Alumina-Doped Zirconia Submicro-Particles: Synthesis, Thermal Stability, and Microstructural Characterization
Abstract
:1. Introduction
2. Results and Discussion
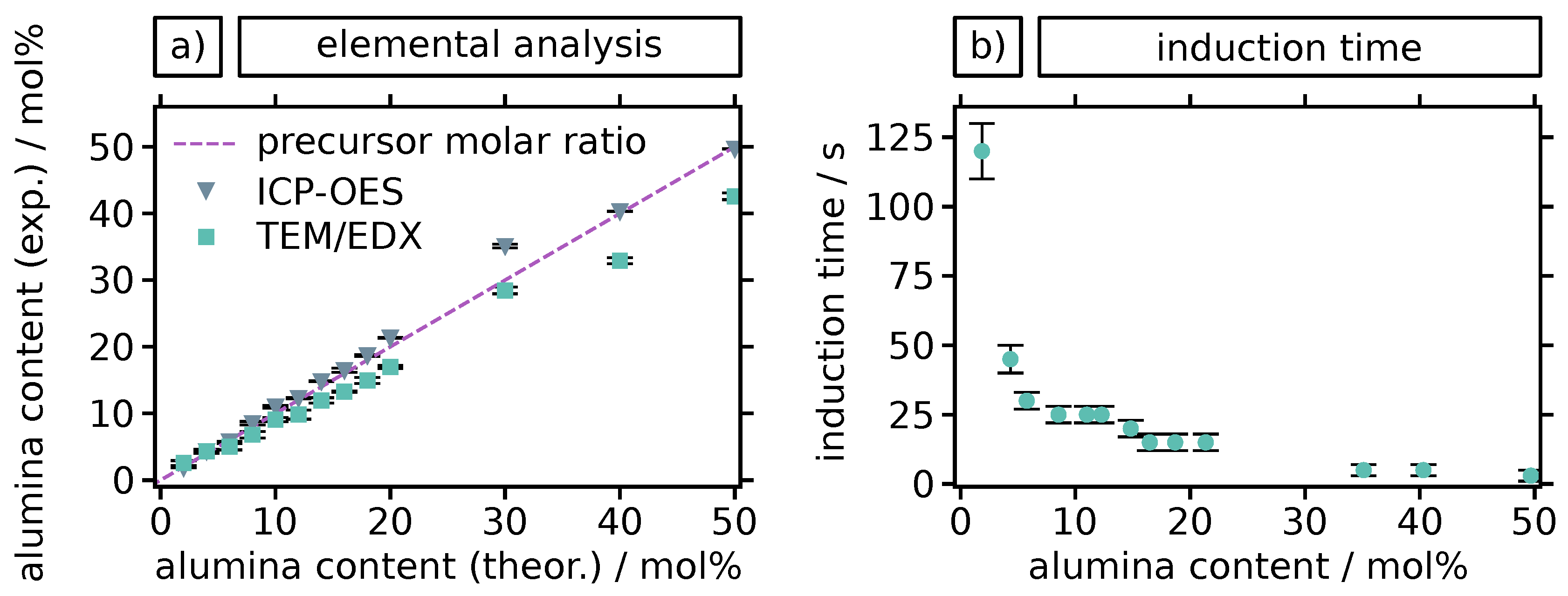
2.1. Thermal Stability of Alumina-Doped Zirconia Particles
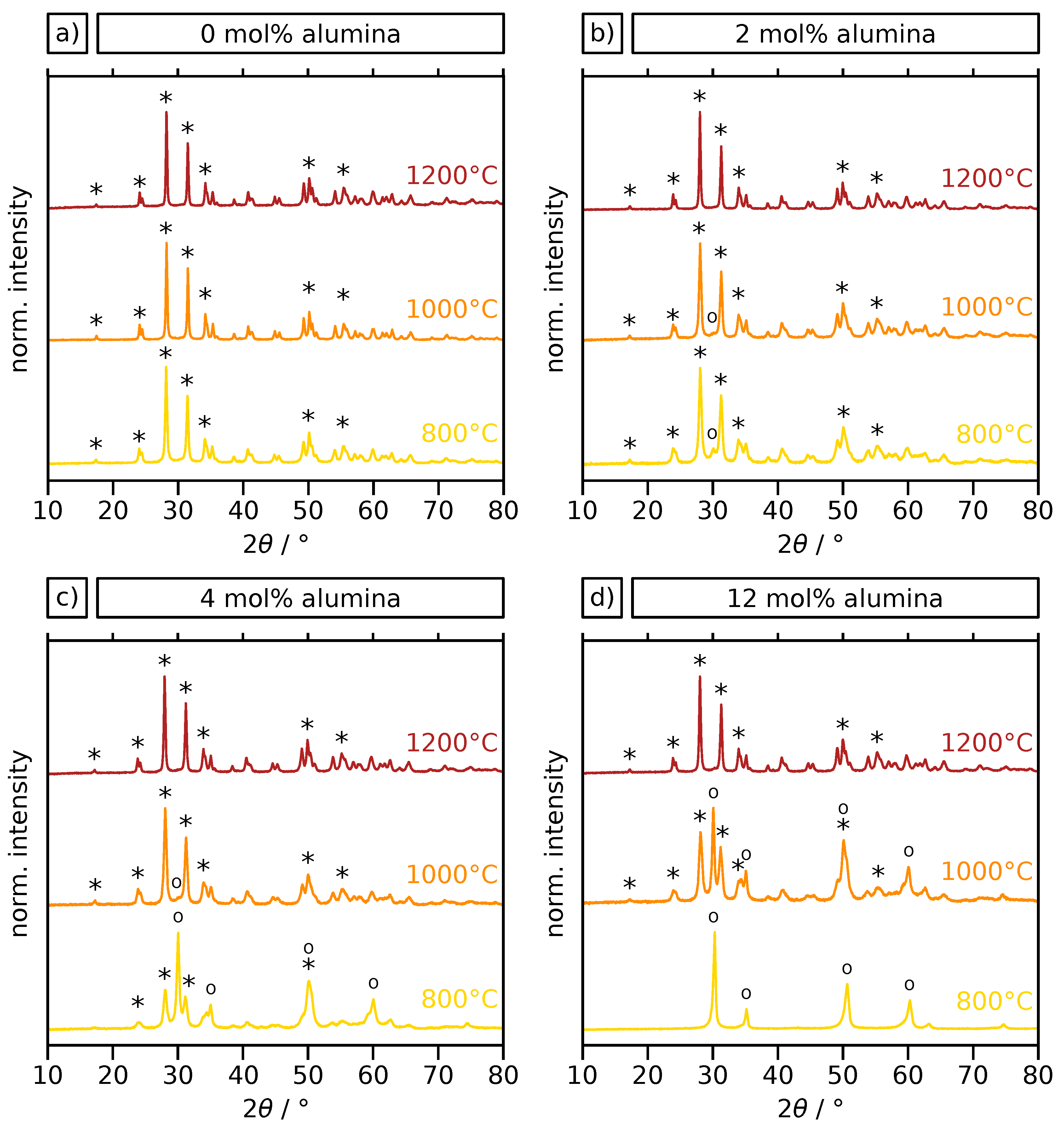
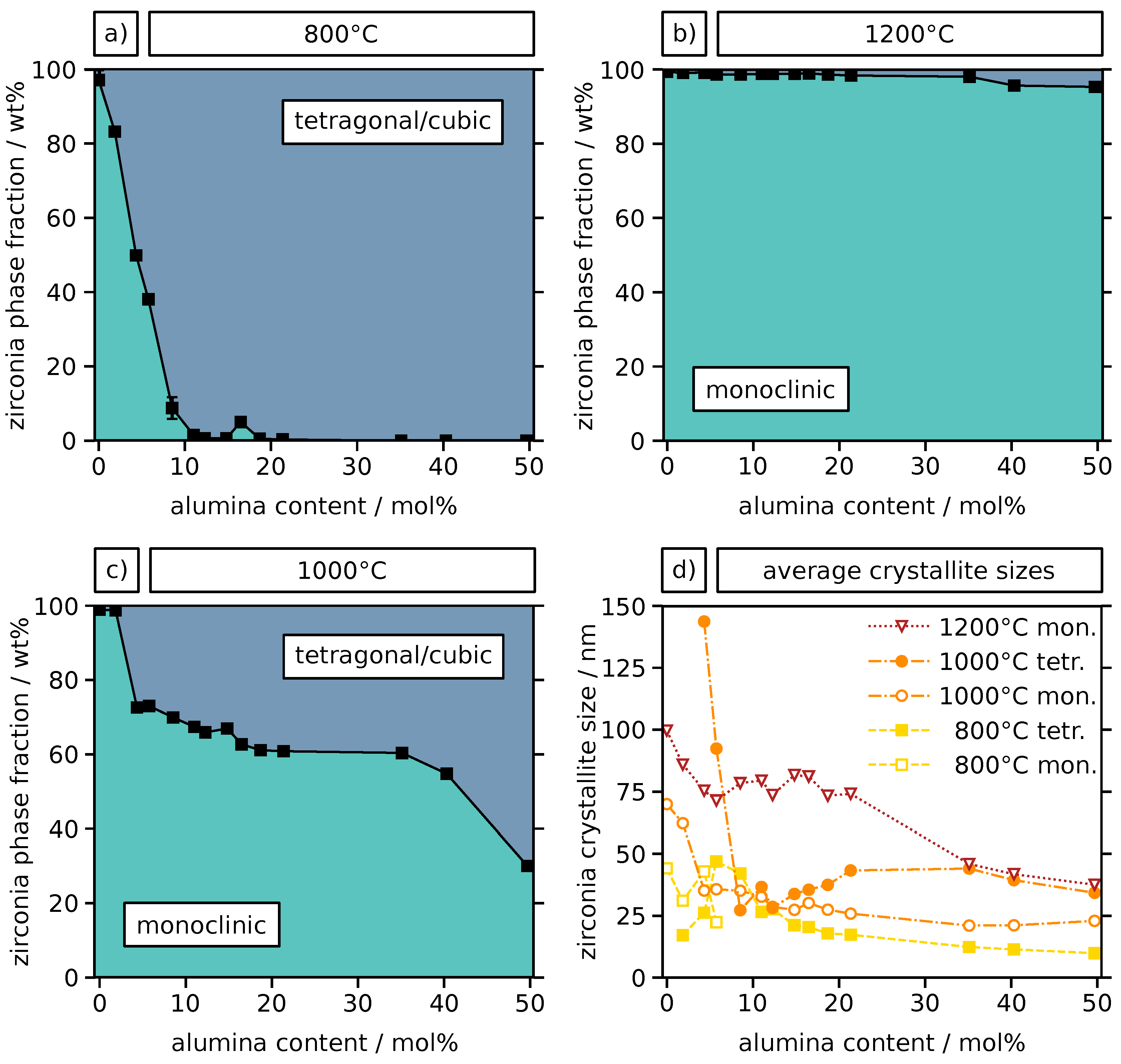
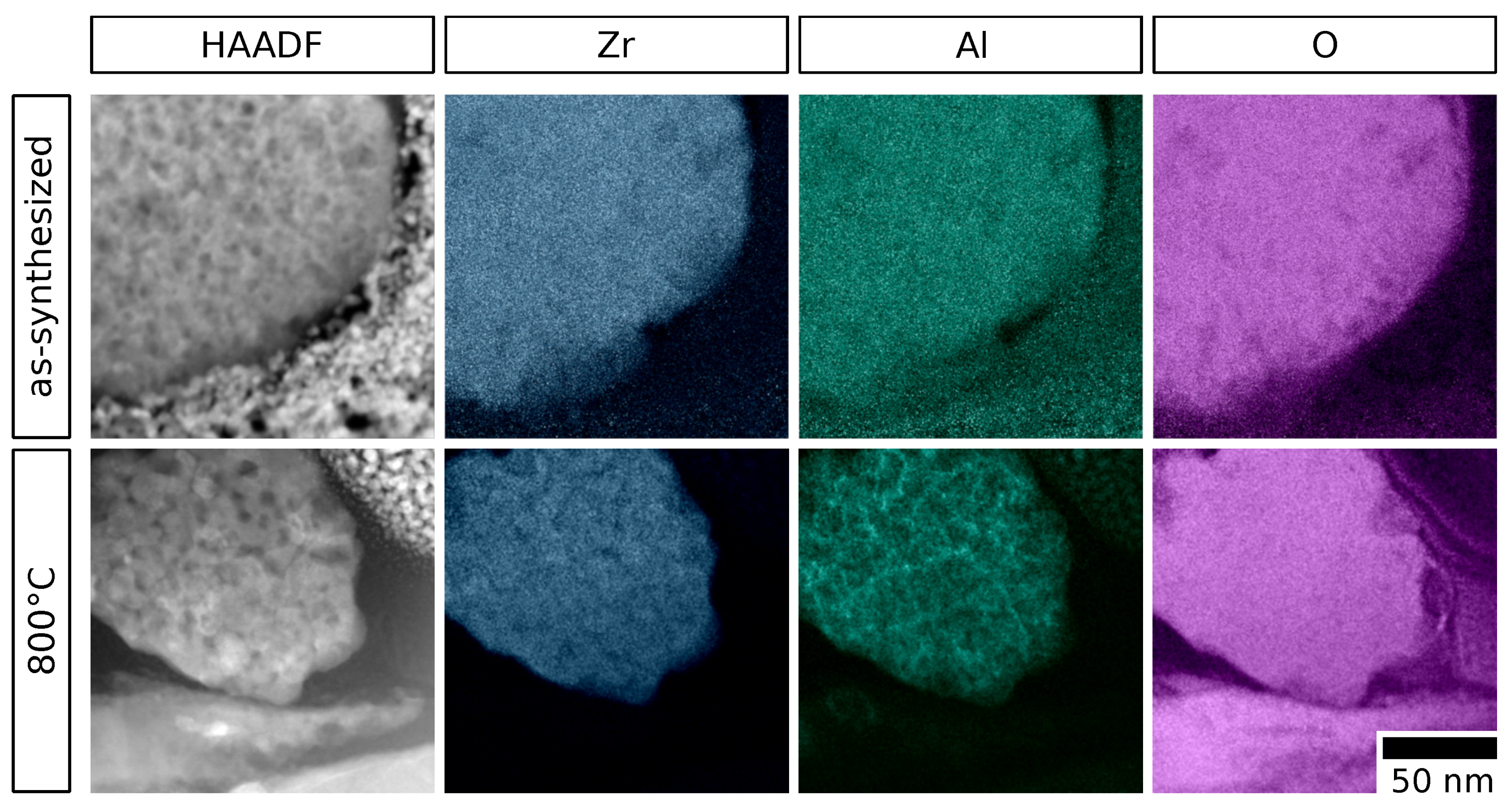
2.2. Crystal Phase and Microstructure Analysis
3. Materials and Methods
3.1. Materials
3.2. Particle Syntheses
3.3. Annealing Experiments
3.4. Characterization
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, A.K. Sol-Gel Based Nanoceramic Materials: Preparation, Properties and Applications; Springer: Cham, Switzerland, 2016; pp. 1–297. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation Toughening in Zirconia-Containing Ceramics. J. Am. Ceram. Soc. 2000, 83, 461–487. [Google Scholar] [CrossRef]
