Influence of Different Pretreatments on the Structure and Hydrolysis Behavior of Bamboo: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
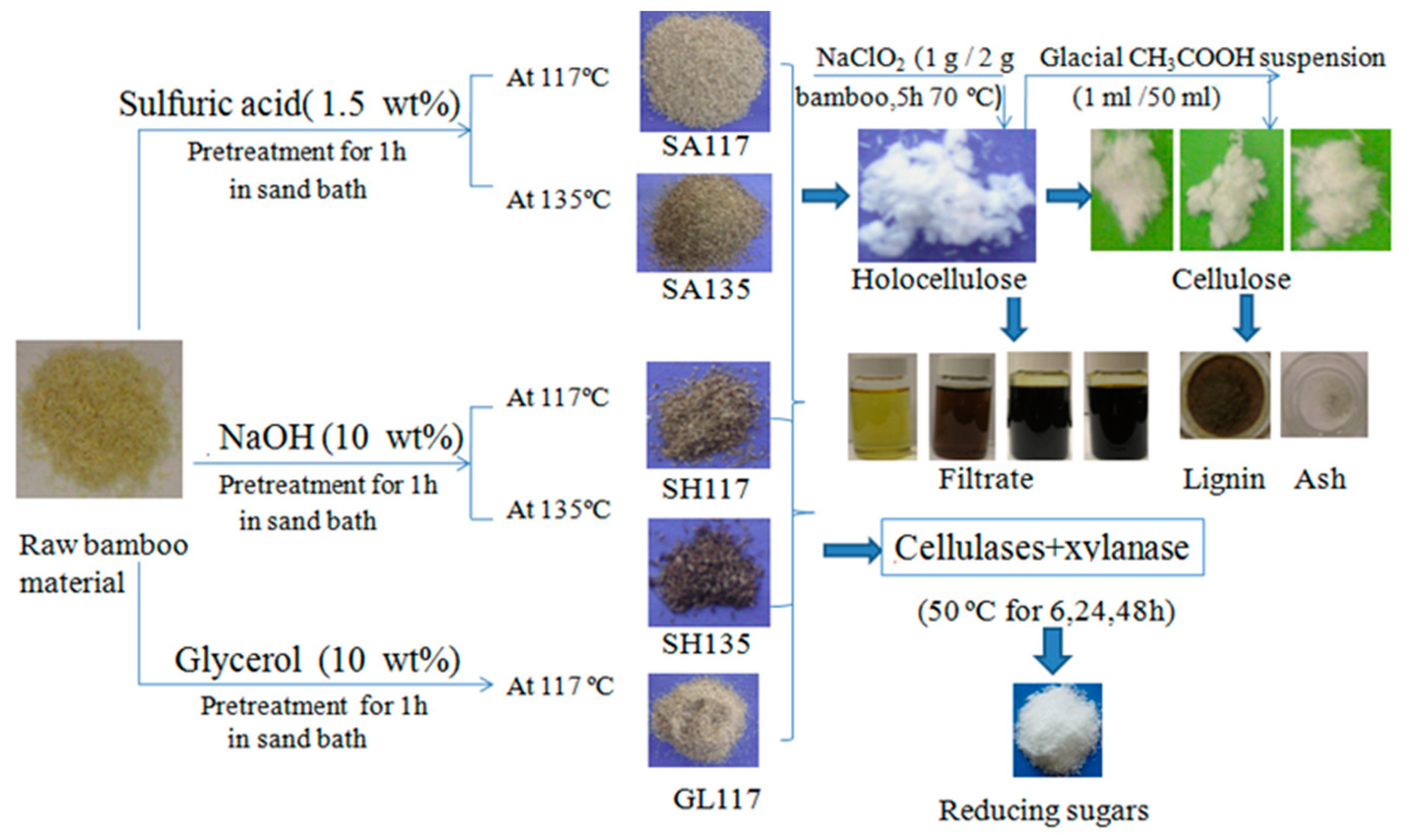
2.2. Pretreatment
2.3. Components Analysis
2.4. Average Degree Of Polymerization (DP)
2.5. FT-IR Spectroscopy
2.6. Analysis of Crystallinity by XRD
2.7. Analysis of the Chemical Structure by Solid-State CP/MAS 13C NMR
2.8. Scanning Electron Microscopy
2.9. TGA
2.10. Enzymatic Hydrolysis
3. Results and Discussion
3.1. Chemical Composition of Untreated and Pretreated Bamboo
3.2. Degree of Polymerization
3.3. Analysis of the Bamboo Samples by FT-IR Spectroscopy
3.4. Analysis of the Bamboo Samples by XRD
3.5. CP/MAS 13C NMR Analysis
3.6. SEM Analysis
3.7. Thermal Stability
3.8. Enzymatic Hydrolysis of the NaOH Pretreated Bamboo Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Z.; Jiang, Z.; Fei, B.; Yu, Y.; Cai, Z. Effective of microwave-KOH pretreatment on enzymatic hydrolysis of bamboo. J. Sustain. Bioenergy Syst. 2012, 2, 104–107. [Google Scholar] [CrossRef]
- Li, K.; Wan, J.; Wang, X.; Wang, J.; Zhang, J. Comparison of dilute acid and alkali pretreatments in production offermentable sugars from bamboo: Effect of Tween 80. Ind. Crop. Prod. 2016, 83, 414–422. [Google Scholar] [CrossRef]
- García-Aparicio, M.; Parawira, W.; van Rensburg, E.; Diedericks, D.; Galbe, M.; Rosslander, C.; Zacchi, G.; Görgens, J. Evaluation of steam-treated giant bamboo for production of fermentable sugars. Biotechnol. Prog. 2011, 27, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Leenakul, W.; Tippayawong, N. Dilute acid pretreatment of bamboo for fermentable sugar production. J. Sustain. Energy Environ. 2010, 1, 117–120. [Google Scholar]
- Peng, P.; She, D. Isolation, structural characterization, and potential applications of hemicelluloses from bamboo: A review. Carbohydr. Polym. 2014, 112, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Han, J.; Han, G.; Ait, G.M.; Wu, Q. Cellulose fibers isolated from energycane bagasse using alkaline and sodium chlorite treatments: Structural, chemical and thermal properties. Ind. Crop. Prod. 2015, 76, 355–363. [Google Scholar] [CrossRef]
- Yue, Y.; Han, J.; Han, G.; Zhang, Q.; Wu, Q. Characterization of cellulose I/II hybrid fibers isolated from energycane bagasse during the delignification process: Morphology, crystallinityandpercentage estimation. Carbohydr. Polym. 2015, 133, 438–447. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, M.; Xin, D.; Wang, J.; Zhang, J. Evaluation of aqueous ammonia pretreatment for enzymatic hydrolysis of different fractions of bamboo shoot and mature bamboo. Bioresour. Technol. 2014, 173, 198–206. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, L. Hydrolysis behavior of bamboo fiber in formic acid reaction system. J. Agric. Food Chem. 2010, 58, 253–2259. [Google Scholar] [CrossRef]
