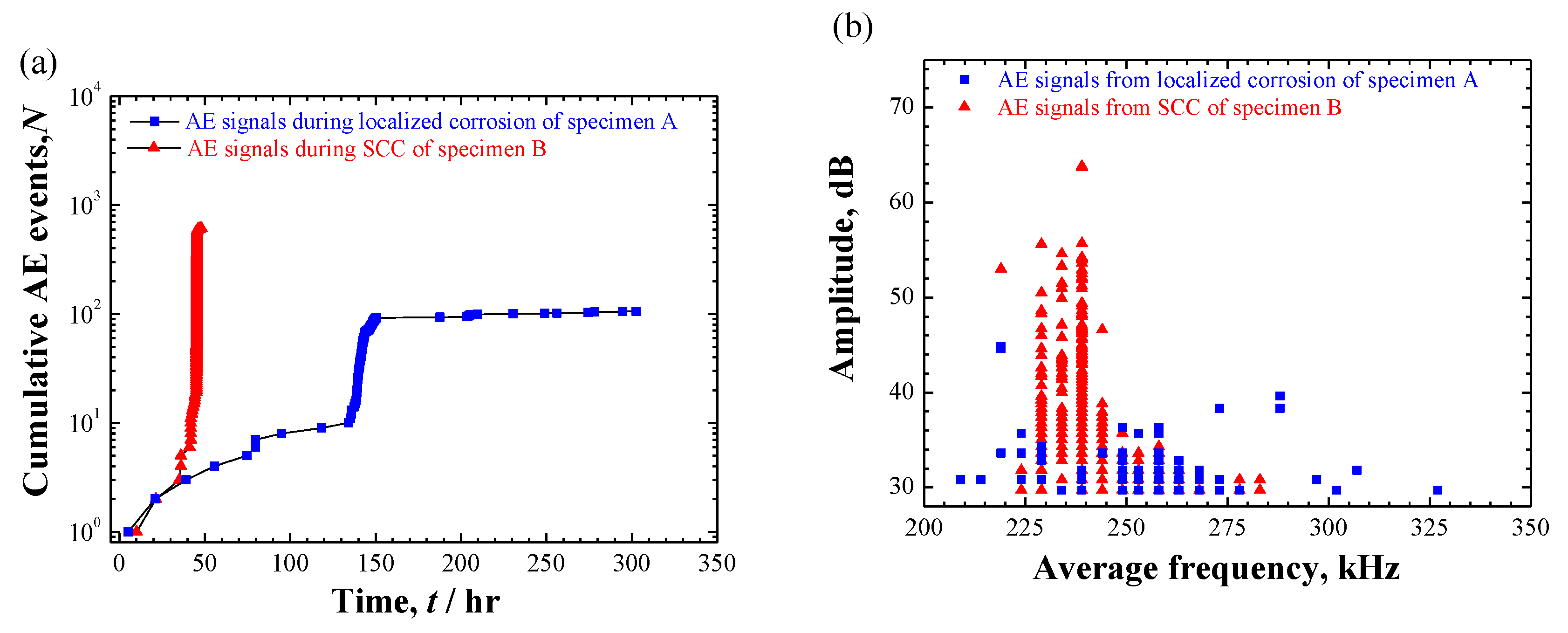
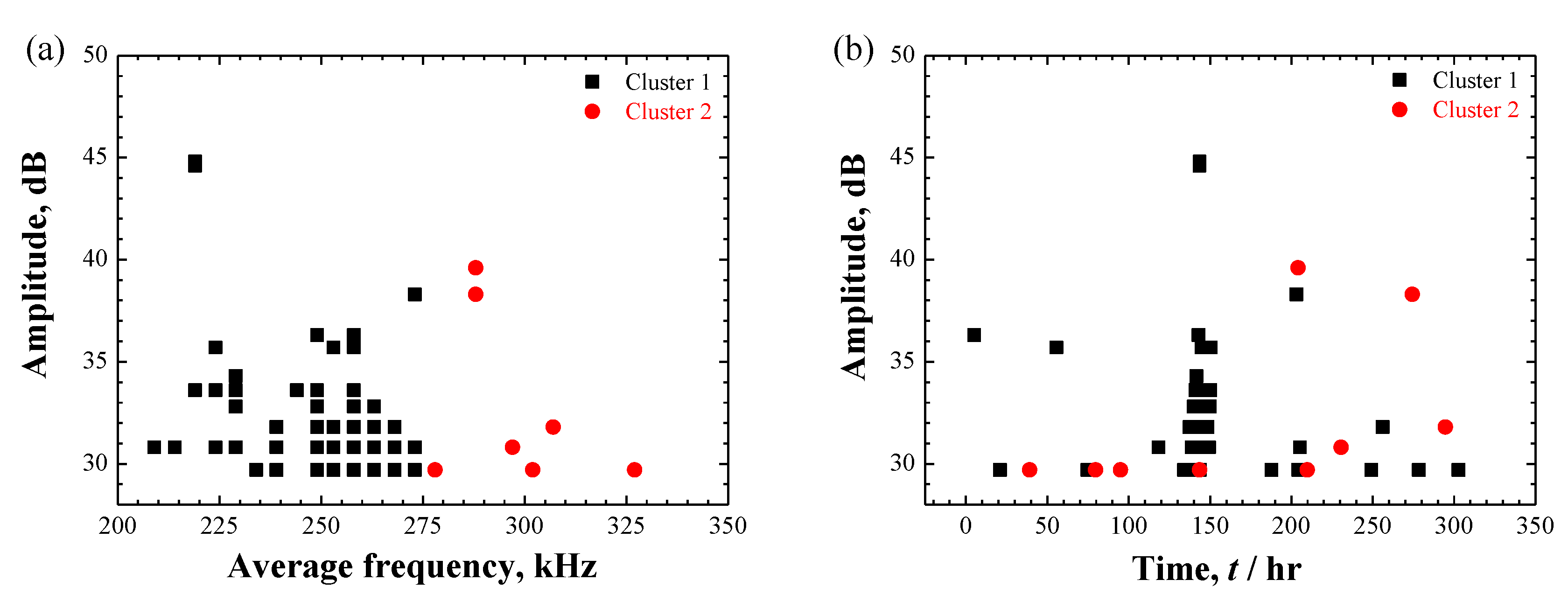
3.4. AE Results
3.4.1. AE Signals during Localized Corrosion in Specimen A
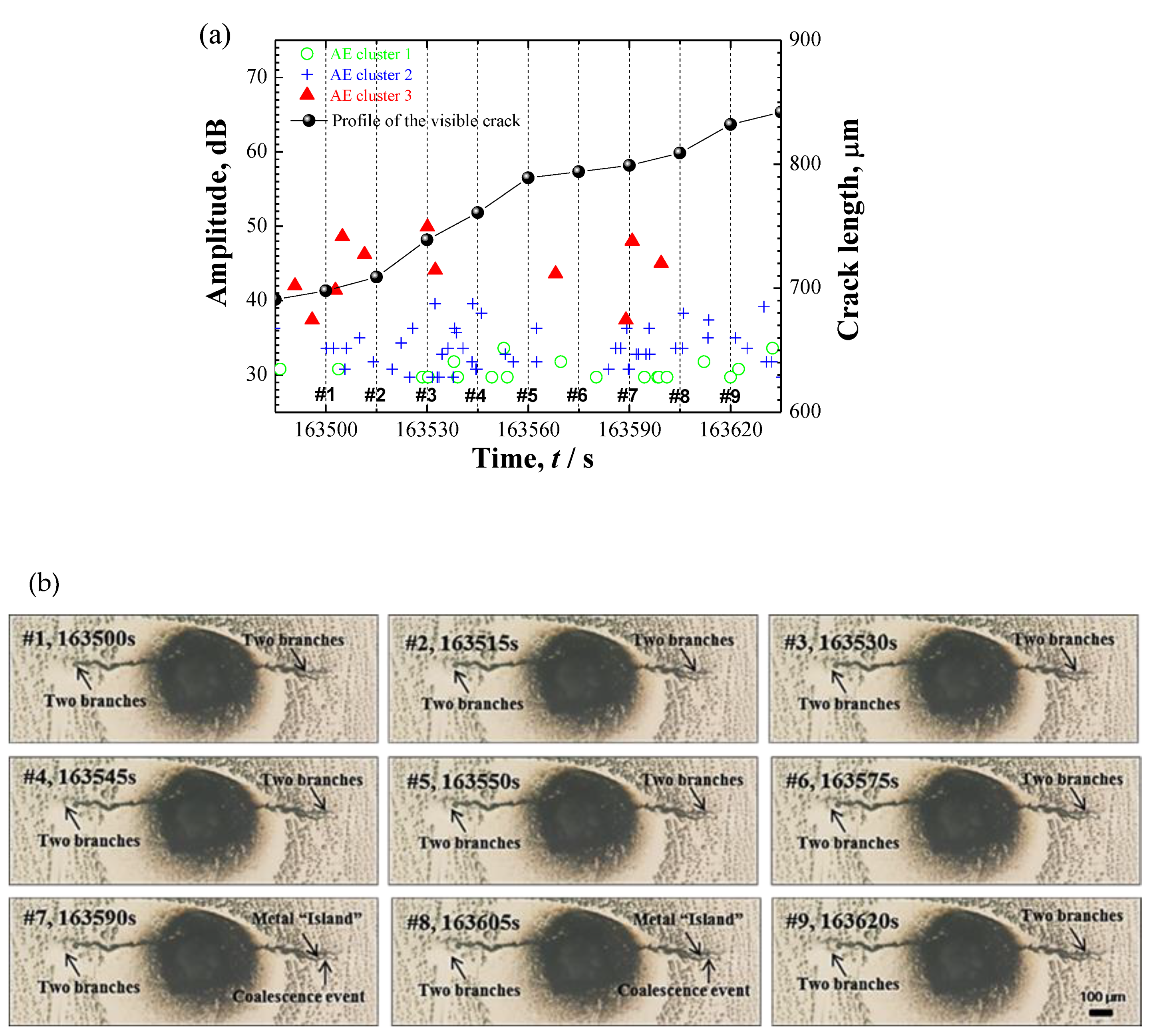
3.4.2. AE Signals during SCC in Specimen B
4. Discussion
4.1. Effect of Heat Treatment on the Corrosion Behavior of 13Cr MSSs
4.2. Relationship between Corrosion Process and AE Behavior
4.3. Uncertainty on AE Classification and Source Identification
5. Conclusions
- (1)
- The corrosion susceptibility of SUS420J2 seemed to largely depend on the microstructure in relation to the Cr-rich M23C6 carbides and residual stress due to phase transformation during quenching treatment. With an improved droplet SCC testing, localized corrosion as the early precursor stage to SCC was intentionally controlled in a 500 °C-tempered steel specimen-A. Initiation and propagation of SCC were intended to be accelerated in the specimen-B of as-quenched condition with high-residual stress and low toughness.
- (2)
- Localized corrosion was featured by an irregular pit with deposited corrosion products containing cracks; while the SCC was observed to be initiated from a corrosion pit and propagated with a 3D tortuous and discontinuous morphology that might be segmented by some voids, rather than the pseudo-continuous from 2D characterization.
- (3)
- AE signals were detected in both cases. In case of localized corrosion, two clusters with different frequency level were recognized. Low-frequency AE cluster was believed to be from the cracking within the deposited corrosion products; high-frequency cluster was proposed to be associated with hydrogen-bubble evolution. Contrastingly, three AE clusters were divided in the case of crack propagation. Except for the high-frequency hydrogen-bubble evolution, plastic deformation and cracking process seemingly could be separated by the use of AE amplitude-parameter. The distinction of AE features between localized corrosion and cracking propagation could be used for in-situ corrosion monitoring against SCC-induced premature failures.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Truman, J.E. Stress-corrosion cracking of martensitic and ferritic stainless steels. Int. Mater. Rev. 1981, 26, 301–349. [Google Scholar] [CrossRef]
- Turnbull, A. Corrosion pitting and environmentally assisted small crack growth. Proc. R. Soc. A 2014, 470, 20140254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono, K. Acoustic emission in materials research–a review. J. Acoust. Emission 2011, 29, 284–308. [Google Scholar]
