Zeolite Nanocrystals Protect the Performance of Organic Additives and Adsorb Acid Compounds during Lubricants Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LTL Nanocrystals
2.3. Oxidation Process
2.4. Characterization
2.4.1. Characterization of Zeolite Nanocrystals
2.4.2. Characterization of Lubricants
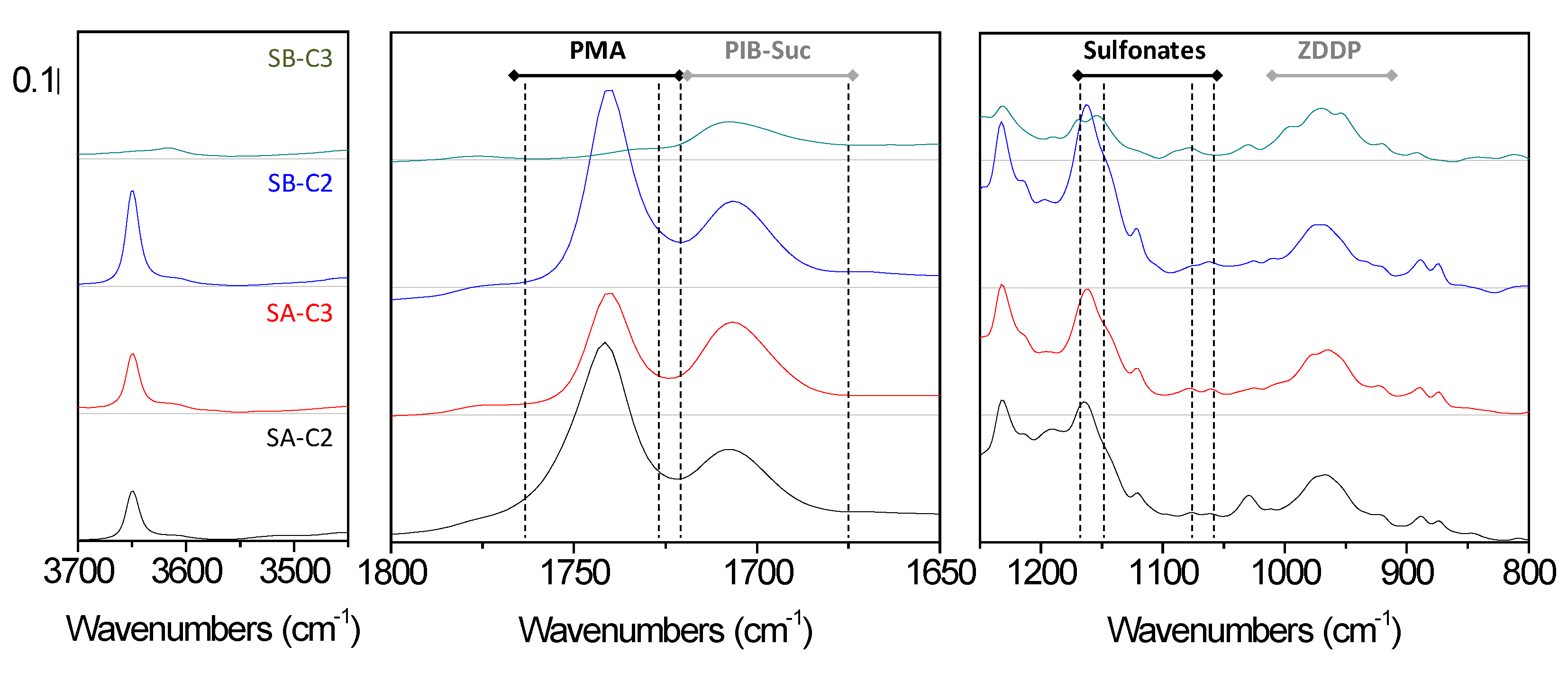
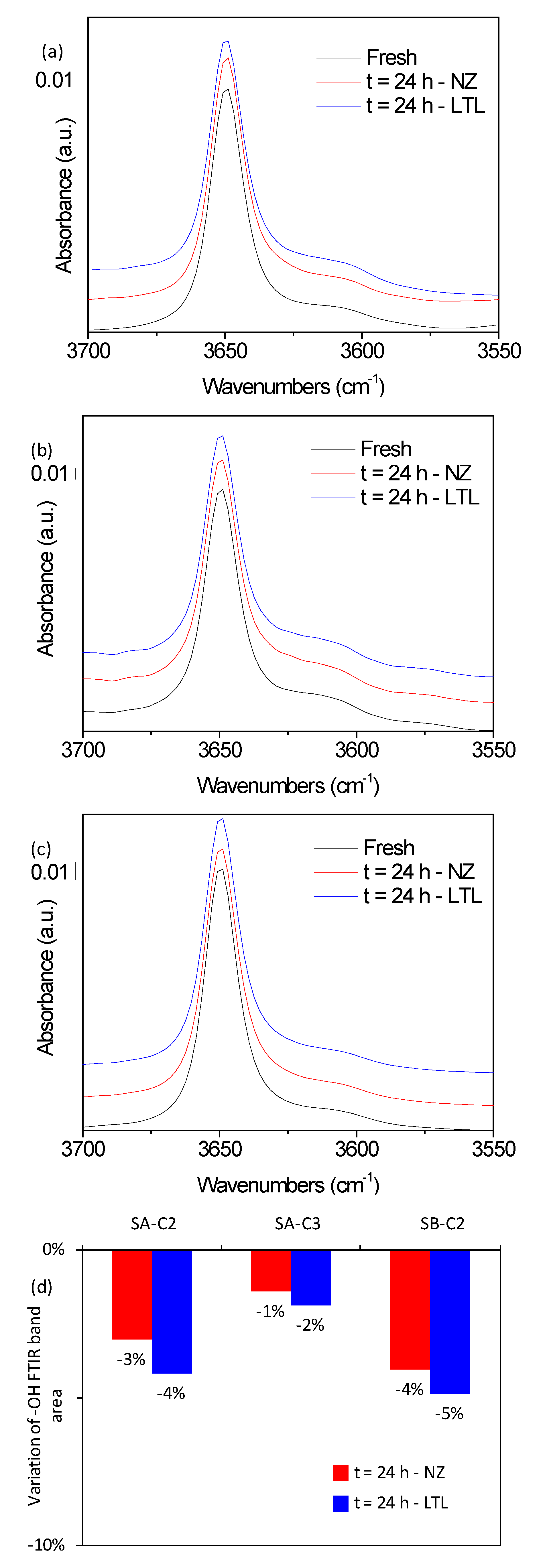
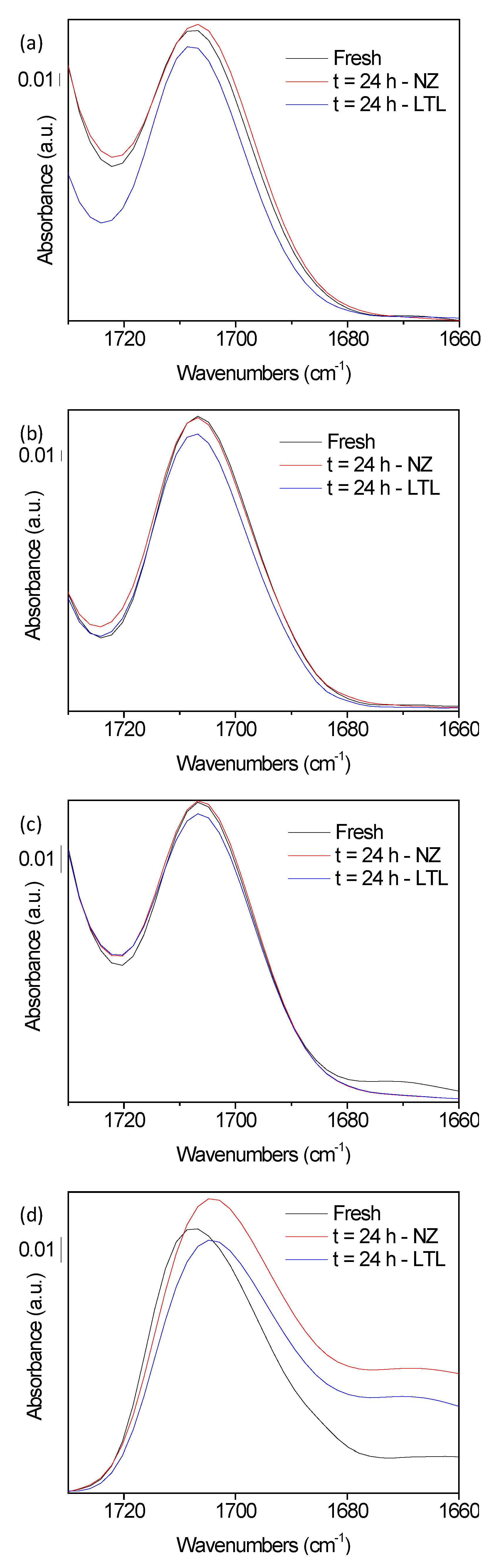
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Boyde, S. Green lubricants. Environmental benefits and impacts of lubrication. Green Chem. 2002, 4, 293–307. [Google Scholar] [CrossRef]
- Majano, G.; Ng, E.P.; Lakiss, L.; Mintova, S. Nanosized molecular sieves utilized as an environmentally friendly alternative to antioxidants for lubricant oils. Green Chem. 2011, 13, 2435–2440. [Google Scholar] [CrossRef]
- He, C.; Yan, H.; Li, X.; Wang, X. In situ fabrication of carbon dots-based lubricants using a facile ultrasonic approach. Green Chem. 2019, 21, 2279–2285. [Google Scholar] [CrossRef]
- Nguele, R.; Al-Salim, H.; Mohammad, K. Modeling and Forecasting of Depletion of Additives in Car Engine Oils Using Attenuated Total Reflectance Fast Transform Infrared Spectroscopy. Lubricants 2014, 2, 206–222. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.; Hils, J. Phosphate Esters, Thiophosphate Esters and Metal Thiophosphates as Lubricant Additives. Lubricants 2013, 1, 132–148. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Pantar, A.V.; Rao, U.S.; Sarma, A.S. Shear stability of polymers used as viscosity modifiers in lubricating oils. Indian J. Chem. Technol. 1998, 5, 309–314. [Google Scholar]
- Schrive, L.; Sarrade, S.; Gourgouillon, D. Method for treating an oil using a liquid in a supercritical state. PCT Pat. Appl. WO2000/052118A1, 8 September 2000. [Google Scholar]
- Wu, H.; Zhao, J.; Xia, W.; Cheng, X.; He, A.; Yun, J.H.; Wang, L.; Huang, H.; Jiao, S.; Huang, L.; et al. A study of the tribological behaviour of TiO2 nano-additive water-based lubricants. Tribol. Int. 2017, 109, 398–408. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Zhao, J.; Luo, L.; Huang, S.; Wang, L.; Zhang, S.; Jiao, S.; Huang, H.; Jiang, Z. Performance Evaluation and Lubrication Mechanism of Water-Based Nanolubricants Containing Nano-TiO2 in Hot Steel Rolling. Lubricants 2018, 6, 57. [Google Scholar] [CrossRef]
