Effective Photocatalytic Activity of Sulfate-Modified BiVO4 for the Decomposition of Methylene Blue Under LED Visible Light
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Fabrication of Sulfate-Modified Bismuth Vanadate (BiVO4)
2.3. Characterization
2.4. Photocatalytic Activity Test
2.5. Active Species Trapping Experiments
2.6. Analysis of Hydroxyl Radical (HO•)
3. Results and Discussion
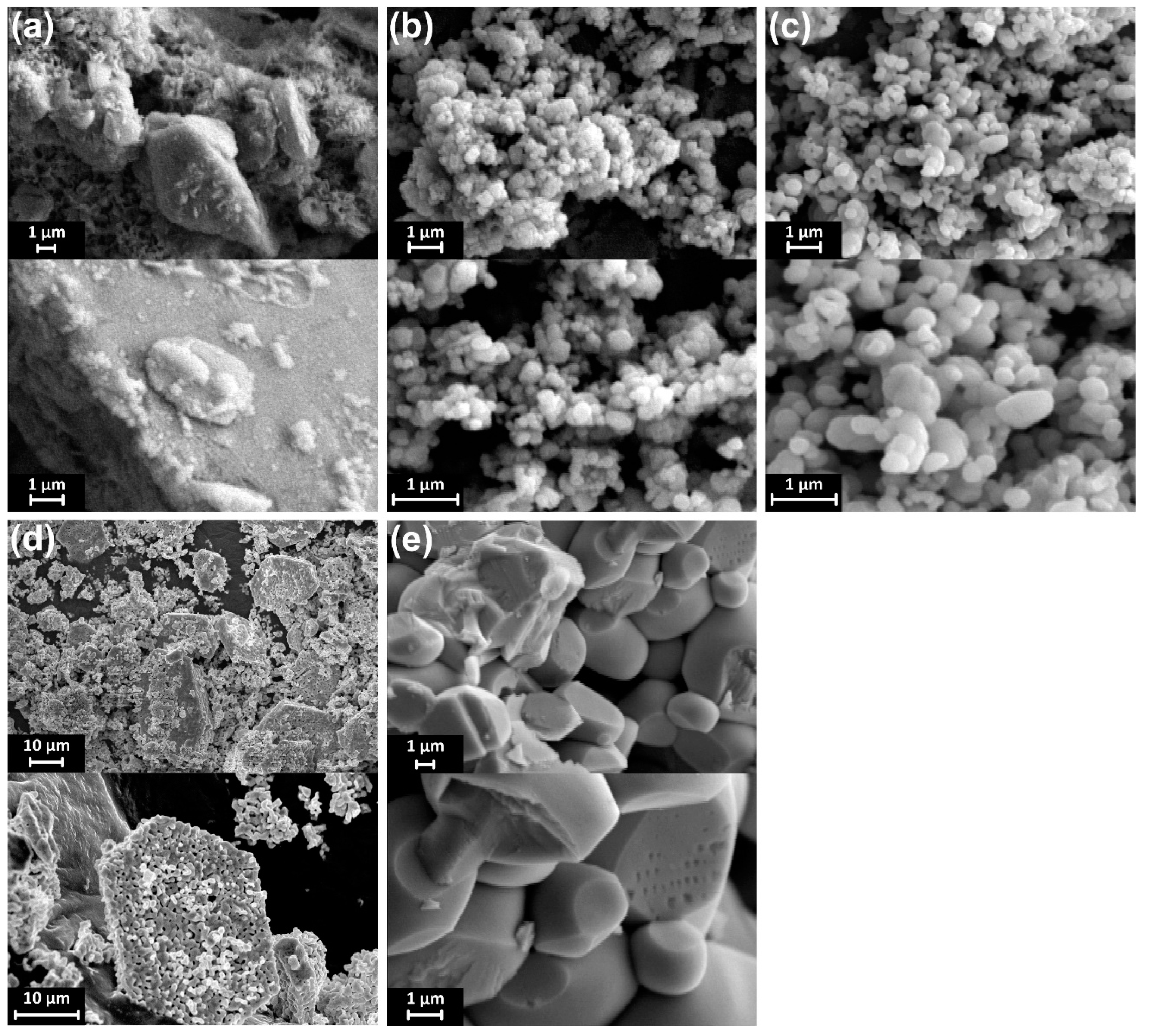
3.1. Characterization of Sulfate-Modified BiVO4 and Pure BiVO4
3.2. Effect of the Annealing Temperature
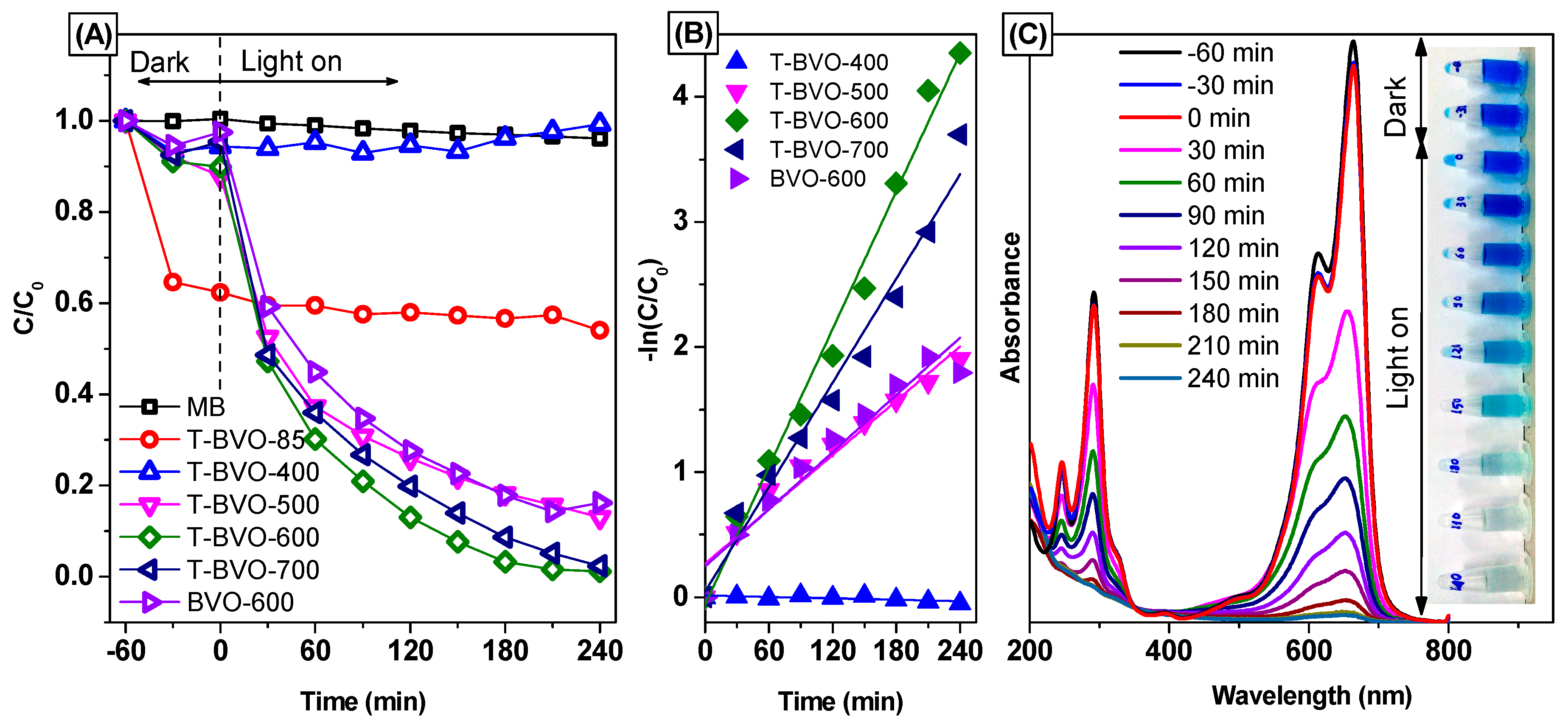
3.3. Photocatalytic Activities
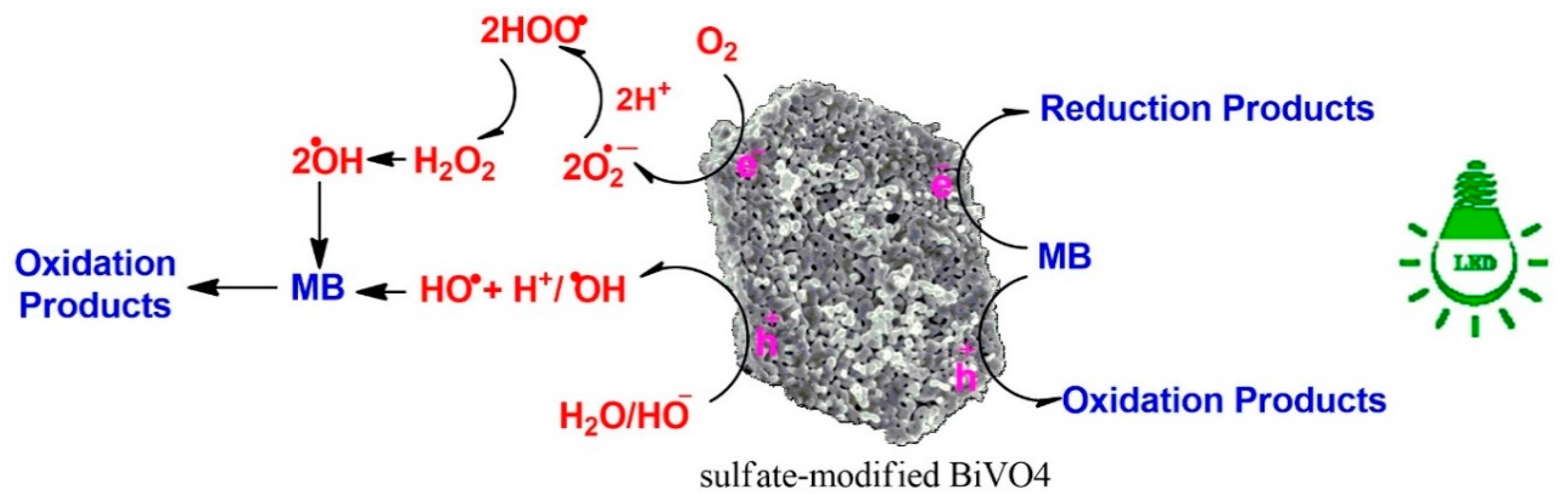
3.4. Investigation of the Mechanisms of Dye Degradation
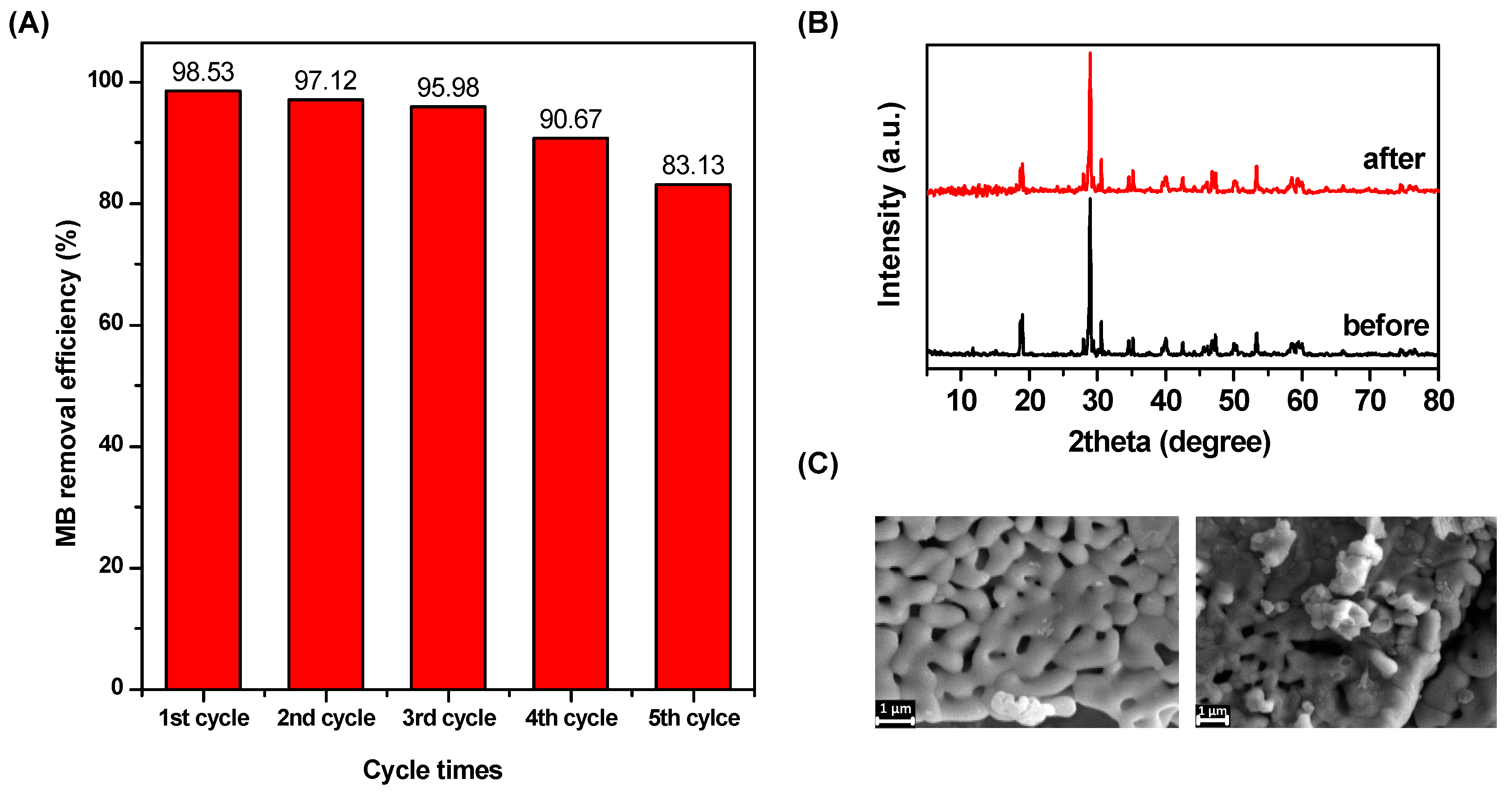
3.5. Reusability and Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Kudo, A.; Omori, K.; Kato, H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999, 121, 11459–11467. [Google Scholar] [CrossRef]
- Gan, J.; Rajeeva, B.B.; Wu, Z.; Penley, D.; Liang, C.; Tong, Y.; Zheng, Y. Plasmon-enhanced nanoporous BiVO4 photoanodes for efficient photoelectrochemical water oxidation. Nanotechnology 2016, 27, 235401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dai, H.; Deng, J.; Liu, Y.; Au, C.T. Enhanced visible-light photocatalytic activities of porous olive-shaped sulfur-doped BiVO4-supported cobalt oxides. Solid State Sci. 2013, 18, 98–104. [Google Scholar] [CrossRef]
- García-Pérez, U.M.; Sepúlveda-Guzmán, S.; Martínez-De La Cruz, A. Nanostructured BiVO 4 photocatalysts synthesized via a polymer-assisted coprecipitation method and their photocatalytic properties under visible-light irradiation. Solid State Sci. 2012, 14, 293–298. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hong, S.S. Synthesis of BiVO4 nanoparticles using microwave process and their photocatalytic activity under visible light irradiation. J. Nanosci. Nanotechnol. 