Optimization of Low-Cost Ti-35421 Titanium Alloy: Phase Transformation, Bimodal Microstructure, and Combinatorial Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Dilatometric Test
2.3. Heat Treatment
2.4. Microstructure Observation
2.5. Mechanical Properties
3. Results and Discussions
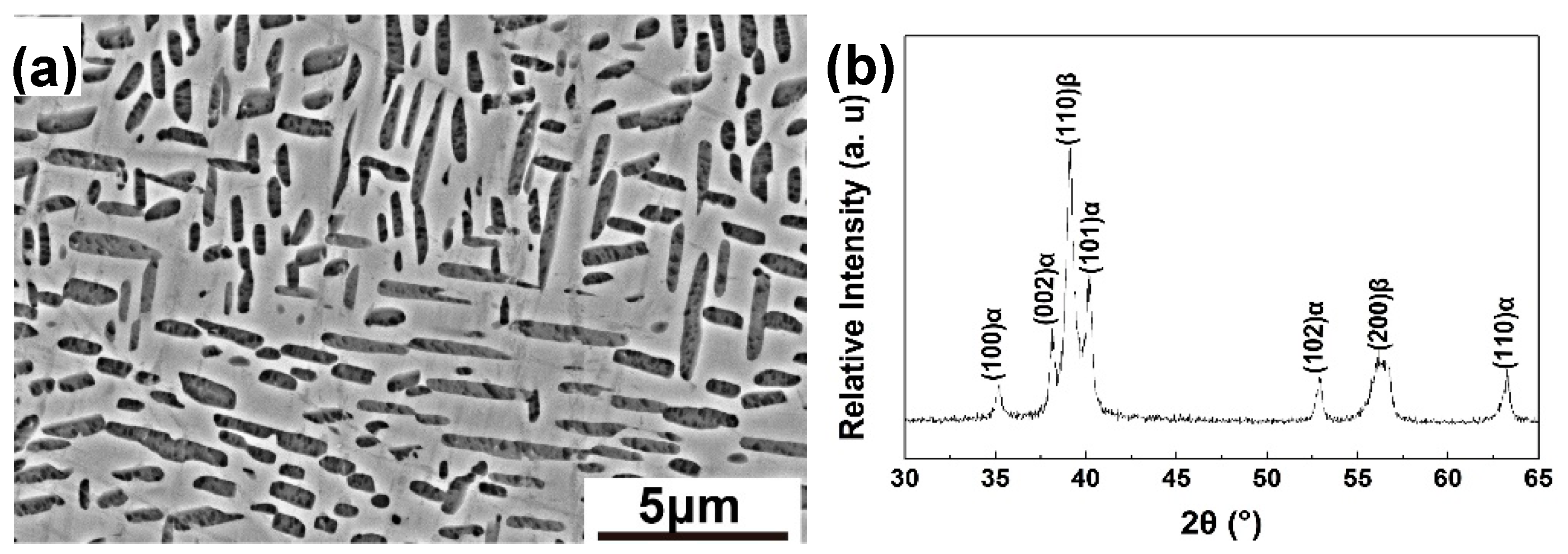
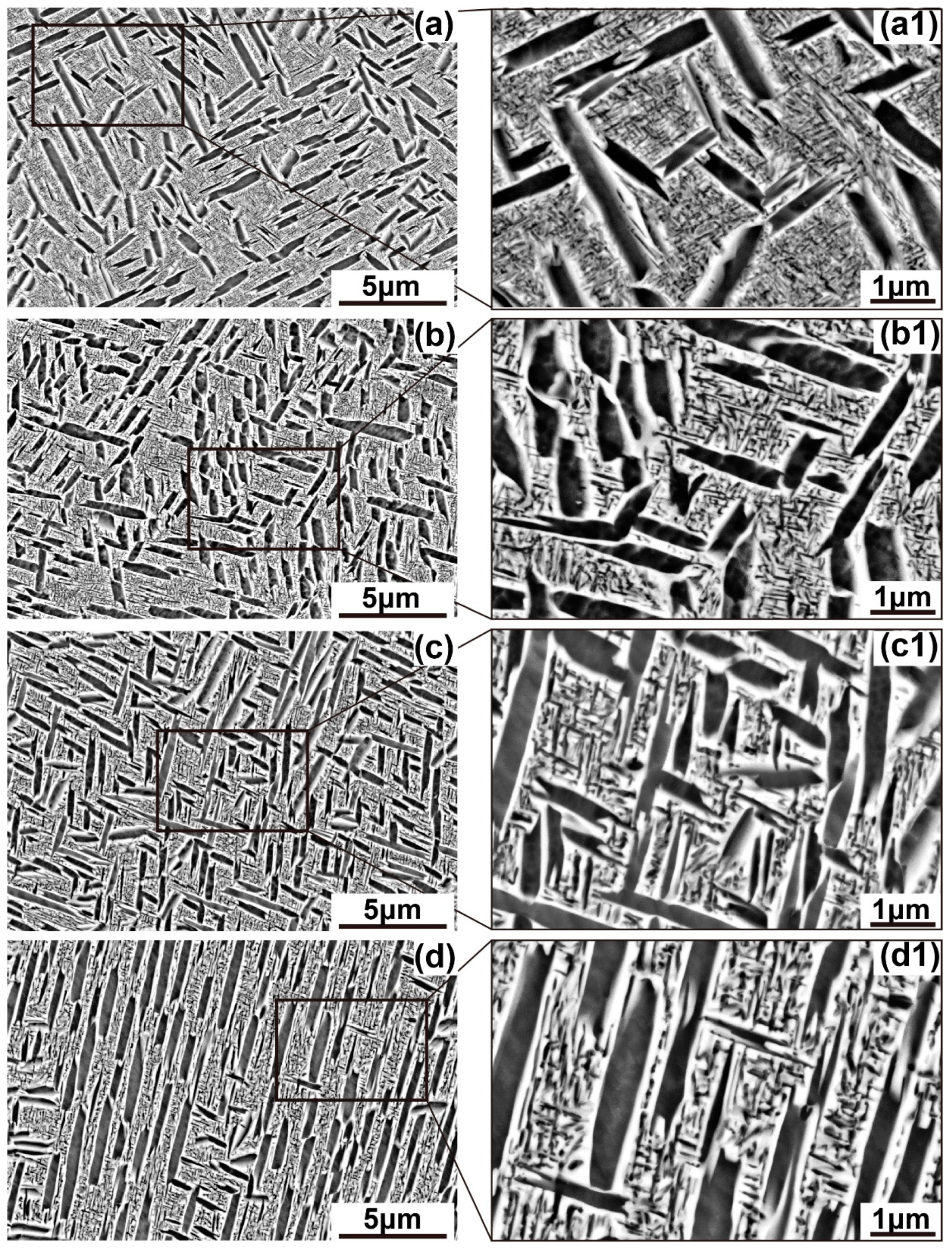
3.1. Initial Microstructure of Solid Solution Treated Alloy
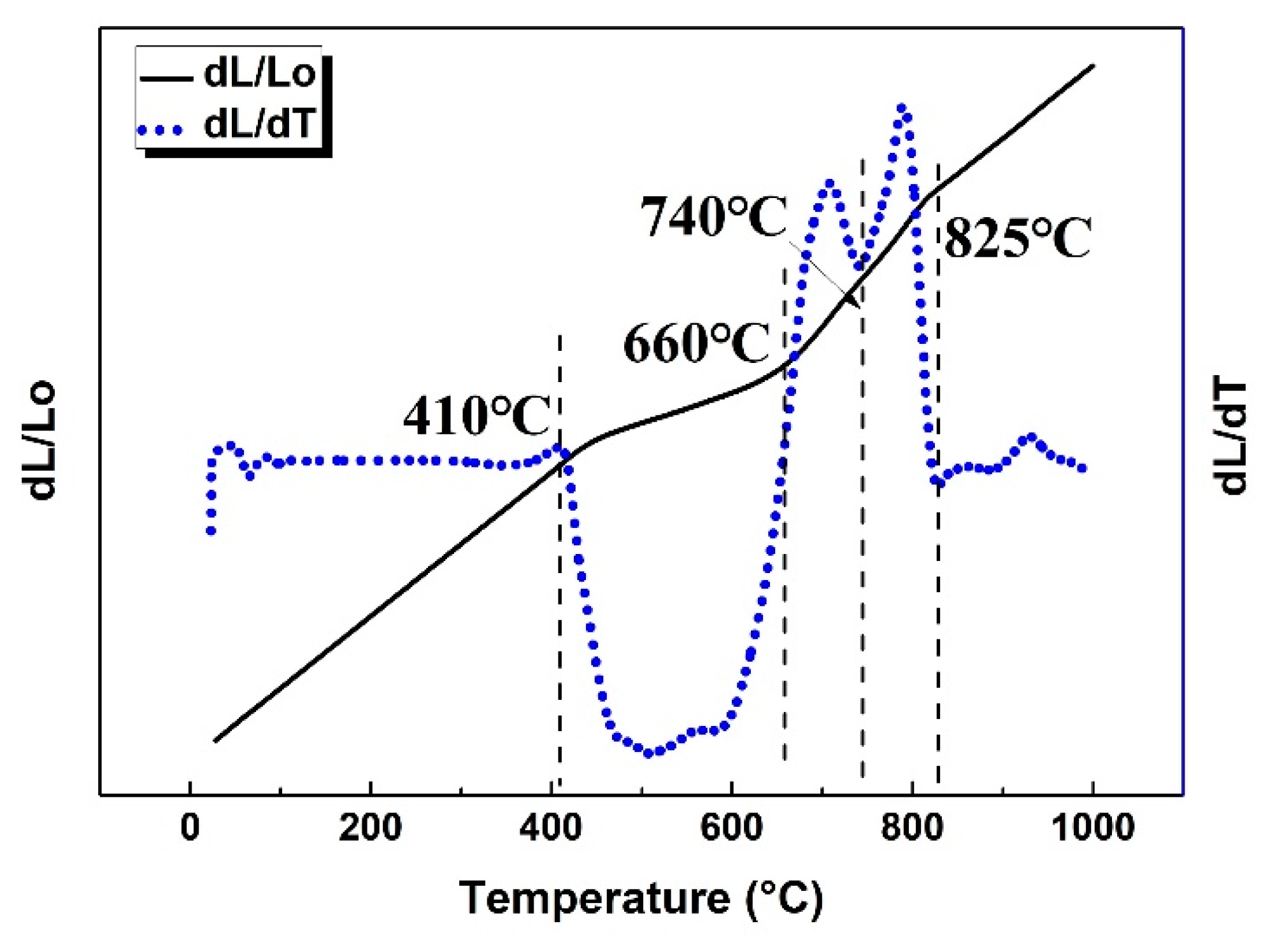
3.2. Phase Transformation Reactions and Temperature Ranges
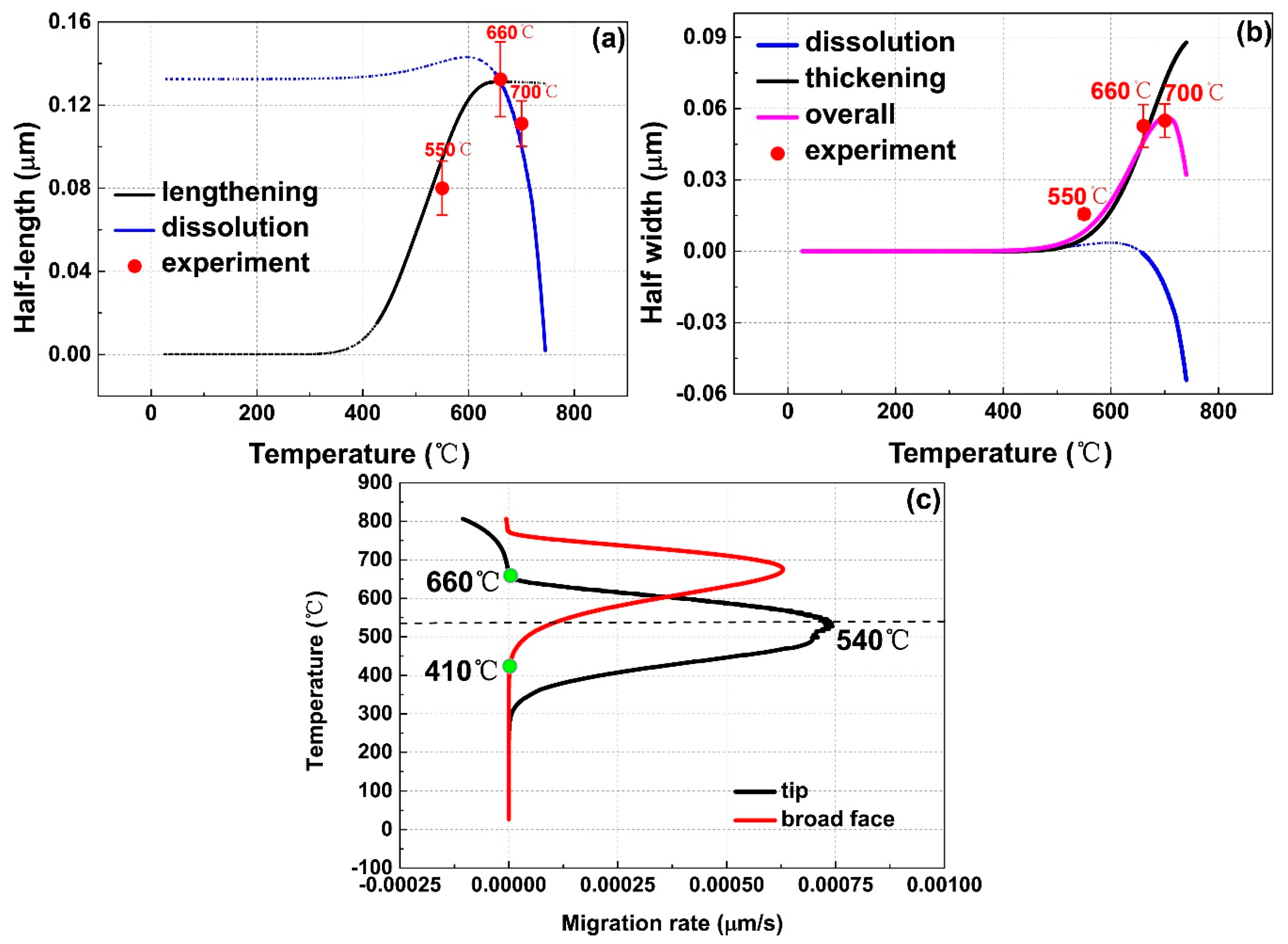
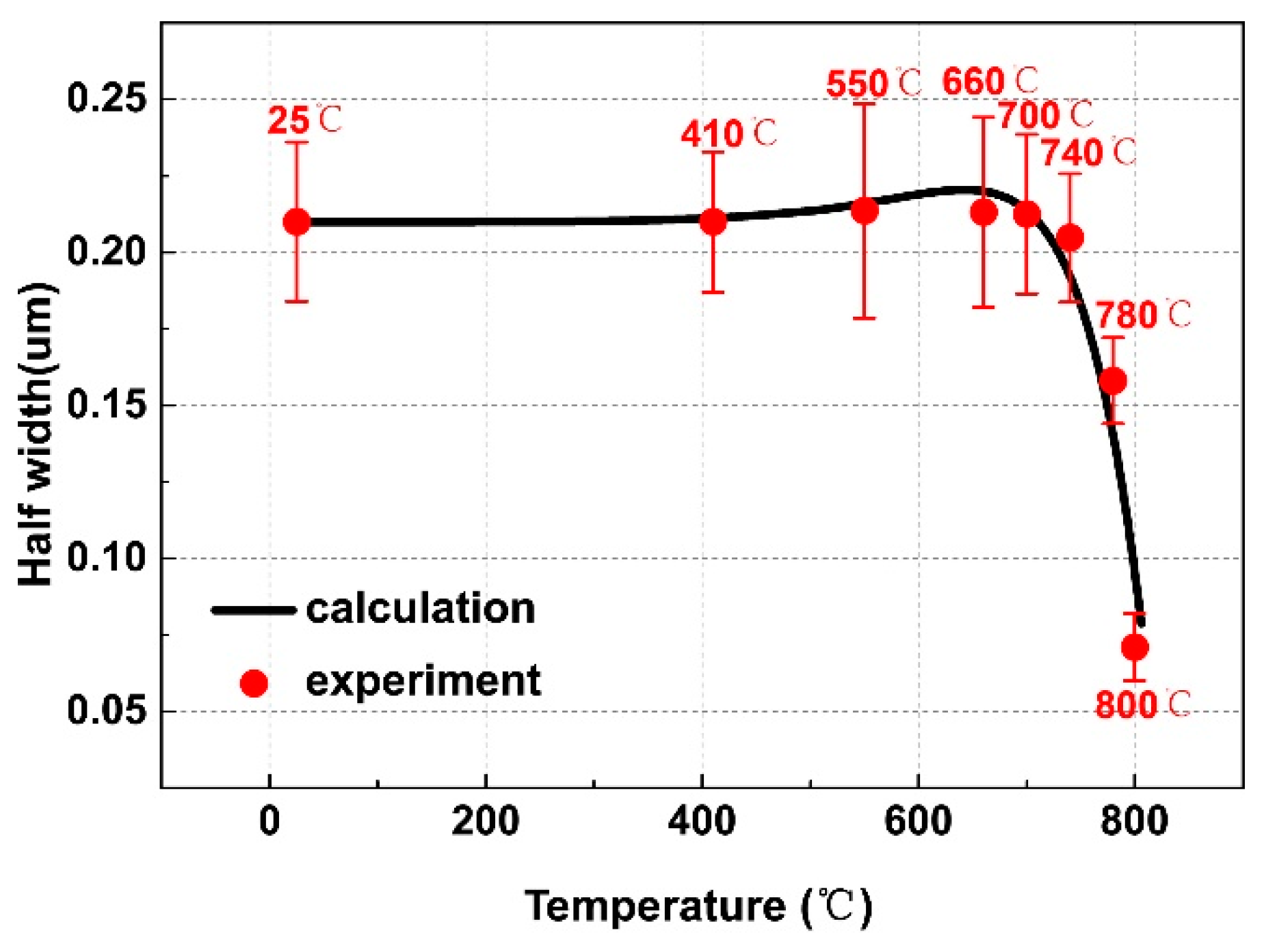
3.3. Thermo-Kinetic Modeling of Growth and Dissolution
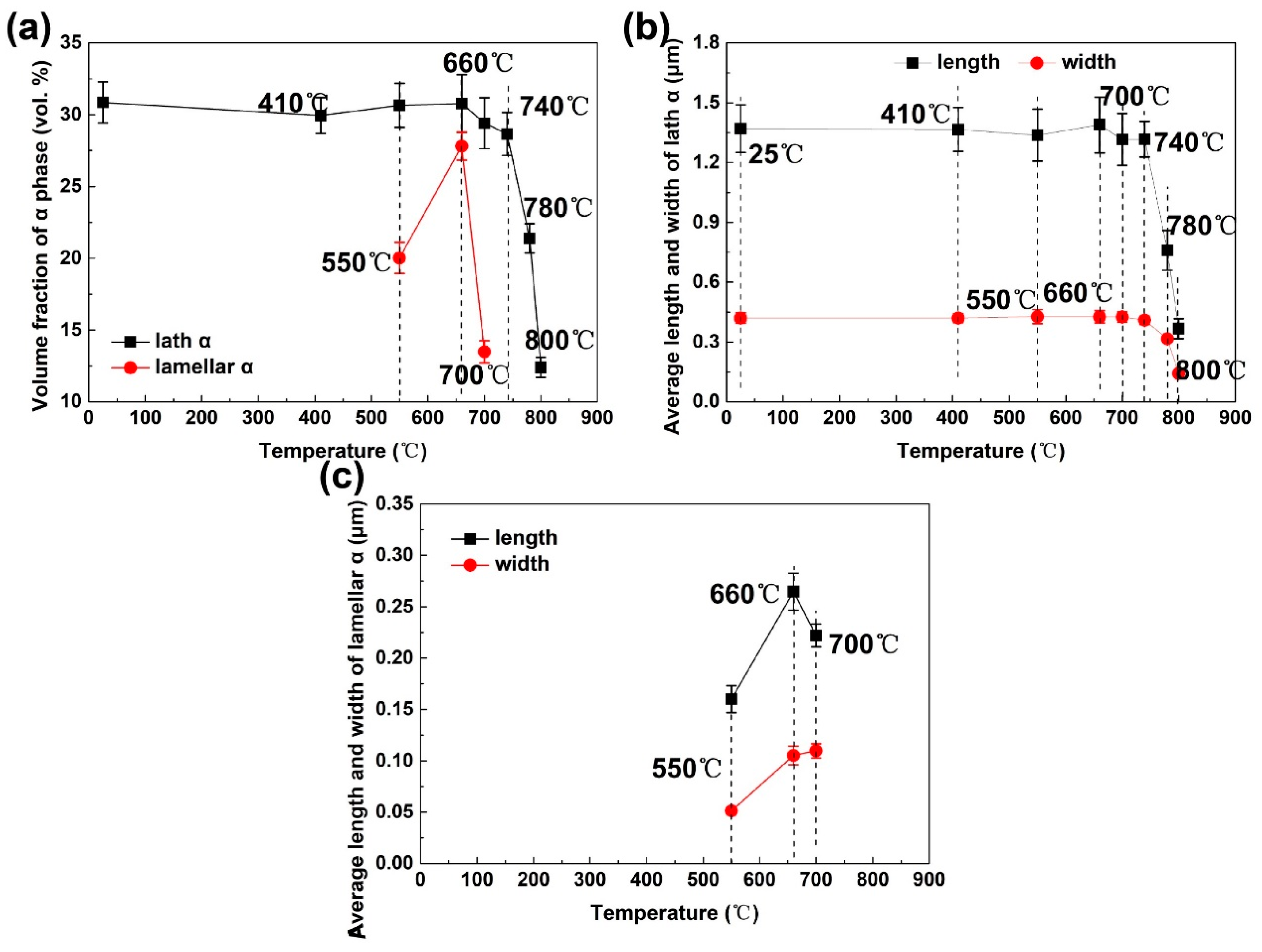
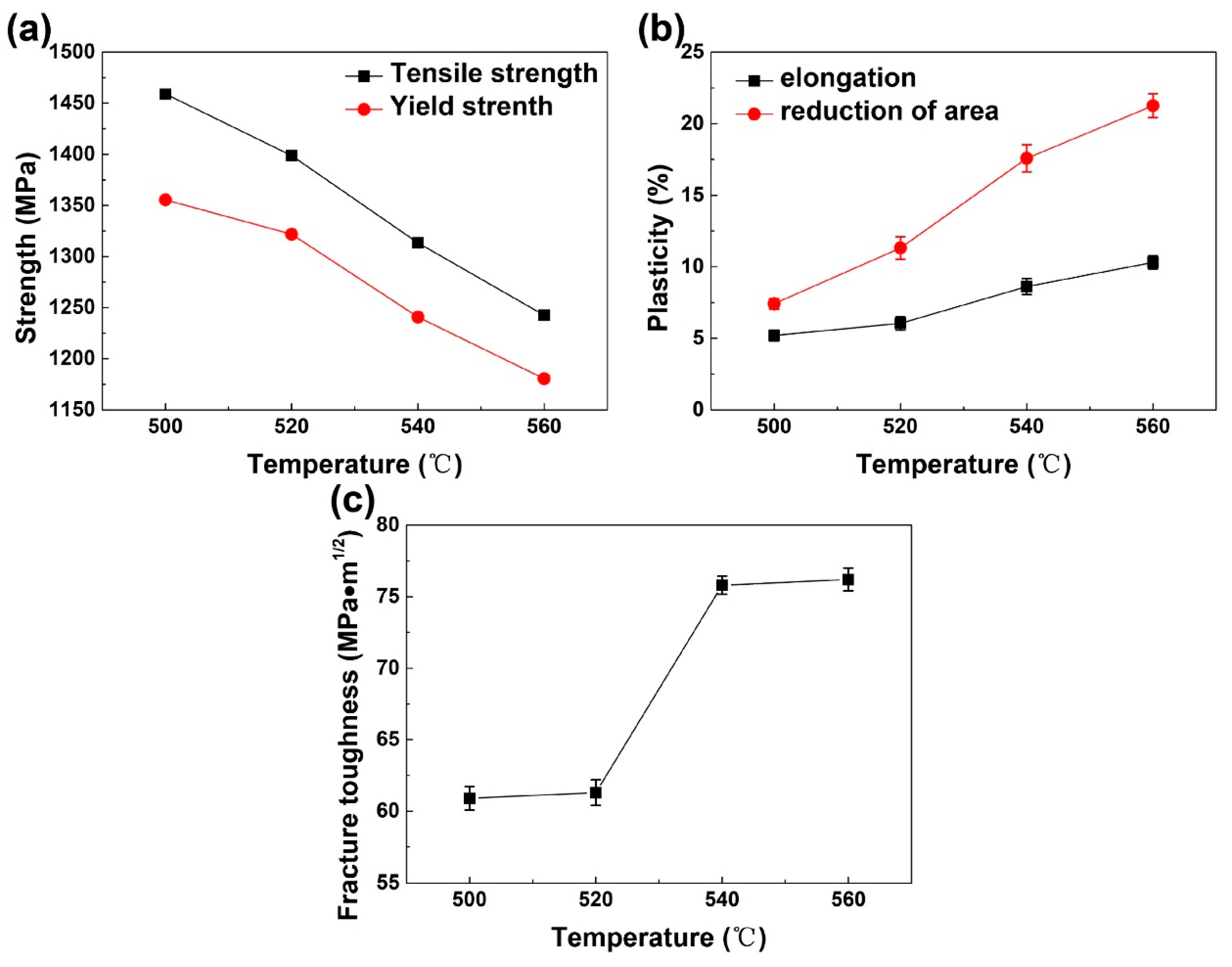
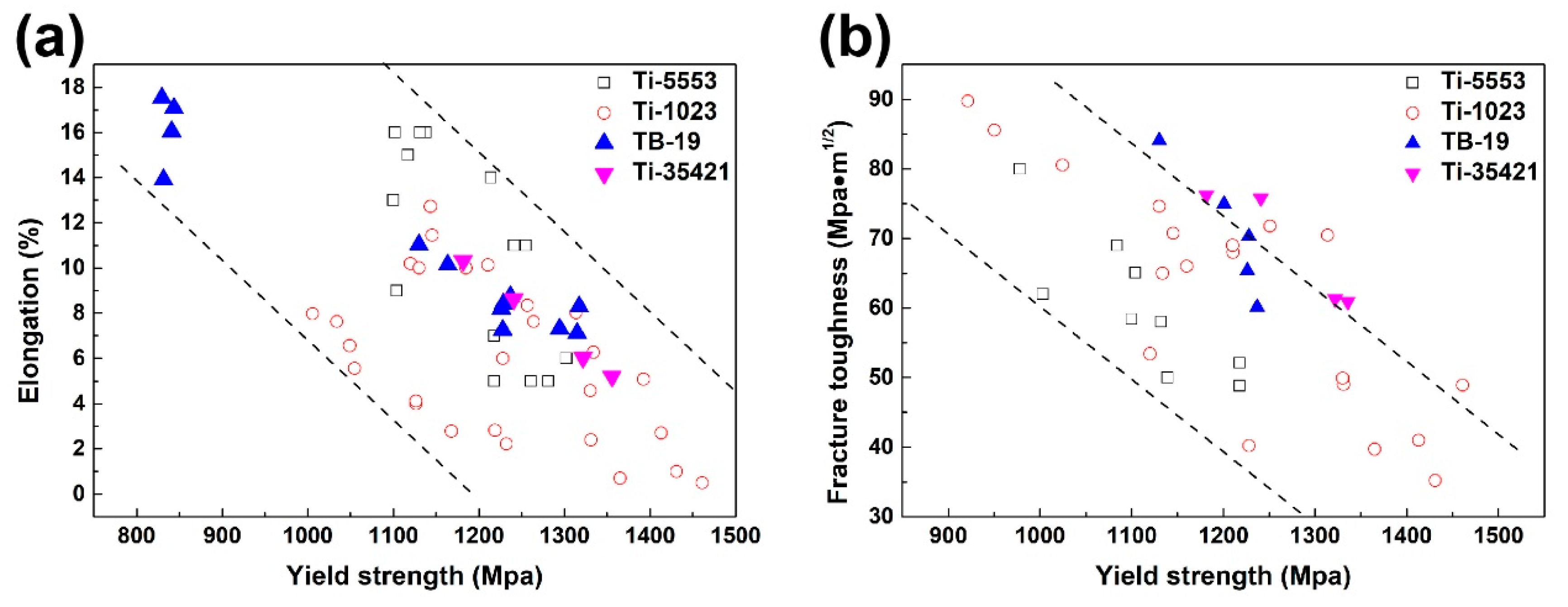
3.4. Mechanical Properties Dependent on Aging Temperature and Microstructure Features
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
A.1. Lengthening Model of αlamellar Tip
A.2. Dissolution Model of αlamellar Tip
A.3. Thickening Model of αlamellar Broad Face
A.4. Dissolution Model of αlamellar Broad Face below Teq
A.5. Dissolution model of αlamellar Broad Face above Teq and αlath Broad Face
References
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar]