- Xin, D.; Yang, Z.; Liu, F.; Xu, X.; Zhang, J. Comparison of aqueous ammonia and dilute acid pretreatment of bamboo fractions: Structure properties and enzymatic hydrolysis. Bioresour. Technol. 2015, 175, 529–536. [Google Scholar] [CrossRef]
- Jiang, W.; Kumar, A.; Adamopoulos, S. Liquefaction of lignocellulosic materials and its applications in wood adhesives—A review. Ind. Crop. Prod. 2018, 124, 25–342. [Google Scholar] [CrossRef]
- Romaní, A.; Ruiz, H.; Pereira, F.; Teixeira, J.; Domingues, L. Optimization of glycerol-organosolv pretreatment for improving enzymatic saccharification of Eucalyptus wood. Chem. Por. Poster Sess. Energy 2014, 13, 1–4. [Google Scholar]
- Abdulkhani, A.; Hosseinzadeh, J.; Ashori, A.; Dadashi, S.; Takzare, Z. Preparation and characterization of modified cellulose nanofibers reinforced polylactic acid nanocomposite. Polym. Test. 2014, 35, 73–79. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Q.; Zhu, J.; Pan, X. Sulfite (SPORL) pretreatment of switchgrass for enzymatic saccharification. Bioresour. Technol. 2013, 29, 127–134. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, X.; Lin, L.; Chen, L.; Survase, S.; Huang, L.; Cao, S. Evaluating effects of benzene–ethanol extraction on molecular weight of lignin isolated from pretreated bamboo substrate. Wood Sci. Technol. 2015, 49, 945–955. [Google Scholar] [CrossRef]
- Yamashita, Y.; Shono, M.; Sasaki, C.; Nakamura, Y. Alkaline peroxide pretreatment for efficient enzymatic saccharification of bamboo. Carbohyd. Polym. 2010, 79, 914–920. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Li, K.; Wang, X.; Wang, J.; Zhang, J. Benefits from additives and xylanase during enzymatic hydrolysis of bamboo shoot and mature bamboo. Bioresour. Technol. 2015, 192, 424–431. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional Analysis of LignocellulosicFeedstocks. 1. Review and Description of Methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, N.; Feng, M. Polyurethane foams derived from liquefied mountain pine beetle-infested barks. Appl. Polym. Sci. 2012, 123, 284–2858. [Google Scholar] [CrossRef]
- Li, M.; Fan, Y.; Xu, F.; Sun, R.; Zhang, X. Cold sodium hydroxide/urea based pretreatment of bamboo for bioethanol production: Characterization of the cellulose rich fraction. Ind. Crop. Prod. 2010, 32, 551–559. [Google Scholar] [CrossRef]
- Yang, M.; Wang, J.; Hou, X.; Wu, J.; Fan, X.; Jiang, F.; Tao, P.; Wang, F.; Peng, P.; Zhang, J.; et al. Exploring surface characterization and electrostatic property of Hybrid Pennisetum during alkaline sulfite pretreatment for enhanced enzymatic hydrolysability. Bioresour. Technol. 2017, 244, 1166–1172. [Google Scholar] [CrossRef]