2017, 17, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.K.; Choi, S.; Gamelin, D.R. Near-complete suppression of surface recombination in solar photoelectrolysis by “co-Pi” catalyst-modified W: BiVO4. J. Am. Chem. Soc. 2011, 133, 18370–18377. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, F.; Wang, D.; Yang, J.; Li, M.; Zhu, J.; Zhou, X.; Han, H.; Li, C. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013, 4, 1432. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gong, H.; Zhang, Y.; Liu, K.; Cao, H.; Yan, H.; Zhu, J. The controllable synthesis of octadecahedral BiVO4 with exposed {111} Facets. Eur. J. Inorg. Chem. 2017, 2017, 2990–2997. [Google Scholar] [CrossRef]
- Cao, J.; Zhou, C.; Lin, H.; Xu, B.; Chen, S. Surface modification of m-BiVO 4 with wide band-gap semiconductor BiOCl to largely improve the visible light induced photocatalytic activity. Appl. Surf. Sci. 2013, 284, 263–269. [Google Scholar] [CrossRef]
- Lopes, O.F.; Carvalho, K.T.G.; Nogueira, A.E.; Avansi, W.; Ribeiro, C. Controlled synthesis of BiVO4 photocatalysts: Evidence of the role of heterojunctions in their catalytic performance driven by visible-light. Appl. Catal. B Environ. 2016, 188, 87–97. [Google Scholar] [CrossRef]
- Han, M.; Sun, T.; Tan, P.Y.; Chen, X.; Tan, O.K.; Tse, M.S. M-BiVO@4γBi2O3 core-shell p-n heterogeneous nanostructure for enhanced visible-light photocatalytic performance. RSC Adv. 2013, 3, 24964–24970. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, S.; Jang, J.S.; Lee, J.S. Heterojunction BiVO4/WO3 electrodes for enhanced photoactivity of water oxidation. Energy Environ. Sci. 2011, 4, 1781–1787. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Wang, Y.; Yang, Y.; Tang, J.; Yang, Y. Carbon spheres supported visible-light-driven CuO-BiVO4 heterojunction: Preparation, characterization, and photocatalytic properties. Appl. Catal. B Environ. 2012, 115–116, 90–99. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Thuan, T.V.; Do, S.T.; Nguyen, D.T.; Vo, D.V.N.; Bach, L.G. High Photocatalytic Activity of Oliver-Like BiVO4 for Rhodamine B Degradation under Visible Light Irradiation. Appli. Mech. Mater. 2018, 876, 52–56. [Google Scholar] [CrossRef]