- Williams, J.C.; Edgar, A.S.J. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Raghunathan, S.L.; Stapleton, A.M.; Dashwood, R.J.; Jackson, M.; Dye, D. Micromechanics of Ti-10V-2Fe-3Al: In situ synchrotron characterisation and modelling. Acta Mater. 2007, 55, 6861–6872. [Google Scholar] [CrossRef]
- Ivasishin, O.M.; Markovsky, P.E.; Matviychuk, Y.V.; Semiatin, S.L.; Ward, C.H.; Fox, S. A comparative study of the mechanical properties of high-strength β-titanium alloys. J. Alloys Compd. 2008, 457, 296–309. [Google Scholar] [CrossRef]
- Chen, F.; Xu, G.; Zhang, X.; Zhou, K. Exploring the Phase Transformation in β-Quenched Ti-55531 alloy during continuous heating via dilatometric measurement, microstructure characterization, and diffusion analysis. Met. Mater. Trans. A 2016, 47, 5383–5394. [Google Scholar] [CrossRef]
- Sachdev, A.K.; Kulkarni, K.; Fang, Z.Z.; Yang, R.; Girshov, V. Titanium for automotive applications: Challenges and opportunities in materials and processing. JOM 2012, 64, 553–565. [Google Scholar] [CrossRef]
- Crowli, G. How to Extract Low-Cost Titanium. Adv. Mater. Process 2003, 161, 25–27. [Google Scholar]
- Ezugwu, E.O.; Wang, Z.M. Titanium alloys and their machinability—A review. J. Mater. Process. Technol. 1997, 68, 262–274. [Google Scholar] [CrossRef]
- Cui, C.; Hu, B.; Zhao, L.; Liu, S. Titanium alloy production technology, market prospects and industry development. Mater. Des. 2011, 32, 1684–1691. [Google Scholar]
