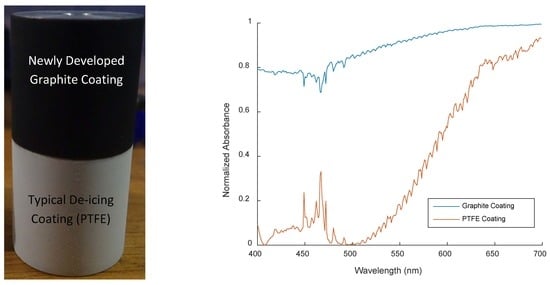
Novel Superhydrophobic Surface with Solar-Absorptive Material for Improved De-Icing Performance
Abstract
:1. Introduction
2. Method and Experiment
2.1. Spectrometer Testing
2.2. Contact and Roll-Off Angle Tests
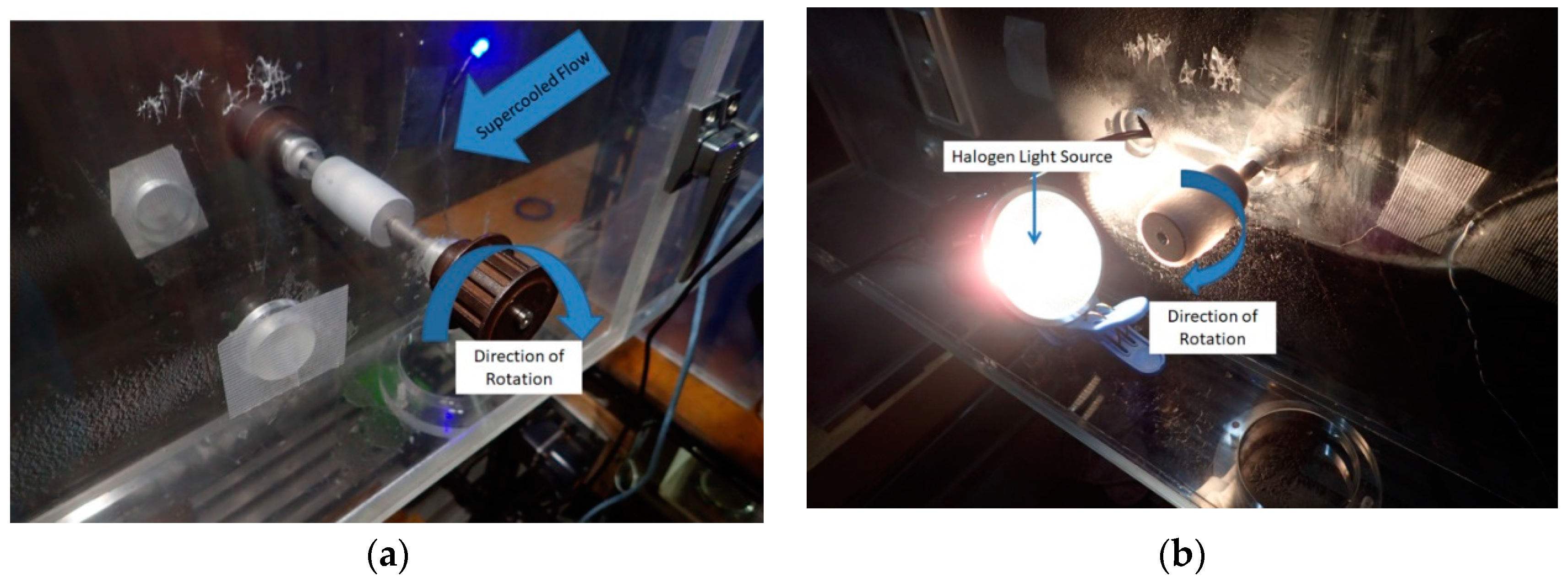
2.3. Icing Wind Tunnel Tests
3. Results
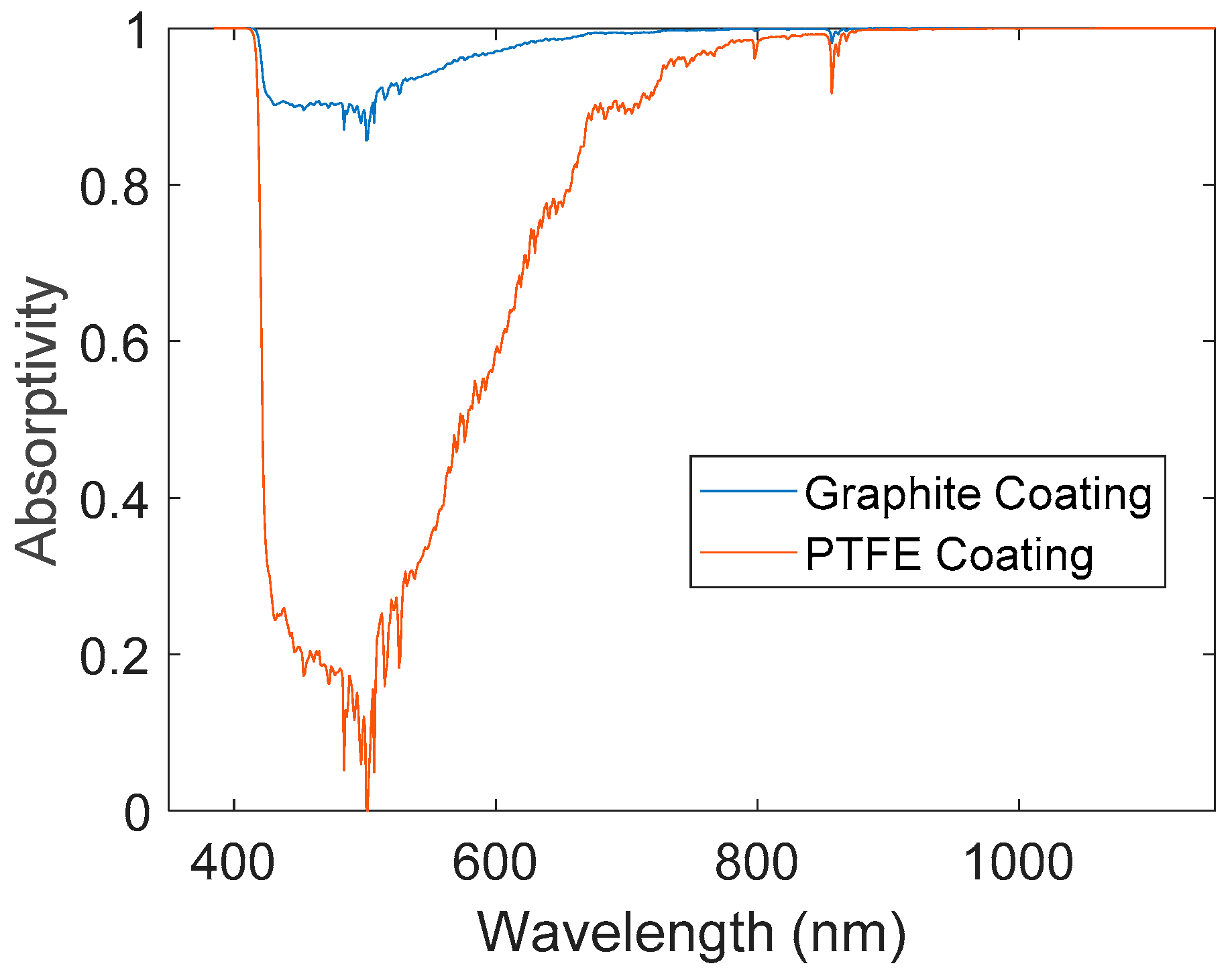
3.1. Energy Absorption Coefficient
3.2. Hydrophobic Performance
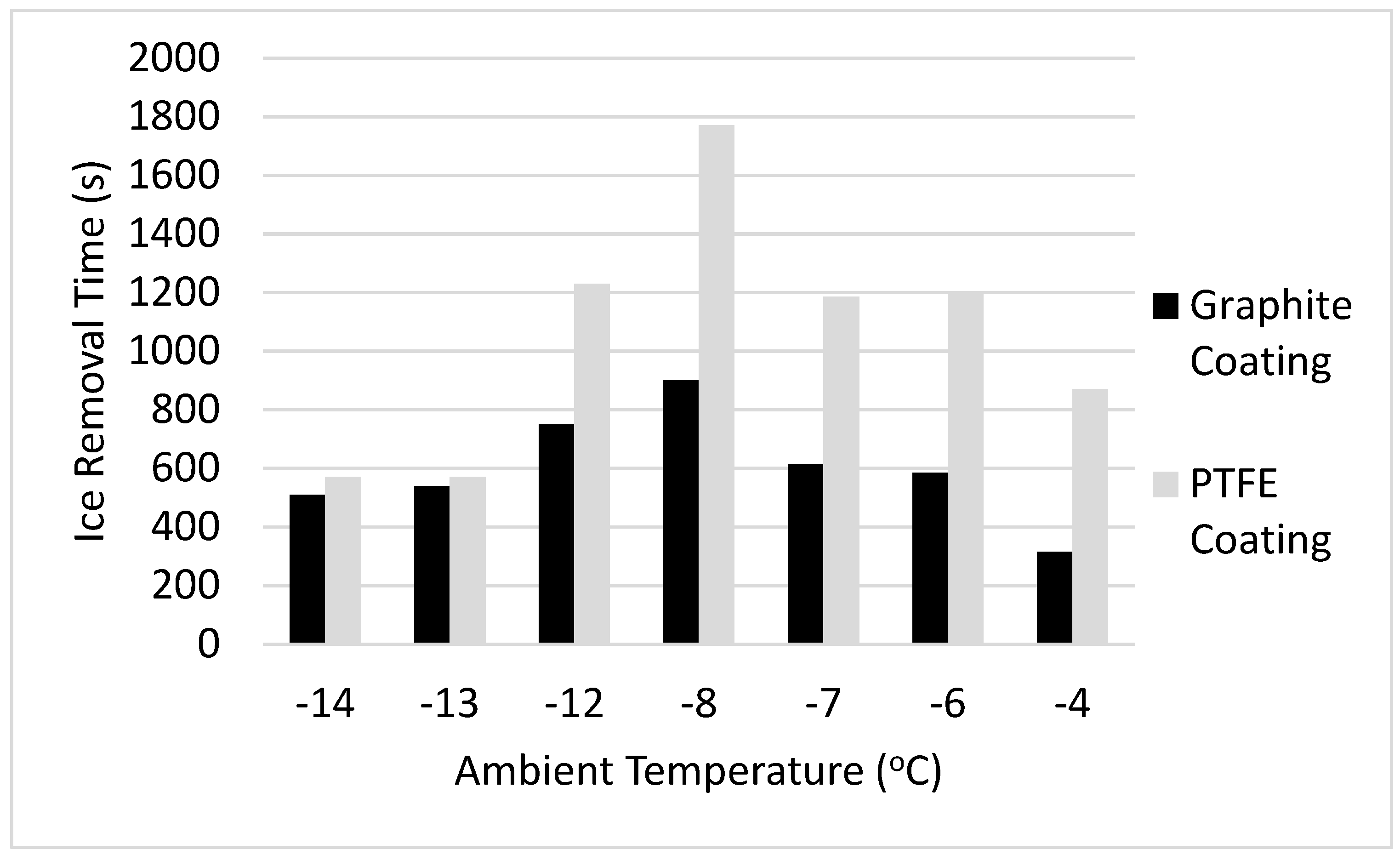
3.3. Icing Wind Tunnel Results
4. Conclusions and Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Boinovich, L.B.; Emelyanenko, A.M. Anti-icing Potential of superhydrophobic coatings. Mendeleev Commun. 2013, 23, 3–10. [Google Scholar] [CrossRef]
- Poots, G.; Skelton, P.L.I. Rime- and glaze-ice accretion due to freezing rain falling vertically on a horizontal thermally insulated overhead line conductor. Int. J. Heat Fluid Fl. 1992, 13, 390–398. [Google Scholar] [CrossRef]
- Petrenko, V.F.; Sullivan, C.R.; Kozlyuk, V. Variable-resistance conductors (VRC) for power-line de-icing. Cold Reg. Sci. Technol. 2011, 65, 23–28. [Google Scholar] [CrossRef]
- Laforte, J.L.; Allaire, M.A.; Laflamme, J. State-of-the-art on power line de-icing. Atmos. Res. 1998, 46, 143–158. [Google Scholar] [CrossRef]
- Susoff, M.; Siegmann, K.; Pfaffenroth, C.; Hirayama, M. Evaluation of icephobic coatings—Screening of different coatings and influence of roughness. Appl. Surf. Sci. 2013, 282, 870–879. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H. Effect of drop size on the impact thermodynamics for supercooled large droplet in aircraft icing. Phys. Fluids 2016, 28. [Google Scholar] [CrossRef]