- Jiang, H.Q.; Endo, H.; Natori, H.; Nagai, M.; Kobayashi, K. Fabrication and photoactivities of spherical-shaped BiVO4 photocatalysts through solution combustion synthesis method. J. Eur. Ceram. Soc. 2008, 28, 2955–2962. [Google Scholar] [CrossRef]
- Guo, M.; Wang, Y.; He, Q.; Wang, W.; Wang, W.; Fu, Z.; Wang, H. Enhanced photocatalytic activity of S-doped BiVO 4 photocatalysts. RSC Adv. 2015, 5, 58633–58639. [Google Scholar] [CrossRef]
- Yin, C.; Zhu, S.; Chen, Z.; Zhang, W.; Gu, J.; Zhang, D. One step fabrication of C-doped BiVO4 with hierarchical structures for a high-performance photocatalyst under visible light irradiation. J. Mater. Chem. A 2013, 1, 8367–8378. [Google Scholar] [CrossRef]
- Tan, G.; Zhang, L.; Ren, H.; Huang, J.; Yang, W.; Xia, A. Microwave hydrothermal synthesis of N-doped BiVO4 nanoplates with exposed (040) facets and enhanced visible-light photocatalytic properties. Ceram. Int. 2014, 40, 9541–9547. [Google Scholar] [CrossRef]
- Jo, W.J.; Jang, J.W.; Kong, K.J.; Kang, H.J.; Kim, J.Y.; Jun, H.; Parmar, K.P.S.; Lee, J.S. Phosphate doping into monoclinic BiVO 4 for enhanced photoelectrochemical water oxidation activity. Angew. Chemie Int. Ed. 2012, 51, 3147–3151. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, H.; Deng, J.; Liu, Y.; Au, C.T. Effect of sulfur doping on the photocatalytic performance of BiVO4 under visible light illumination. Cuihua Xuebao Chinese J. Catal. 2013, 34, 1617–1626. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, P.; Kuang, S. Fabrication and enhanced visible-light photocatalytic activities of BiVO4/Bi2WO6 composites. RSC Adv. 2014, 4, 46054–46059. [Google Scholar] [CrossRef]