- Philippart, I.; Rack, H.J. High temperature dynamic yielding in metastable Ti-6.8 Mo-4.5 F-1.5 Al. Mater. Sci. Eng. A 1998, 243, 196–200. [Google Scholar] [CrossRef]
- Hu, Y.M.; Floer, W.; Krupp, U.; Christ, H.J. Microstructurally short fatigue crack initiation and growth in Ti-6.8 Mo-4.5 Fe-1.5 Al. Mater. Sci. Eng. A 2000, 278, 170–180. [Google Scholar] [CrossRef]
- Koch, B.; Birgit, S. Strain controlled fatigue testing of the metastable β-titanium alloy Ti-6.8 Mo-4.5 Fe-1.5 Al (Timetal LCB). Mater. Sci. Eng. A 2011, 528, 5999–6005. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, H.; Xie, Y.; Ding, L.; Li, H.; Cui, Y.; Zhou, L. Hot deformation behavior of two kinds of TC4-xFe alloys based on processing map. Rare Met. Mater. Eng. 2017, 46, 204–210. [Google Scholar]
- Chang, H.; Zeng, W.; Luo, Y.; Zhou, Y.; Zhou, L. Phase transformation and microstructure evolution in near beta Ti-B19 titanium alloy during aging treatment. Rare Met. Mater. Eng. 2006, 35, 1589–1592. [Google Scholar]
- Li, D. A Study on Microstructure and Mechanical Properties of Ti-35421 Alloy with High Strength and Toughness. Master’s Thesis, Nanjing Tech University, Nanjing, China, 2017. [Google Scholar]
- Shekhar, S.; Sarkar, R.; Kar, S.K. Bhattacharjee, A. Effect of solution treatment and aging on microstructure and tensile properties of high strength β titanium alloy, Ti-5Al-5V-5Mo-3Cr. Mater. Des. 2015, 66, 596–610. [Google Scholar] [CrossRef]