- Padgurskas, J.; Rukuiza, R.; Prosyčevas, I.; Kreivaitis, R. Tribological properties of lubricant additives of Fe, Cu and Co nanoparticles. Tribol. Int. 2013, 60, 224–232. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Guo, J.; Yang, X.; Xu, B. Tribological property of onion-like fullerenes as lubricant additive. Mater. Lett. 2008, 62, 2524–2527. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Chu, H.-Y.; Jeng, Y.-R.; Huang, Y.-H.; Wang, M.H.; Chang, C.K. In situ de-agglomeration and surface functionalization of detonation nanodiamond, with the polymer used as an additive in lubricant oil. J. Mater. Chem. 2011, 21, 13213–13222. [Google Scholar] [CrossRef]
- Rapoport, L.; Fleischer, N.; Tenne, R. Fullerene-like WS2 Nanoparticles: Superior Lubricants for Harsh Conditions. Adv. Mater. 2003, 15, 651–655. [Google Scholar] [CrossRef]
- Morishita, H.; Saito, Y.; Fukutomi, I.; Murakami, M.; Miyasaka, K.; Ohmiya, Y.; Moritani, H.; Tohyama, M.; Tatsuda, N. Oil Degradation Prevention Device. U.S. Patent 2015/0083655 A1, 26 March 2015. [Google Scholar]
- Ohmiya, Y.; Tohyama, M.; Moritani, H.; Tatsuda, N.; Yano, K.; Harada, K.; Fukutomi, I.; Murakami, M.; Inami, N.; Koike, R. Material for Trapping Target Substance, Filter for Trapping Target Substance, Container for Liquid Organic Compound, and Engine Oil. U.S. Patent 2012/0312731 A1, 13 December 2012. [Google Scholar]
- Lockledge, S.P.; Brownawell, D.W. Oil Filters Containing Strong Base and Methods of Their Use. U.S. Patent WO2009/099882 A2, 13 August 2009. [Google Scholar]
- Klein, M.; Vaillant, C.; Amirnasr, E.; Gohl, P.; Ahuja, R.; Desjardins, M. Filter, Filter Element, and Filter Housing. U.S. Patent 2016/0354714 A1, 08 December 2016. [Google Scholar]
- Lockledge, S.P.; Brownawell, D.W. Materals and Processes for Reducing Combustion By-Products in a Lubrication System for an Internal Combuston Engine. U.S. Patent 2006/0260874 A1, 23 November 2006. [Google Scholar]