- Haddadi, S.A.; Mahdavian, M.; Karimi, E. Evaluation of the corrosion protection properties of an epoxy coating containing sol-gel surface modified nano-zirconia on mild steel. RSC Adv. 2015, 5, 28769–28777. [Google Scholar] [CrossRef]
- He, J.; Chen, J.; Ren, L.; Wang, Y.; Teng, C.; Hong, M.; Zhao, J.; Jiang, B. Fabrication of monodisperse porous zirconia microspheres and their phosphorylation for friedel-crafts alkylation of indoles. ACS Appl. Mater. Interfaces 2014, 6, 2718–2725. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, A.; Subramanian, A. Impact of porogens on the pore characteristics of zirconia particles made by polymer-induced colloid aggregation. Int. J. Appl. Ceram. Technol. 2011, 8, 94–111. [Google Scholar] [CrossRef]
- Persson, A.; Lekholm, V.; Thornell, G.; Klintberg, L. A high-temperature calorimetric flow sensor employing ion conduction in zirconia. Appl. Phys. Lett. 2015, 106, 194103. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Sun, R.; Yang, X.; Liang, X.; Wang, C.; Sun, P.; Liu, F.; Zhao, C.; Sun, Y.; Lu, G. Mixed-potential type NOx sensor using stabilized zirconia and MoO3–In2O3 nanocomposites. Ceram. Int. 2016, 42, 12503–12507. [Google Scholar] [CrossRef]