- Li, C.L.; Mi, X.J.; Ye, W.J.; Hui, S.X.; Yu, Y.; Wang, W.Q. A study on the microstructures and tensile properties of new beta high strength titanium alloy. J. Alloys Compd. 2013, 550, 23–30. [Google Scholar] [CrossRef]
- Welk, B.A. Microstructural and Property Relationships in Titanium Alloy Ti-5553. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2010. [Google Scholar]
- Srinivasu, G.; Natraj, Y.; Bhattacharjee, A.; Nandy, T.K.; Rao, G.N. Tensile and fracture toughness of high strength β Titanium alloy, Ti-10V-2Fe-3Al, as a function of rolling and solution treatment temperatures. Mater. Des. 2013, 47, 323–330. [Google Scholar] [CrossRef]
- Fan, J.K.; Li, J.S.; Kou, H.C.; Hua, K.; Tang, B. The interrelationship of fracture toughness and microstructure in a new near β titanium alloy Ti-7Mo-3Nb-3Cr-3Al. Mater. Charact. 2014, 96, 93–99. [Google Scholar] [CrossRef]
- ASTM International. ASTM E8/E8M–09, Standard Test Methods for Tension Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM International. ASTM E399-06. Standard Test Method for Linear-Elastic Plane-Strain Toughness KIC of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Chen, F.W.; Xu, G.; Zhang, X.Y.; Zhou, K.C.; Cui, Y. Effect of α morphology on the diffusional β ↔ α transformation in Ti-55531 during continuous heating: Dissection by dilatometer test, microstructure observation and calculation. J. Alloys Compd. 2017, 702, 352–365. [Google Scholar] [CrossRef]