- Magaino, S.; Kawaguchi, A.; Hirata, A.; Osaka, T. Spectrum analysis of corrosion potential fluctuations for localized corrosion of type 304 stainless steel. J. Electrochem. Soc. 1987, 134, 2993–2997. [Google Scholar] [CrossRef]
- Wu, K.; Jung, W.S.; Byeon, J.W. In-situ monitoring of pitting corrosion on vertically positioned 304 stainless steel by analyzing acoustic-emission energy parameter. Corros. Sci. 2016, 105, 8–16. [Google Scholar] [CrossRef]
- Wu, K.; Byeon, J.W. Morphological estimation of pitting corrosion on vertically positioned 304 stainless steel using acoustic-emission duration parameter. Corros. Sci. 2019, 148, 331–337. [Google Scholar] [CrossRef]
- Jirarungsatian, C.; Prateepasen, A. Pitting and uniform corrosion source recognition using acoustic emission parameters. Corros. Sci. 2010, 52, 187–197. [Google Scholar] [CrossRef]
- Shiwa, M.; Masuda, H.; Yamawaki, H.; Ito, K.; Enoki, M. In-situ observation and acoustic emission analysis for corrosion pitting of MgCl2 droplet in SUS304 stainless steel. Mater. Trans. 2012, 53, 1069–1074. [Google Scholar] [CrossRef]
- Fregonese, M.; Idrissi, H.; Mazille, H.; Renaud, L.; Cetre, Y. Initiation and propagation steps in pitting corrosion of austenitic stainless steels: monitoring by acoustic emission. Corros. Sci. 2001, 43, 627–641. [Google Scholar] [CrossRef]
- Darowicki, K.; Mirakowski, A.; Krakowiak, S. Investigation of pitting corrosion of stainless steel by means of acoustic emission and potentiodynamic methods. Corros. Sci. 2003, 45, 1747–1756. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Han, E.H. Acoustic emission during pitting corrosion of 304 stainless steel. Corros. Sci. 2011, 53, 1537–1546. [Google Scholar] [CrossRef]
- Wu, K.; Jung, W.S.; Byeon, J.W. Acoustic emission of hydrogen bubbles on the counter electrode during pitting corrosion of 304 stainless steel. Mater. Trans., 2015, 56, 587–592. [Google Scholar] [CrossRef]
- Wu, K.; Jung, W.S.; Byeon, J.W. Evaluation of corrosion critical variables of 304 stainless steel by delay time of acoustic emission. Mater. Trans., 2015, 56, 398–403. [Google Scholar] [CrossRef]