- Sakaue, H.; Morita, K.; Kimura, S. International Project Summary of ICE-WIPS—A hybrid aircraft ice-protection system using an icephobic coating and an electric heater. In Proceedings of the AIAA Aviation and Aeronautics Forum and Exposition, GA, Atlanta, USA, 25–28 June 2018. [Google Scholar]
- Janjua, Z.A.; Turnbull, B.; Hibberd, S.; Choi, K.-S. Mixed ice accretion on aircraft wings. Phys. Fluids 2018, 30, 027101. [Google Scholar] [CrossRef] [Green Version]
- Lesbayev, B.T.; Nazhipkyzy, M.; Prikhodko, N.G.; Temirgaliyeva, T.S.; Ustaeva, G.S.; Nurgozhaeva, A.; Beksultan, J.; Turganbaeva, A.; Mansurov, Z.A. Creating of anti-icing coatings based on nanoscale powders of silicon dioxide obtained from silicone waste. Procedia Manuf. 2017, 12, 22–27. [Google Scholar] [CrossRef]
- Farhadi, S.; Farzaneh, M.; Kulinich, S.A. Anti-icing performance of superhydrophobic surfaces. Appl. Surf. Sci. 2011, 257, 6264–6269. [Google Scholar] [CrossRef]
- Silva, C.; Pinto da Cunha, J.; Pereira, A.; Chepel, V.; Lopes, M.I.; Solovov, V.; Neves, F. Reflectance of polytetrafluoroethylene for xenon scintillation light. J. Appl. Phys. 2010, 107, 064902. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Kim, C.H.; Choi, W.J.; Lee, T.G.; Cho, S.K.; Yang, Y.S.; Lee, J.H.; Lee, S.-J. Fluorocarbon thin films fabricated using carbon nanotube/polytetrafluoroethylene composite polymer targets via mid-frequency sputtering. Sci. Rep. 2017, 7, 1451. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar Uddin, A.S.M.; Yaqoob, U.; Phan, D.-T.; Chung, G.-S. A novel flexible acetylene gas sensor based on PI/PTFE-supported Ag-loaded vertical ZnO nanorods array. Sensor. Actuat. B-Chem. 2016, 222, 536–542. [Google Scholar] [CrossRef]
- Sakai, Y.; Sadaoka, Y.; Ikeuchi, K. Humidity sensors composed of grafted copolymers. Sensor. Actuat. 1986, 9, 125–131. [Google Scholar] [CrossRef]
- Sakai, Y.; Sadaoka, Y.; Matsuguchi, M. Humidity sensors based on polymer thin films. Sensor. Actuat. B-Chem. 1996, 35, 85–90. [Google Scholar] [CrossRef]
- Bruschi, P.; Cacialli, F.; Nannini, A. Sensing properties of polypyrrole-polytetrafluoroethylene composite thin films from granular metal-polymer precursors. Sensor. Actuat. A-Phys. 1992, 32, 313–317. [Google Scholar] [CrossRef]
- Wang, K.K.; Wu, Z.Z.; Peng, C.J.; Wang, K.P.; Cheng, B.; Song, C.L.; Han, G.R.; Liu, Y. A facile process to prepare crosslinked nano-graphites uniformly dispersed in titanium oxide films as solar selective absorbers. Sol. Energy Mat. Sol. C. 2015, 143, 198–204. [Google Scholar] [CrossRef]
- Charlot, A.; Bruguier, O.; Toquer, G.; Grandjean, A.; Deschanels, X. Nanocomposites derived from silica and carbon for low temperature photothermal conversion. Thin Solid Films 2014, 553, 157–160. [Google Scholar] [CrossRef]
- Morita, K.; Gonzales, J.; Sakaue, H. Effect of PTFE particle size on superhydrophobic coating for supercooled water prevention. Coatings 2018, 8, 426. [Google Scholar] [CrossRef]
- Morita, K.; Sakaue, H. Characterization method of hydrophobic anti-icing coatings. Rev. Sci. Instrum. 2015, 86, 115108. [Google Scholar] [CrossRef]