- Liu, Z.; Chang, Y.A. On the applicability of the Ivantsov growth equation. J. Appl. Phys. 1997, 82, 4838–4841. [Google Scholar] [CrossRef]
- Shiflet, G.J.; Aaronson, H.I.; Courtney, T.H. Kinetics of coarsening by the ledge mechanism. Acta Met. 1979, 27, 377–385. [Google Scholar] [CrossRef]
- Aaron, H.B.; Kotler, G.R. Second phase dissolution. Met. Trans. 1971, 2, 393–408. [Google Scholar] [CrossRef]
- Madelung, O. Landolt-Börnstein New Series Group III; Springer: Berlin/Heidelberg, Germany, 1982. [Google Scholar]
- Chen, C.C.; Hall, J.A.; Boyer, R.R. High strength beta titanium alloy forgings for aircraft structural applications. In Titanium 80: Science and Technology, Proceedings of the Fourth International Conference on Titanium, Kyoto, Japan, 19–22 May 1980; Kimura, H., Izumi, O., Eds.; Stanford University Press: Palo Alto, CA, USA, 1980. [Google Scholar]
- Chen, J.; Chang, H.; Li, H. Effect of heat treatment on optimization matching between strength and toughness of Ti-B19 alloy. Acta Met. Sin. 2002, 38, 108–111. [Google Scholar]
- Fanning, J.C. Properties of TIMETAL 555 (Ti-5Al-5Mo-5V-3Cr-0.6 Fe). J. Mater. Eng. Perform. 2005, 14, 788–791. [Google Scholar] [CrossRef]