- Albuquerque, R.Q.; Calzaferri, G. Proton activity inside the channels of zeolite L. Chem. A Eur. J. 2007, 13, 8939–8952. [Google Scholar] [CrossRef] [PubMed]
- Zaarour, M.; Dong, B.; Naydenova, I.; Retoux, R.; Mintova, S. Progress in zeolite synthesis promotes advanced applications. Microporous Mesoporous Mater. 2014, 189, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Mintova, S.; Jaber, M.; Valtchev, V. Nanosized microporous crystals: Emerging applications. Chem. Soc. Rev. 2015, 44, 7207–7233. [Google Scholar] [CrossRef]
- Tan, K.H.; Awala, H.; Mukti, R.R.; Wong, K.L.; Ling, T.C.; Mintova, S.; Ng, E.P. Zeolite nanoparticles as effective antioxidant additive for the preservation of palm oil-based lubricant. J. Taiwan Inst. Chem. Eng. 2016, 58, 565–571. [Google Scholar] [CrossRef]
- Tan, K.-H.; Cham, H.-Y.; Awala, H.; Ling, T.C.; Mukti, R.R.; Wong, K.-L.; Mintova, S.; Ng, E.-P. Effect of Extra-Framework Cations of LTL Nanozeolites to Inhibit Oil Oxidation. Nanoscale Res. Lett. 2015, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.H.; Awala, H.; Mukti, R.R.; Wong, K.L.; Rigaud, B.; Ling, T.C.; Aleksandrov, H.A.; Koleva, I.Z.; Vayssilov, G.N.; Mintova, S.; et al. Inhibition of palm oil oxidation by zeolite nanocrystals. J. Agric. Food Chem. 2015, 63, 4655–4663. [Google Scholar] [CrossRef]
- Fois, E.; Tabacchi, G.; Calzaferri, G. Interactions, behavior, and stability of fluorenone inside zeolite nanochannels. J. Phys. Chem. C 2010, 114, 10572–10579. [Google Scholar] [CrossRef]
- Majano, G.; Mintova, S. Mineral oil regeneration using selective molecular sieves as sorbents. Chemosphere 2010, 78, 591–598. [Google Scholar] [CrossRef]
- Laurent, S.; Ng, E.P.; Thirifays, C.; Lakiss, L.; Goupil, G.M.; Mintova, S.; Burtea, C.; Oveisi, E.; Hébert, C.; De Vries, M.; et al. Corona protein composition and cytotoxicity evaluation of ultra-small zeolites synthesized from template free precursor suspensions. Toxicol. Res. (Camb) 2013, 2, 270–279. [Google Scholar] [CrossRef]