- Yonezu, A.; Cho, H.; Takemoto, M. Monitoring of stress corrosion cracking in stainless steel weldments by acoustic and electrochemical measurements. Meas. Sci. Technol. 2006, 17, 2447–2454. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Galeano, M.; Proverbio, E.; Pietro, D.D.; Cappuccini, F. Identification of damage evolution during SCC on 17–4 pH stainless steel by combining electrochemical noise and acoustic emission techniques. Corros. Sci. 2015, 98, 573–584. [Google Scholar] [CrossRef]
- Calabrese, L.; Galeano, M.; Proverbio, E.; Pietro, D.D.; Cappuccini, F.; Donato, A. Monitoring of 13% Cr martensitic stainless steel corrosion in chloride solution in presence of thiosulphate by acoustic emission technique. Corros. Sci. 2016, 111, 151–161. [Google Scholar] [CrossRef]
- Xu, J.; Wu, X.; Han, E.-H. Acoustic emission response of sensitized 304 stainless steel during intergranular corrosion and stress corrosion cracking. Corros. Sci. 2013, 73, 262–273. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, X.; Tan, J. In-situ monitoring of stress corrosion cracking of 304 stainless steel in high-temperature water by analyzing acoustic emission waveform. Corros. Sci. 2019, 146, 90–98. [Google Scholar] [CrossRef]
- Shiwa, M.; Masuda, H.; Yamawaki, H.; Ito, K.; Enoki, M. In-situ observation and acoustic emission analysis for SCC of MgCl2 droplet in SUS304 stainless steel. Mater. Trans. 2014, 55, 285–289. [Google Scholar] [CrossRef]
- Cho, H.; Takemoto, M. Acoustic Emission from Rust in Stress Corrosion Cracking. In Proceedings of the 26th European Conference on Acoustic Emission Testing, Berlin, Germany, 15–17 September 2004. [Google Scholar]
- Yonezu, A.; Taniyama, Y.; Cho, H.; Ogawa, T.; Takemoto, M. Acoustic emission and potential fluctuation during chloride SCC with oxide in its surface. J. Soc. Mater. Sci. Jpn. 2006, 55, 211–217. [Google Scholar] [CrossRef]
- Ito, K.; Yamawaki, H.; Masuda, H.; Shiwa, M.; Enoki, M. SCC monitoring of chloride droplets on thin SUS304 plate specimens by analysis of continuous recorded AE waveform. Mater. Trans. 2010, 51, 1409–1413. [Google Scholar] [CrossRef]
- Takemoto, M.; Ueno, S.; Nakamura, M. Source of AEs from IG-SCC of face centered cubic metals under static or dynamic straining. J. Acoust. Emission. 2017, S32–S37. [Google Scholar]
- Smanio, V.; Kittel, J.; Fregonese, M.; Cassagne, T.; Normand, B.; Ropital, F. Acoustic emission monitoring of wet H2S cracking of linepipe steels: Application to hydrogen-induced cracking and stress-oriented hydrogen-induced cracking. Corrosion 2011, 67, 065002-1–065002-12. [Google Scholar] [CrossRef]