- Mæland, D.; Suciu, C.; Wærnhus, I.; Hoffmann, A.C. Sintering of 4YSZ (ZrO2 + 4 mol% Y2O3) nanoceramics for solid oxide fuel cells (SOFCs), their structure and ionic conductivity. J. Eur. Ceram. Soc. 2009, 29, 2537–2547. [Google Scholar] [CrossRef]
- Mahato, N.; Gupta, A.; Balani, K. Doped zirconia and ceria-based electrolytes for solid oxide fuel cells: A review. Nanomater. Energy 2012, 1, 27–45. [Google Scholar] [CrossRef]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- McEntire, B.J.; Bal, B.S.; Rahaman, M.N.; Chevalier, J.; Pezzotti, G. Ceramics and ceramic coatings in orthopaedics. J. Eur. Ceram. Soc. 2015, 35, 4327–4369. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Imran, A.B.; Seki, T.; Ishii, M.; Nakamura, H.; Takeoka, Y. Angle-independent structural color in colloidal amorphous arrays. ChemPhysChem 2010, 11, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, P.N.; Do Rosário, J.J.; Leib, E.W.; Petrov, A.Y.; Kubrin, R.; Schneider, G.A.; Weller, H.; Vossmeyer, T.; Eich, M. Ceramic Photonic Glass for Broadband Omnidirectional Reflection. ACS Photonics 2014, 1, 1127–1133. [Google Scholar] [CrossRef]
- Dyachenko, P.N.; do Rosário, J.J.; Leib, E.W.; Petrov, A.Y.; Störmer, M.; Weller, H.; Vossmeyer, T.; Schneider, G.A.; Eich, M. Tungsten band edge absorber/emitter based on a monolayer of ceramic microspheres. Opt. Express 2015, 23, A1236–A1244. [Google Scholar] [CrossRef] [PubMed]
- Fegley, B.; Barringer, E.A. Synthesis, Characterization, and Processing of Monosized Ceramic Powders. MRS Proc. 1984, 32, 187–197. [Google Scholar] [CrossRef]
- Ogihara, T.; Mizutani, N.; Kato, M. Processing of monodispersed ZrO2 powders. Ceram. Int. 1987, 13, 35–40. [Google Scholar] [CrossRef]
- Ogihara, T.; Mizutani, N.; Kato, M. Growth Mechanism of Monodispersed ZrO2 Particles. J. Am. Ceram. Soc. 1989, 72, 421–426. [Google Scholar] [CrossRef]