- Kar, S.K.; Suman, S.; Shivaprasad, S.; Chaudhuri, A.; Bhattacharjee, A. Processing-microstructure-yield strength correlation in a near β Ti alloy, Ti-5Al-5Mo-5V-3Cr. Mater. Sci. Eng. A 2014, 610, 171–180. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, J.R.; Lei, J.F.; Cao, M.Z.; Liu, Y.Y. Effects of primary and secondary a phase on tensile property and fracture toughness of Ti-1023 alloy. Acta Met. Sin. 2007, 43, 1129–1137. [Google Scholar]
- Trivedi, R. The role of interfacial free energy and interface kinetics during the growth of precipitate plates and needles. Met. Trans. 1970, 1, 921–927. [Google Scholar]
- Trivedi, R.; Pound, G.M. Growth Kinetics of plate-like precipitates. J. Appl. Phys. 1969, 40, 4293–4300. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, N.; Wu, K.; Wang, Y. Quantitative phase field modeling of diffusion-controlled precipitate growth and dissolution in Ti-Al-V. Scr. Mater. 2004, 50, 471–476. [Google Scholar] [CrossRef]



| Ti/wt.% | Al/wt.% | Mo/wt.% | Cr/wt.% | Zr/wt.% | Fe/wt.% | O/wt.% |
|---|---|---|---|---|---|---|
| Balance | 3.03 | 5.02 | 3.80 | 1.87 | 0.995 | 0.087 |
| Temperature/°C | αlath | αlamellar | ||||
|---|---|---|---|---|---|---|
| Average Length/μm | Average Width/μm | Volume Fraction/vol. % | Average Length/μm | Average Width/μm | Volume Fraction/vol. % | |
| 25 | 1.370 ± 0.120 | 0.420 ± 0.026 | 30.86 ± 1.43 | |||