- Du, G.; Li, J.; Wang, W.K.; Jiang, C.; Song, S.Z. Detection and characterization of stress-corrosion cracking on 304 stainless steel by electrochemical noise and acoustic emission techniques. Corros. Sci. 2011, 53, 2918–2926. [Google Scholar] [CrossRef]
- Kovac, J.; Alaux, C.; Marrow, T.J.; Govekar, E.; Legat, A. Correlations of electrochemical noise, acoustic emission and complementary monitoring techniques during intergranular stress-corrosion cracking of austenitic stainless steel. Corros. Sci. 2010, 52, 2015–2025. [Google Scholar] [CrossRef]
- ASTM G129-00. Standard Practice for Slow Strain Rate Testing to Evaluate the Susceptibility of Metallic Materials to Environmentally Assisted Cracking; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM G36-94. Standard Practice for Evaluating Stress-Corrosion-Cracking Resistance of Metals and Alloys in a Boiling Magnesium Chloride Solution; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- NACE TM0177. Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments; NACE International (NACE): Houston, TX, USA, 2016. [Google Scholar]
- Wu, K.; Briffod, F.; Ito, K.; Shinozaki, I.; Chivavibul, P.; Enoki, M. In-situ observation and acoustic emission monitoring of the initiation-to-propagation transition of stress corrosion cracking in SUS420J2 stainless steel. Mater. Trans. 2019. [Google Scholar] [CrossRef]
- Wu, K.; Ito, K.; Shinozaki, I.; Enoki, M. Stress corrosion cracking monitoring of 13Cr martensitic stainless steel under chloride solution droplet by in-situ observation and acoustic emission clustering analysis. Corros. Sci. (under review).
- Connolly, B.J.; Horner, D.A.; Fox, S.J.; Davenport, A.J.; Padovani, C.; Zhou, S.; Turnbull, A.; Preuss, M.; Stevens, N.P.; Marrow, T.J.; et al. X-ray microtomography studies of localised corrosion and transitions to stress corrosion cracking. Mater. Sci. Technol. 2006, 22, 1076–1085. [Google Scholar] [CrossRef]
- Horner, D.A.; Connolly, B.J.; Zhou, S.; Crocker, L.; Turnbull, A. Novel images of the evolution of stress corrosion cracks from corrosion pits. Corros. Sci. 2011, 53, 3466–3485. [Google Scholar] [CrossRef]
- Zhu, L.K.; Yan, Y.; Li, J.X.; Qiao, L.J.; Volinsky, A.A. Stress corrosion cracking under low stress: Continuous or discontinuous cracks? Corros. Sci. 2014, 80, 350–358. [Google Scholar] [CrossRef]