- Gotić, M.; Musić, S.; Ivanda, M.; Šoufek, M.; Popović, S. Synthesis and characterisation of bismuth(III) vanadate. J. Mol. Struct. 2005, 744–747, 535–540. [Google Scholar] [CrossRef]
- Zulkifili, A.; Fujiki, A.; Kimijima, S. Flower-like BiVO4 Microspheres and Their Visible Light-Driven Photocatalytic Activity. Appl. Sci. 2018, 8, 216. [Google Scholar] [CrossRef]
- Nam, S.H.; Kim, T.K.; Boo, J.H. Physical property and photo-catalytic activity of sulfur doped TiO 2 catalysts responding to visible light. Catal. Today 2012, 185, 259–262. [Google Scholar] [CrossRef]
- Phu, N.D.; Hoang, L.H.; Vu, P.K.; Kong, M.H.; Chen, X.B.; Wen, H.C.; Chou, W.C. Control of crystal phase of BIVO4nanoparticles synthesized by microwave assisted method. J. Mater. Sci. Mater. Electron. 2016, 27, 6452–6456. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. Determination of vanadium-oxygen bond distances and bond orders by Raman spectroscopy. J. Phys. Chem. 2005, 95, 5031–5041. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hong, S.S. The effect of solvent on the synthesis of BiVO4 using solvothermal method and their photocatalytic activity under visible light irradiation. Top. Catal. 2017, 60, 782–788. [Google Scholar] [CrossRef]
- Chen, G.; Ford, T.E.; Clayton, C.R. Interaction of sulfate-reducing bacteria with molybdenum dissolved from sputter-deposited molybdenum thin films and pure molybdenum powder. J. Colloid Interface Sci. 1998, 204, 237–246. [Google Scholar] [CrossRef]
- Strohmeier, B.R.; Hercules, D.M. Surface spectroscopic characterization of manganese/aluminum oxide catalysts. J. Phys. Chem. 2005, 88, 4922–4929. [Google Scholar] [CrossRef]