| 410 | 1.373 ± 0.112 | 0.420 ± 0.023 | 29.95 ± 1.25 | |||
| 550 | 1.341 ± 0.133 | 0.427 ± 0.035 | 30.66 ± 1.54 | 0.160 ± 0.013 | 0.031 ± 0.002 | 20.02 ± 1.09 |
| 660 | 1.389 ± 0.144 | 0.426 ± 0.031 | 30.78 ± 2.01 | 0.265 ± 0.018 | 0.105 ± 0.009 | 27.81 ± 0.98 |
| 700 | 1.320 ± 0.143 | 0.425 ± 0.026 | 29.41 ± 1.78 | 0.222 ± 0.011 | 0.109 ± 0.007 | 13.49 ± 0.78 |
| 740 | 1.321 ± 0.096 | 0.409 ± 0.021 | 28.65 ± 1.49 | |||
| 780 | 0.764 ± 0.101 | 0.316 ± 0.021 | 21.39 ± 1.02 | |||
| 800 | 0.372 ± 0.053 | 0.142 ± 0.011 | 12.40 ± 0.69 | |||
| Temperature/°C | αlath | αlamellar | ||||
|---|---|---|---|---|---|---|
| Average Length/μm | Average Width/μm | Volume Fraction/vol. % | Average Length/μm | Average Width/μm | Volume Fraction/vol. % | |
| 500 | 1.404 ± 0.151 | 0.425 ± 0.051 | 29.16 ± 1.24 | 0.065 ± 0.011 | 0.031 ± 0.005 | 33.49 ± 1.27 |
| 520 | 1.390 ± 0.112 | 0.421 ± 0.049 | 29.96 ± 1.17 | 0.092 ± 0.010 | 0.037 ± 0.006 | 29.18 ± 1.49 |
| 540 | 1.359 ± 0.11 | 0.429 ± 0.056 | 30.26 ± 1.41 | 0.161 ± 0.017 | 0.072 ± 0.009 | 26.55 ± 1.28 |
| 560 | 1.316 ± 0.12 | 0.430 ± 0.061 | 29.01 ± 1.38 | 0.218 ± 0.021 | 0.089 ± 0.009 | 25.21 ± 0.98 |
| Temperature/°C | Strength | Plasticity | Fracture Toughness KIC/MPa·m1/2 | ||
|---|---|---|---|---|---|
| Tensile Strength σs/MPa | Yield Strength σy/MPa | Elongation δ/% | Reduction of Area ψ/% | ||