- Seo, K.; Kim, M.; Ahn, J.K.; Kim, D.H. Effects of drop size and measuring condition on static contact angle measurement on a superhydrophobic surface with goniometric technique. Korean J. Chem. Eng. 2015, 32, 2394–2399. [Google Scholar] [CrossRef]
- Oleskiw, M.; Knezevici, D.; Kind, R. Determination of Median Volume Diameter (MVD) and Liquid Water Content (LWC) by Multiple Rotating Cylinders. In Proceedings of the 43rd AIAA Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10–13 January 2005. [Google Scholar] [CrossRef]
- Hatfield, J.; Giorgis, R.; Flocchini, R. A simple solar radiation model for computing direct and diffuse spectral fluxes. Sol. Energy 1981, 27, 323–329. [Google Scholar] [CrossRef]




| PTFE Coating | Graphite Coating |
|---|---|
| 2 g PTFE nanoparticles (1 μm diameter) | 1 g PTFE nanoparticles (1 μm diameter) |
| 60 μL KP-109 Silicon | 60 μL KP-109 Silicon |
| 10 mL Novec 7300 Engineered Fluid | 10 mL Novec 7300 Engineered Fluid |
| 1 mL KR-400 Hardener | 1 mL KR-400 Hardener |
| - | 25 g graphite microparticles (<20 μm diameter) |
| Condition | Rime Ice | Glaze Ice |
|---|---|---|
| Temperature Range (°C) | [−14, −13] | [−12, −4] |
| Velocity (m/s) | 10 | 40, 80 |
| Humidity (%) | [45,50] | [40,45,55] |
| LWC (g/m3) | 1.035 | 1.035 |
| MVD (μm) | 26.6 | 95.5 |
| Air Volume (L/min) | 20 | 20 |
| Water Volume (mL/min) | 20 | 15 |
| Coated Surface | αoverall (solar) | αoverall (halogen) |
|---|---|---|
| PTFE coating | 0.719 | 0.907 |
| Graphite coating | 0.971 | 0.992 |