- Wang, M.; Niu, C.; Liu, J.; Wang, Q.; Yang, C.; Zheng, H. Effective visible light-active nitrogen and samarium co-doped BiVO4 for the degradation of organic pollutants. J. Alloys Compd. 2015, 648, 1109–1115. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, G.; Chen, Y.; Shi, Y.; Tian, C.; Pan, K.; Fu, H. Growth rate controlled synthesis of hierarchical Bi2S 3/In2S3 core/shell microspheres with enhanced photocatalytic activity. Sci. Rep. 2014, 4, 4027. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.P.; Baikie, T.; Chiam, S.Y.; Ren, Y.; Prabhakar, R.R.; Batabyal, S.K.; Loo, S.C.J.; Barber, J.; Wong, L.H. Catalytic effect of Bi5+ in enhanced solar water splitting of tetragonal BiV0.8Mo0.2O4. Appl. Catal. A Gen. 2016, 526, 21–27. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chanéac, C.; Durupthy, O.; Mariey, L.; Maugé, F.; Brezová, V.; Jolivet, J.P. New insights into BiVO 4 properties as visible light photocatalyst. J. Phys. Chem. C 2015, 119, 12967–12977. [Google Scholar] [CrossRef]
- Vila, M.; Díaz-Guerra, C.; Lorenz, K.; Piqueras, J.; Alves, E.; Nappini, S.; Magnano, E. Structural and luminescence properties of Eu and Er implanted Bi2O3nanowires for optoelectronic applications. J. Mater. Chem. C 2013, 1, 7920–7929. [Google Scholar] [CrossRef]
- Yousif, A.; Jafer, R.M.; Som, S.; Duvenhage, M.M.; Coetsee, E.; Swart, H.C. Ultra-broadband luminescent from a Bi doped CaO matrix. RSC Adv. 2015, 5, 54115–54122. [Google Scholar] [CrossRef]
- Usai, S.; Obregón, S.; Becerro, A.I.; Colón, G. Monoclinic-tetragonal heterostructured BiVO4 by yttrium doping with improved photocatalytic activity. J. Phys. Chem. C 2013, 117, 24479–24484. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Hong, J.; Xia, X.; Wang, Y.; Xu, R. Mesoporous carbon nitride with in situ sulfur doping for enhanced photocatalytic hydrogen evolution from water under visible light. J. Mater. Chem. 2012, 22, 15006–15012. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Controllable synthesis of mesoporous sulfur-doped carbon nitride materials for enhanced visible light photocatalytic degradation. Langmuir 2017, 33, 7062–7078. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wang, N.; Chen, M.L.; Yang, T.; Wang, J.H. One step preparation of proton-functionalized photoluminescent graphitic carbon nitride and its sensing applications. RSC Adv. 2016, 6, 98893–98898. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Wang, Y.; Zhao, J.; Wang, D.; Li, X.; Guo, Z.; Wang, H.; Deng, Y.; Niu, C.; et al. Novel ternary heterojunction photcocatalyst of Ag nanoparticles and g-C3N4 nanosheets co-modified BiVO4 for wider spectrum visible-light photocatalytic degradation of refractory pollutant. Appli. Catalys. B Environ. 2017, 205, 133–147. [Google Scholar] [CrossRef]
- Wang, A.; Lee, C.; Bian, H.; Li, Z.; Zhan, Y.; He, J.; Wang, Y.; Lu, J.; Li, Y.Y. Synthesis of g-C3N4/Silica Gels for White-Light-Emitting Devices. Part. Part. Syst. Charact. 2017, 34, 1600258. [Google Scholar] [CrossRef]
- Oh, W.D.; Lok, L.W.; Veksha, A.; Giannis, A.; Lim, T.T. Enhanced photocatalytic degradation of bisphenol A with Ag-decorated S-doped g-C 3 N 4 under solar irradiation: Performance and mechanistic studies. Chem. Eng. J. 2018, 333, 739–749. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Krivtsov, I.; García, J.R.; Palmisano, L. Selective photocatalytic oxidation of aromatic alcohols in water by using P-doped g-C3N4. Appl. Catal. B Environ. 2018, 220, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Sleight, A.W.; Chen, H.Y.; Ferretti, A.; Cox, D.E. Crystal growth and structure of BiVO4. Mater. Res. Bull. 1979, 14, 1571–1581. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Z.; Zheng, G.; Yao, Z.; Mu, J. Superior visible light photocatalytic performance of reticular BiVO4 synthesized: Via a modified sol-gel method. RSC Adv. 2018, 8, 10654–10664. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Hong, S.S. Synthesis of Metal Ion-Doped TiO 2 Nanoparticles Using Two-Phase Method and Their Photocatalytic Activity Under Visible Light Irradiation. J. Nanosci. Nanotechnol. 2015, 16, 1911–1915. [Google Scholar] [CrossRef]