- Oh, S.Y.; Yoo, D.I.; Shin, Y.; Kim, H.C.; Kim, H.Y.; Chung, Y.S.; Park, W.H.; Youk, J.H. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydr. Res. 2005, 340, 2376–2391. [Google Scholar] [CrossRef]
- Foreman, D.W.; Jakes, K.A. X-ray Diffractometric Measurement of Microcrystallite Size, Unit Cell Dimensionsand Crystallinity: Application to Cellulosic Marine Textiles. Text. Res. J. 1993, 63, 455–464. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Mooney, C.; Saddler, J.N. Substrate and enzyme characteristics that limit cellulose hydrolysis. Biotechnol. Progr. 1999, 15, 804–816. [Google Scholar] [CrossRef]
- Hall, M.; Bansal, P.; Lee, J.H.; Realff, M.J.; Bommarius, A.S. Cellulose crystallinity—A key predictor of the enzymatic hydrolysis rate. FEBS J. 2010, 277, 1571–1582. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H. X-ray diffraction study of bamboo fibers treated with NaOH. Fiber Polym. 2008, 9, 735–739. [Google Scholar] [CrossRef]
- Wen, J.; Sun, S.; Yuan, T.; Xu, F.; Sun, R. Fractionation of bamboo culms by auto hydrolysis, organosolv delignification and extended delignification: Understanding the fundamental chemistry of the lignin during the integrated process. Bioresour. Technol. 2013, 150, 278–286. [Google Scholar] [CrossRef]
- Sun, X.F.; Sun, R.C.; Su, Y.; Sun, J.X. Comparative study of crude and purified cellulose from wheat straw. J. Agric. Food Chem. 2004, 52, 839–847. [Google Scholar] [CrossRef]
- Zuckerstätter, G.; Schild, G.; Wollboldt, P.; Röder, T.; Weber, H.K.; Sixta, H. The elucidation of cellulose supramolecular structure by 13C CP-MAS NMR. Lenzing. Ber. 2009, 87, 38–46. [Google Scholar]
- Goshadrou, A.; Karimi, K.; Taherzadeh, M.J. Bioethanol production fromsweet sorghum bagasse by Mucorhiemalis. Ind. Crop. Prod. 2011, 34, 1219–1225. [Google Scholar] [CrossRef]
- Rezende, C.A.; de Lima, M.A.; Maziero, P.; deAzevedo, E.R.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 1–18. [Google Scholar] [CrossRef]
- Mandal, A.; Chakrabarty, D. Isolation of nanocellulose from waste sugarcane bagasse (SCB) and its characterization. Carbohydr. Polym. 2011, 86, 1291–1299. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of cellulose andpreparation of nanocellulose from sisal fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Zhang, J.; Tuomainen, P.; Siika-Aho, M.; Viikari, L. Comparison of the synergistic action of two thermostablexylanases from GH families 10 and 11 with thermostablecellulases in lignocellulose hydrolysis. Bioresour. Technol. 2011, 102, 9090–9095. [Google Scholar] [CrossRef]
- Wang, J.; Xin, D.; Hou, X.; Wu, J.; Fan, X.; Li, K.; Zhang, J. Structural properties and hydrolysabilities of Chinese Pennisetum and Hybrid Pennisetum: Effect of aqueous ammonia pretreatment. Bioresour. Technol. 2016, 199, 211–219. [Google Scholar] [CrossRef]






| Pretreatment | Temperature °C | Holocellulose (%) | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Ash (%) | Extractives (%) |