- Joshi, D.; NL, C. Infrared Spectroscopy Technique for Differentiation of Genuine and Counterfeit Engine Oil—A Forensic Aspect. MOJ Civ. Eng. 2017, 3, 00073. [Google Scholar]
- Wei, C.; Di, Z.; Zhang, D.; Liu, Z.; Li, S.; Piao, J.; Wang, H. Synthesis of modified CeO2 nanoparticles highly stabilized in organic solvent using higee technology. Chem. Eng. J. 2016, 304, 573–578. [Google Scholar] [CrossRef]
- Peng, P.; Hong, S.-Z.; Lu, W.-Z. The degradation of zinc dialkyldithiophosphate additives in fully formulated engine oil as studied by P-31 NMR spectroscopy. Lubr. Eng. 1994, 50, 230–235. [Google Scholar]
- Marshall, G.L. Characterization of Lubricants Using 31P Fourier Transform Nuclear Magnetic Resonance Spectroscopy. Appl. Spectrosc. 1984, 38, 522–526. [Google Scholar] [CrossRef]
- Pirouz, S.; Wang, Y.; Chong, J.M.; Duhamel, J. Chemical Modification of Polyisobutylene Succinimide Dispersants and Characterization of Their Associative Properties. J. Phys. Chem. B 2015, 119, 12202–12211. [Google Scholar] [CrossRef] [Green Version]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaarour, M.; El Siblani, H.; Arnault, N.; Boullay, P.; Mintova, S. Zeolite Nanocrystals Protect the Performance of Organic Additives and Adsorb Acid Compounds during Lubricants Oxidation. Materials 2019, 12, 2830. https://doi.org/10.3390/ma12172830
Zaarour M, El Siblani H, Arnault N, Boullay P, Mintova S. Zeolite Nanocrystals Protect the Performance of Organic Additives and Adsorb Acid Compounds during Lubricants Oxidation. Materials. 2019; 12(17):2830. https://doi.org/10.3390/ma12172830
Chicago/Turabian StyleZaarour, Moussa, Hussein El Siblani, Nicolas Arnault, Philippe Boullay, and Svetlana Mintova. 2019. "Zeolite Nanocrystals Protect the Performance of Organic Additives and Adsorb Acid Compounds during Lubricants Oxidation" Materials 12, no. 17: 2830. https://doi.org/10.3390/ma12172830
APA StyleZaarour, M., El Siblani, H., Arnault, N., Boullay, P., & Mintova, S. (2019). Zeolite Nanocrystals Protect the Performance of Organic Additives and Adsorb Acid Compounds during Lubricants Oxidation. Materials, 12(17), 2830. https://doi.org/10.3390/ma12172830