- Frankel, G.S.; Sridhar, N. Understanding localized corrosion. Mater. Today 2008, 11, 38–44. [Google Scholar] [CrossRef]
- JIS G0567. Method of Elevated Temperature Tensile Test for Steels and Heat-Resisting Alloys; Japanese Industrial Standards Committee: Tokyo, Japan, 2019. [Google Scholar]
- Lu, S.Y.; Yao, K.F.; Chen, Y.B.; Wang, M.H.; Liu, X.; Ge, X. The effect of tempering temperature on the microstructure and electrochemical properties of a 13 wt.% Cr-type martensitic stainless steel. Electrochim. Acta 2015, 165, 45–55. [Google Scholar] [CrossRef]
- Davis, J.R. Corrosion Testing: Tests for Stress-Corrosion Cracking. In Metals Handbook Desk Edition, 2nd ed.; Davis, J.R. ASM International: Ohio, OH, USA, 2001; pp. 1348–1351. [Google Scholar]
- Ito, K.; Enoki, M. Acquisition and analysis of continuous acoustic emission waveform for classification of damage sources in ceramic fiber mat. Mater. Trans. 2007, 48, 1221–1226. [Google Scholar] [CrossRef]
- Anantha, K.H.; Örnek, C.; Ejnermark, S.; Thuvander, A.; Medvedeva, A.; Sjöström, J.; Pan, J. Experimental and modelling study of the effect of tempering on the susceptibility to environment-assisted cracking of AISI 420 martensitic stainless steel. Corros. Sci. 2019, 148, 83–93. [Google Scholar] [CrossRef]
- Bonagani, S.K.; Bathula, V.; Kain, V. Influence of tempering treatment on microstructure and pitting corrosion of 13 wt.% Cr martensitic stainless steel. Corros. Sci. 2018, 131, 340–354. [Google Scholar] [CrossRef]
- Li, S.X.; Hihara, L.H. Atmospheric corrosion initiation on steel from predeposited NaCl salt particles in high humidity atmospheres. Corros. Eng. Sci. Techn. 2010, 45, 49–56. [Google Scholar] [CrossRef]
- Sanchez, J.; Andrade, C.; Fullea, J. Reasons for crack arrest in stress corrosion cracking tests-crack propagation rate in high-strength steels. Corrosion 2009, 65, 368–375. [Google Scholar] [CrossRef]
- Qian, L.; Toda, H.; Uesugi, K.; Kobayashi, M.; Kobayashi, T. Direct observation and image-based simulation of three-dimensional tortuous crack evolution inside opaque materials. Phys. Rev. Lett. 2008, 100, 115505. [Google Scholar] [CrossRef]
- Galvele, J.R. Transport processes and the mechanism of pitting of metals. J. Electrochem. Soc. 1976, 123, 464–474. [Google Scholar] [CrossRef]
- Pistorius, P.C.; Burstein, G.T. Metastable pitting corrosion of stainless steel and the transition to stability. Philos. T. R. Soc. A 1992, 341, 531–559. [Google Scholar]