|---|---|---|---|---|---|---|---|
| Native | - | 60.45 ± 5.0 | 46.94 ± 0.1 | 15.59 ± 0.4 | 25.96 ± 0.3 | 8.39 ± 3.9 | 11.54 ± 0.9 |
| Glycerol | 117 | 67.53 ± 0.8 | 51.94 ± 0.2 | 15.59 ± 0.6 | 18.39 ± 0.1 | 9.54 ± 3.5 | 3.19 ± 0.1 |
| H2SO4 | 117 | 77.01 ± 0.6 | 57.12 ± 0.3 | 19.89 ± 0.3 | 15.68 ± 1.7 | 5.99 ± 0.0 | 1.87 ± 0.2 |
| 135 | 78.07 ± 0.3 | 61.24 ± 0.2 | 16.83 ± 0.1 | 14.71 ± 2.9 | 5.11 ± 0.0 | 2.11 ± 0.1 | |
| NaOH | 117 | 82.13 ± 0.7 | 68.15 ± 0.4 | 13.98 ± 0.3 | 13.29 ± 0.2 | 1.48 ± 2.5 | 2.66 ± 0.3 |
| 135 | 87.38 ± 0.3 | 73.94 ± 0.1 | 14.44 ± 0.2 | 8.93 ± 0.6 | 3.12 ± 1.9 | 1.03 ± 0.1 |
| Pretreatment | Temperature | DP | η (mL/g) |
|---|---|---|---|
| Native | - | 396 | 132.62 |
| Glycerol | 117 °C | 362 | 121.63 |
| H2SO4 | 117 °C | 372 | 129.62 |
| 135 °C | 363 | 122.96 | |
| NaOH | 117 °C | 336 | 110.67 |
| 135 °C | 329 | 103.88 |
| Pretreatment | Temperature | 2θ (°) | CrI (%) | d (nm) | LOI a | HBI b |
|---|---|---|---|---|---|---|
| Native | - | 21.26 | 36.91 | 0.40 | 0.70 | 1.05 |
| Glycerol | 117 °C | 22.28 | 38.09 | 0.39 | 0.74 | 1.04 |
| H2SO4 | 117 °C | 22.48 | 42.66 | 0.39 | 0.84 | 1.01 |
| 135 °C | 22.20 | 40.63 | 0.38 | 0.71 | 1.01 | |
| NaOH | 117 °C | 22.86 | 48.42 | 0.36 | 0.91 | 1.00 |
| 135 °C | 22.19 | 46.50 | 0.38 | 0.88 | 0.99 |
| Pretreatment | Temperature | The Mild Degraded Stage | The Main Pyrolysis Stage | The Charring Stage | |||
|---|---|---|---|---|---|---|---|
| T1 a | WL d (%/°C) | T2 b | WL (%/S) | T3 c | Ar e (%/°C) | ||
| Native | - | 210 | 0.29 | 279 (368) f | 1.18 | 365 | 0.33 |
| Glycerol | 117 °C | 212 | 0.25 | 310 | 0.88 | 369 | 0.15 |
| H2SO4 | 117 °C | 194 | 0.21 | 312 | 0.62 | 356 | 0.13 |
| 135 °C | 215 | 0.2 | 325 | 0.79 | 405 | 0.13 | |
| NaOH | 117 °C | 235 | 0.14 | 346 | 0.69 | 439 | 0.12 |
| 135 °C | 230 | 0.26 | 330 | 1.28 | 383 | 0.15 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, X.; Chu, J.; Jia, L.; Kumar, A. Influence of Different Pretreatments on the Structure and Hydrolysis Behavior of Bamboo: A Comparative Study. Materials 2019, 12, 2570. https://doi.org/10.3390/ma12162570
Qi X, Chu J, Jia L, Kumar A. Influence of Different Pretreatments on the Structure and Hydrolysis Behavior of Bamboo: A Comparative Study. Materials. 2019; 12(16):2570. https://doi.org/10.3390/ma12162570
Chicago/Turabian StyleQi, Xuemin, Jie Chu, Liangliang Jia, and Anuj Kumar. 2019. "Influence of Different Pretreatments on the Structure and Hydrolysis Behavior of Bamboo: A Comparative Study" Materials 12, no. 16: 2570. https://doi.org/10.3390/ma12162570
APA StyleQi, X., Chu, J., Jia, L., & Kumar, A. (2019). Influence of Different Pretreatments on the Structure and Hydrolysis Behavior of Bamboo: A Comparative Study. Materials, 12(16), 2570. https://doi.org/10.3390/ma12162570