| 500 | 1459.03 ± 4.36 | 1355.54 ± 4.67 | 5.20 ± 0.38 | 7.42 ± 0.37 | 60.9 ± 0.82 |
| 520 | 1398.66 ± 3.23 | 1321.74 ± 4.82 | 6.04 ± 0.47 | 11.31 ± 0.78 | 61.3 ± 0.89 |
| 540 | 1313.39 ± 3.48 | 1240.81 ± 4.98 | 8.62 ± 0.56 | 17.58 ± 0.96 | 75.8 ± 0.64 |
| 560 | 1242.52 ± 3.94 | 1180.40 ± 3.67 | 10.31 ± 0.46 | 21.26 ± 0.83 | 76.2 ± 0.79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Xu, G.; Cui, Y.; Chang, H. Optimization of Low-Cost Ti-35421 Titanium Alloy: Phase Transformation, Bimodal Microstructure, and Combinatorial Mechanical Properties. Materials 2019, 12, 2791. https://doi.org/10.3390/ma12172791
Chen F, Xu G, Cui Y, Chang H. Optimization of Low-Cost Ti-35421 Titanium Alloy: Phase Transformation, Bimodal Microstructure, and Combinatorial Mechanical Properties. Materials. 2019; 12(17):2791. https://doi.org/10.3390/ma12172791
Chicago/Turabian StyleChen, Fuwen, Guanglong Xu, Yuwen Cui, and Hui Chang. 2019. "Optimization of Low-Cost Ti-35421 Titanium Alloy: Phase Transformation, Bimodal Microstructure, and Combinatorial Mechanical Properties" Materials 12, no. 17: 2791. https://doi.org/10.3390/ma12172791
APA StyleChen, F., Xu, G., Cui, Y., & Chang, H. (2019). Optimization of Low-Cost Ti-35421 Titanium Alloy: Phase Transformation, Bimodal Microstructure, and Combinatorial Mechanical Properties. Materials, 12(17), 2791. https://doi.org/10.3390/ma12172791