| - | PTFE Coating | Graphite Coating | Ideal |
|---|---|---|---|
| Contact Angle (o) | 157 ± 4 | 155 ± 4 | 180 |
| Roll-off Angle (o) | 4 ± 1.3 | 4 ± 1.2 | 0 |
| Ambient Temperature (°C) | Graphite Coating Ice Accumulation (g) | PTFE Coating Ice Accumulation (g) |
|---|---|---|
| −4 | 11.6562 | 11.2918 |
| −6 | 12.865 | 12.0634 |
| −7 | 13.3347 | 12.8716 |
| −8 | 11.6562 | 11.2718 |
| −12 | 7.3577 | 7.2755 |
| −13 | 1.92785 | 1.88485 |
| −14 | 1.8611 | 1.7961 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzales, J.; Kurihara, D.; Maeda, T.; Yamazaki, M.; Saruhashi, T.; Kimura, S.; Sakaue, H. Novel Superhydrophobic Surface with Solar-Absorptive Material for Improved De-Icing Performance. Materials 2019, 12, 2758. https://doi.org/10.3390/ma12172758
Gonzales J, Kurihara D, Maeda T, Yamazaki M, Saruhashi T, Kimura S, Sakaue H. Novel Superhydrophobic Surface with Solar-Absorptive Material for Improved De-Icing Performance. Materials. 2019; 12(17):2758. https://doi.org/10.3390/ma12172758
Chicago/Turabian StyleGonzales, Joseph, Daiki Kurihara, Tetsuro Maeda, Masafumi Yamazaki, Takahito Saruhashi, Shigeo Kimura, and Hirotaka Sakaue. 2019. "Novel Superhydrophobic Surface with Solar-Absorptive Material for Improved De-Icing Performance" Materials 12, no. 17: 2758. https://doi.org/10.3390/ma12172758
APA StyleGonzales, J., Kurihara, D., Maeda, T., Yamazaki, M., Saruhashi, T., Kimura, S., & Sakaue, H. (2019). Novel Superhydrophobic Surface with Solar-Absorptive Material for Improved De-Icing Performance. Materials, 12(17), 2758. https://doi.org/10.3390/ma12172758