- Robertson, I.M. The effect of hydrogen on dislocation dynamics. Eng. Fract. Mech. 1999, 64, 649–673. [Google Scholar] [CrossRef]
- Martin, M.L.; Dadfarnia, M.; Nagao, A.; Wang, S.; Sofronis, P. Enumeration of the hydrogen-enhanced localized plasticity mechanism for hydrogen embrittlement in structural materials. Acta Mater. 2019, 165, 734–750. [Google Scholar] [CrossRef]
- Scruby, C.B.; Wadley, H.N.G.; Hill, J.J. Dynamic elastic displacements at the surface of an elastic half-space due to defect sources. J. Phys. D Appl. Phys. 1983, 16, 1069–1083. [Google Scholar] [CrossRef]
- Barsoum, F.F.; Suleman, J.; Korcak, A.; Hill, E.V. Acoustic emission monitoring and fatigue life prediction in axially loaded notched steel specimens. J. Acoust. Emission 2009, 27, 40–63. [Google Scholar]












| C | Si | Mn | P | S | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|
| 0.32 | 0.17 | 0.38 | 0.031 | 0.002 | 0.34 | 12.85 | Bal |
| Specimen # | Heat Treatment | Mechanical Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Annealing | Quenching | Tempering | E (GPa) | σ0.2 (MPa) | UTS (MPa) | EL (%) | RA (%) | HV | |
| A | 950 °C/2 h | Argon gas cooling | 500 °C/20 h | 218 | 1027 | 1169 | 12.5 | 45.6 | 367 |
| B | 950 °C/2 h | - | 211 | 1119 | 1699 | 9.6 | 28.4 | 511 | |
| Specimen # | Corrosion Test | Exposure | Corrosion Behavior | AE Events |
|---|---|---|---|---|
| A | U-bend, 1% NaCl-droplet 99% RH, RT. | 311 h | Localized corrosion | 106 |
| B | 48.5 h | SCC | 610 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, K.; Ito, K.; Shinozaki, I.; Chivavibul, P.; Enoki, M. A Comparative Study of Localized Corrosion and Stress Corrosion Cracking of 13Cr Martensitic Stainless Steel Using Acoustic Emission and X-ray Computed Tomography. Materials 2019, 12, 2569. https://doi.org/10.3390/ma12162569
Wu K, Ito K, Shinozaki I, Chivavibul P, Enoki M. A Comparative Study of Localized Corrosion and Stress Corrosion Cracking of 13Cr Martensitic Stainless Steel Using Acoustic Emission and X-ray Computed Tomography. Materials. 2019; 12(16):2569. https://doi.org/10.3390/ma12162569
Chicago/Turabian StyleWu, Kaige, Kaita Ito, Ippei Shinozaki, Pornthep Chivavibul, and Manabu Enoki. 2019. "A Comparative Study of Localized Corrosion and Stress Corrosion Cracking of 13Cr Martensitic Stainless Steel Using Acoustic Emission and X-ray Computed Tomography" Materials 12, no. 16: 2569. https://doi.org/10.3390/ma12162569