- Pingmuang, K.; Chen, J.; Kangwansupamonkon, W.; Wallace, G.G.; Phanichphant, S.; Nattestad, A. Composite photocatalysts containing BiVO 4 for degradation of cationic dyes. Sci. Rep. 2017, 7, 8929. [Google Scholar] [CrossRef]
- Huang, H.; Liu, L.; Zhang, Y.; Tian, N. Novel BiIO4/BiVO4 composite photocatalyst with highly improved visible-light-induced photocatalytic performance for rhodamine B degradation and photocurrent generation. RSC Adv. 2015, 5, 1161–1167. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, H.; Zhang, Y.; Tang, W.; Cheng, X.; Liu, H. Activation of peroxymonosulfate by BiVO4 under visible light for degradation of Rhodamine B. Chem. Phys. Lett. 2016, 653, 101–107. [Google Scholar] [CrossRef]
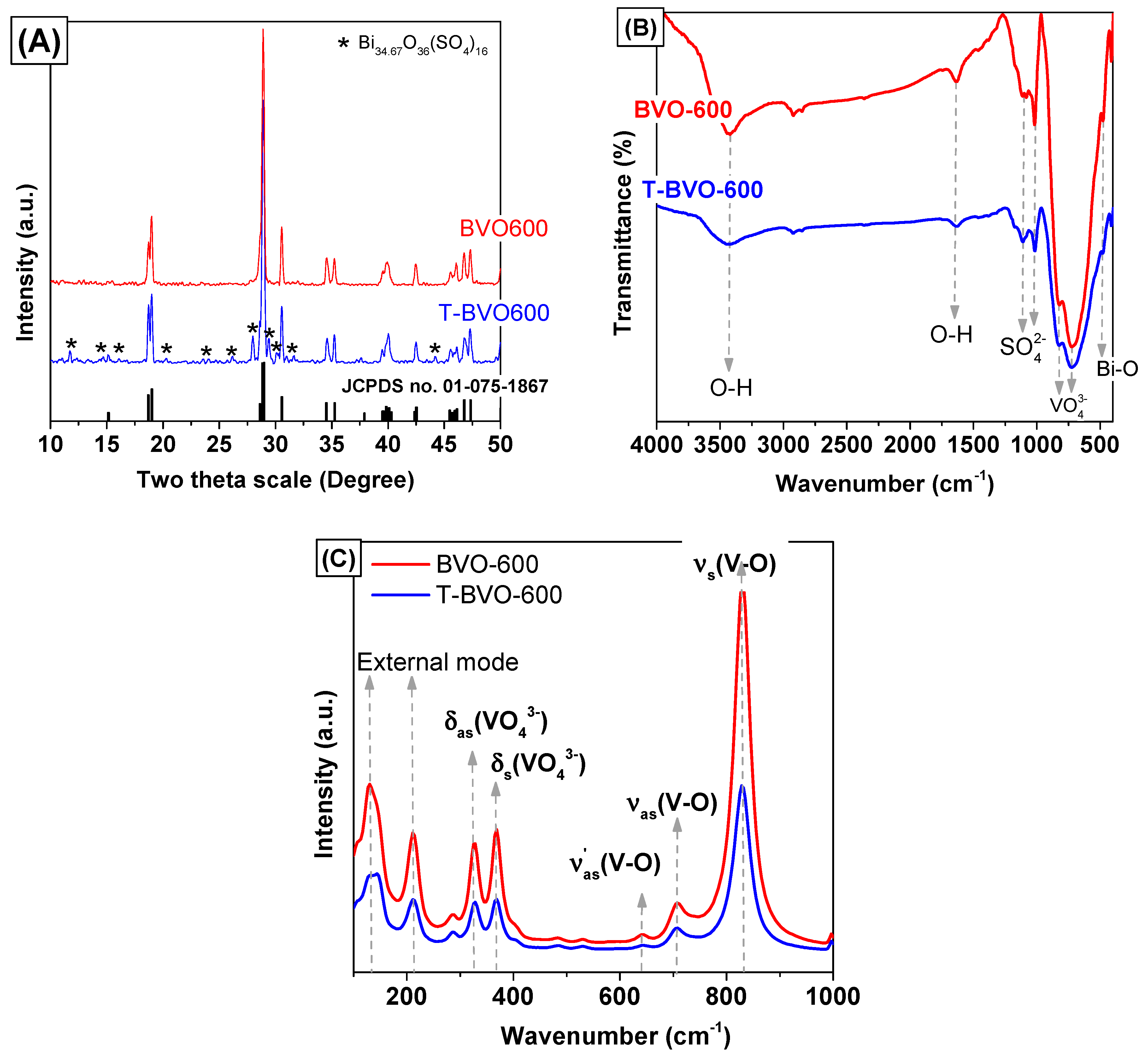
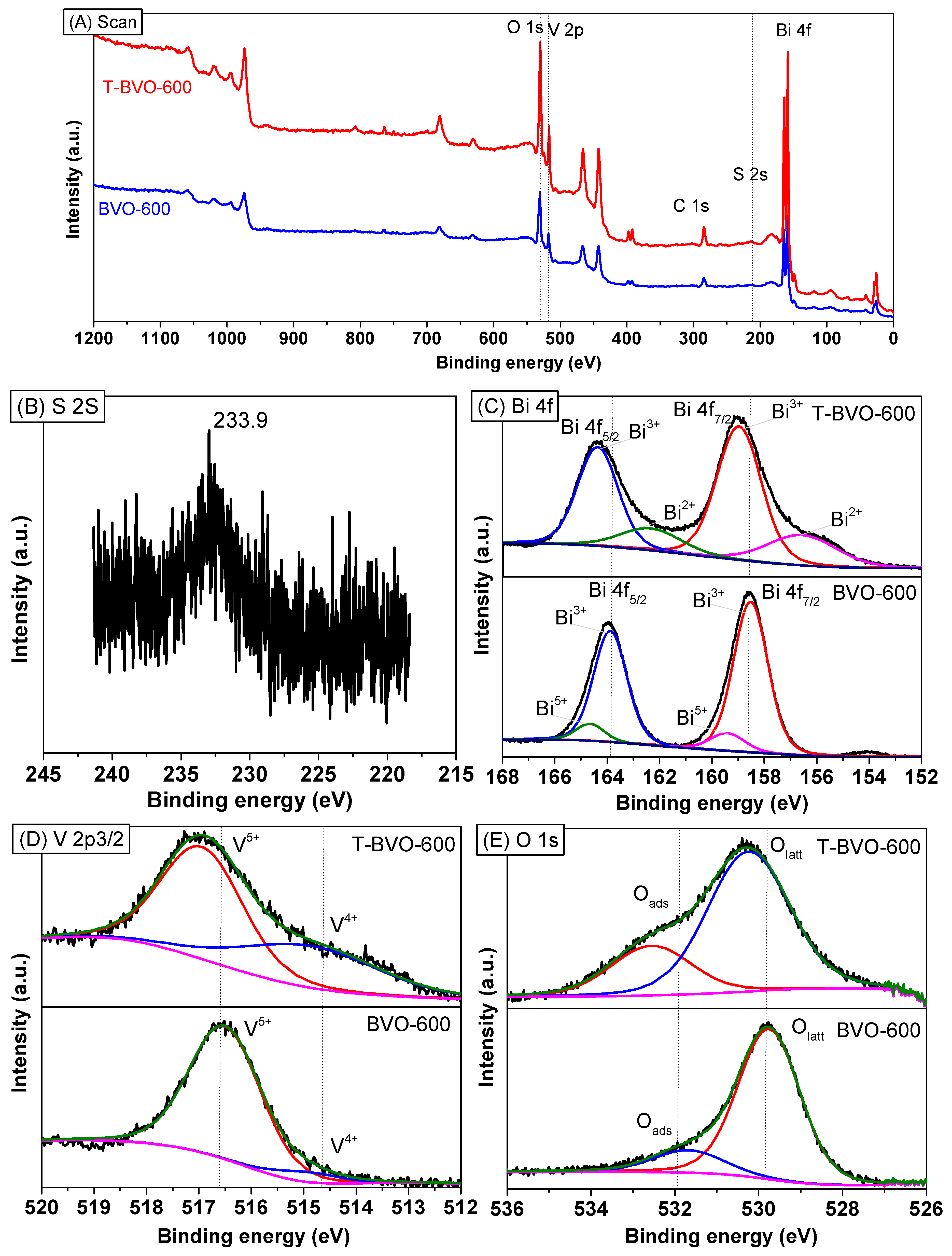

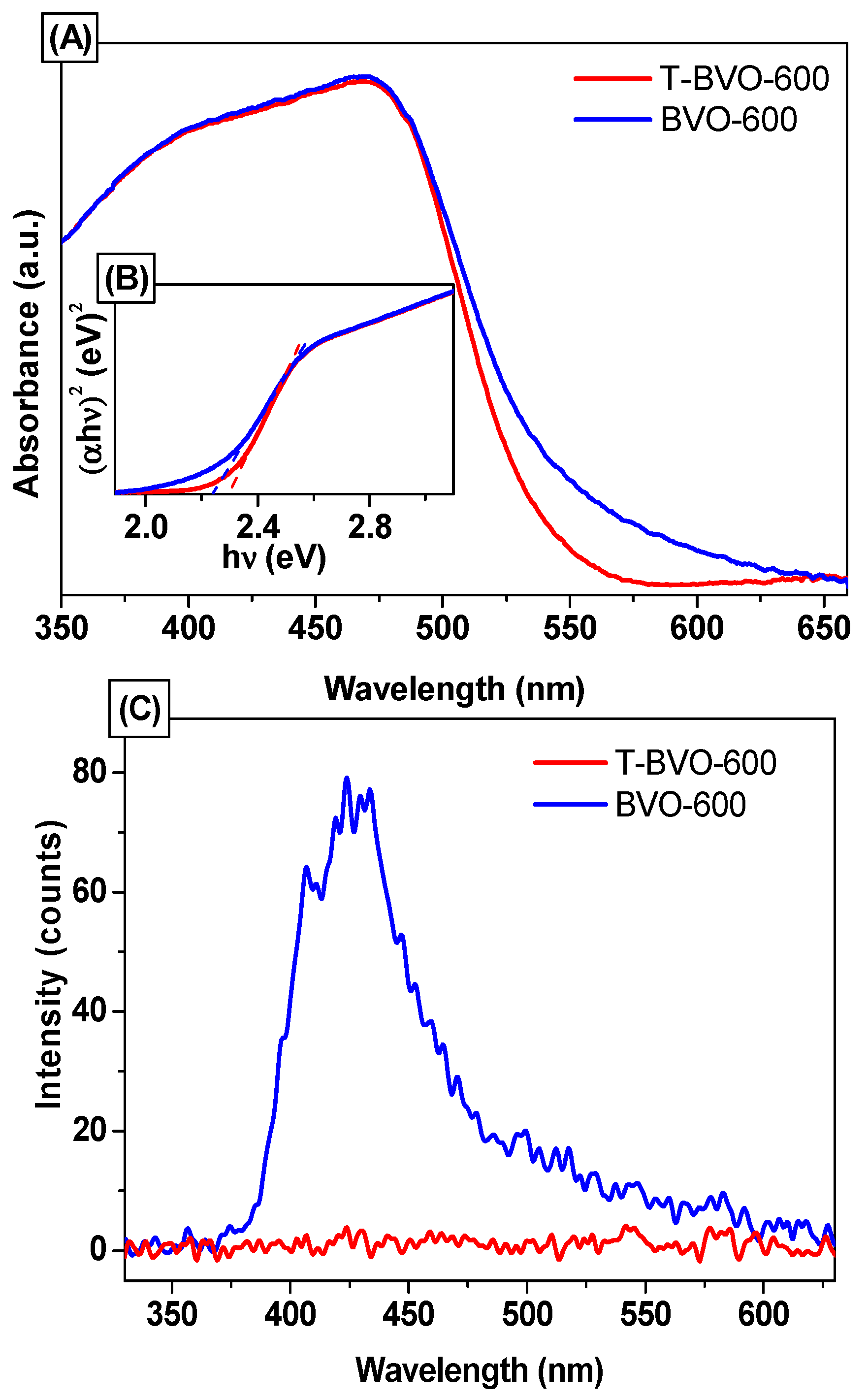
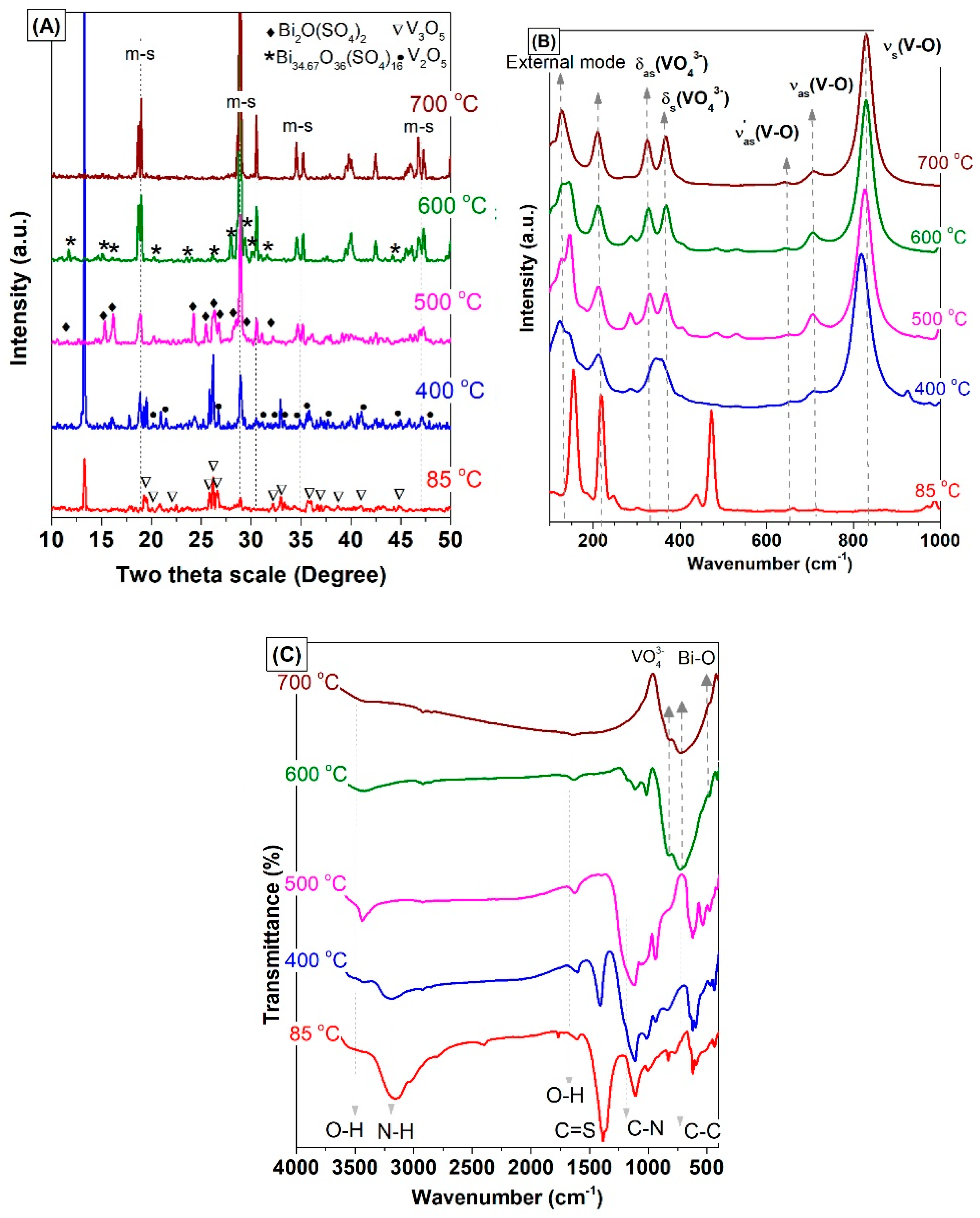


| Sample No. | Preparation Condition (°C) | Cell Parameters a | Crystallite Size a (nm) | Bond Length b (Å) | ||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | β | Vcell | ||||
| (Å) | (Å) | (Å) | (Å) | (Å3) | V–O | |||
| T-BVO-85 | 85 | − | − | − | − | − | − | − |
| T-BVO-400 | 400 | 5.136 | 5.094 | 11.686 | 90.257 | 305.277 | 36.890 | 1.7002 ± 0.0005 |
| T-BVO-500 | 500 | 5.174 | 5.101 | 11.664 | 90.222 | 307.809 | 34.420 | 1.6961 ± 0.0002 |
| T-BVO-600 | 600 | 5.186 | 5.093 | 11.669 | 90.174 | 308.195 | 33.700 | 1.6940 ± 0.0002 |
| T-BVO-700 | 700 | 5.192 | 5.094 | 11.667 | 90.198 | 308.576 | 32.740 | 1.6937 ± 0.0006 |
| BVO-600 | 600 (without thiourea) | 5.192 | 5.095 | 11.664 | 90.206 | 308.501 | 33.190 | 1.6935 ± 0.0006 |
| Sample No. | Atomic % a | ||||
|---|---|---|---|---|---|
| O 1s | C 1s | Bi 4f | V 2p3 | S 2s | |
| BVO-600 | 46.55 | 29.25 | 15.85 | 8.36 | − |
| T-BVO-600 | 50.94 | 28.44 | 11.67 | 6.22 | 2.72 |
| Sample No. | Preparation Condition (°C) | Bandgap c (Eg) | First Kinetics | |||
|---|---|---|---|---|---|---|
| K × 10−3 | R2 | t1/2 | t90 | |||
| eV | (min−1) | (min) | (min) | |||
| T-BVO-85 | 85 | − | 4.313 × 10−4 | 0.824 | 1.607 × 106 | 5.339 × 106 |
| T-BVO-400 | 400 | 2.230 | 1.881 × 10−4 | 0.506 | 3.685 × 106 | 12.241 × 106 |
| T-BVO-500 | 500 | 2.112 | 7.240 | 0.955 | 95.738 | 318.037 |
| T-BVO-600 | 600 | 2.288 | 18.370 | 0.989 | 37.733 | 125.345 |
| T-BVO-700 | 700 | 2.277 | 13.900 | 0.977 | 49.867 | 165.654 |
| BVO-600 | 600 (without thiourea) | 2.246 | 7.620 | 0.945 | 90.964 | 302.176 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.H.; Bui, Q.T.P.; Vo, D.-V.N.; Lim, K.T.; Bach, L.G.; Do, S.T.; Nguyen, T.V.; Doan, V.-D.; Nguyen, T.-D.; Nguyen, T.D. Effective Photocatalytic Activity of Sulfate-Modified BiVO4 for the Decomposition of Methylene Blue Under LED Visible Light. Materials 2019, 12, 2681. https://doi.org/10.3390/ma12172681
Nguyen VH, Bui QTP, Vo D-VN, Lim KT, Bach LG, Do ST, Nguyen TV, Doan V-D, Nguyen T-D, Nguyen TD. Effective Photocatalytic Activity of Sulfate-Modified BiVO4 for the Decomposition of Methylene Blue Under LED Visible Light. Materials. 2019; 12(17):2681. https://doi.org/10.3390/ma12172681
Chicago/Turabian StyleNguyen, Vinh Huu, Quynh Thi Phuong Bui, Dai-Viet N. Vo, Kwon Taek Lim, Long Giang Bach, Sy Trung Do, Tuyen Van Nguyen, Van-Dat Doan, Thanh-Danh Nguyen, and Trinh Duy Nguyen. 2019. "Effective Photocatalytic Activity of Sulfate-Modified BiVO4 for the Decomposition of Methylene Blue Under LED Visible Light" Materials 12, no. 17: 2681. https://doi.org/10.3390/ma12172681
APA StyleNguyen, V. H., Bui, Q. T. P., Vo, D.-V. N., Lim, K. T., Bach, L. G., Do, S. T., Nguyen, T. V., Doan, V.-D., Nguyen, T.-D., & Nguyen, T. D. (2019). Effective Photocatalytic Activity of Sulfate-Modified BiVO4 for the Decomposition of Methylene Blue Under LED Visible Light. Materials, 12(17), 2681. https://doi.org/10.3390/ma12172681