- Van Cantfort, O.; Michaux, B.; Pirard, R.; Pirard, J.P.; Lecloux, A.J. Synthesis and Characterization of Monodisperse Spherical Zirconia Particles. J. Sol-Gel Sci. Technol. 1997, 8, 207–211. [Google Scholar] [CrossRef]
- Widoniak, J.; Eiden-Assmann, S.; Maret, G. Synthesis and characterisation of monodisperse zirconia particles. Eur. J. Inorg. Chem. 2005, 2005, 3149–3155. [Google Scholar] [CrossRef]
- Lerot, L.; Legrand, F.; De Bruycker, P. Chemical control in precipitation of spherical zirconia particles. J. Mater. Sci. 1991, 26, 2353–2358. [Google Scholar] [CrossRef]
- Yan, B.; McNeff, C.V.; Carr, P.W.; McCormick, A.V. Synthesis and characterization of submicron-to-micron scale, monodisperse, spherical, and nonporous zirconia particles. J. Am. Ceram. Soc. 2005, 88, 707–713. [Google Scholar] [CrossRef]
- Leib, E.W.; Vainio, U.; Pasquarelli, R.M.; Kus, J.; Czaschke, C.; Walter, N.; Janssen, R.; Müller, M.; Schreyer, A.; Weller, H.; et al. Synthesis and thermal stability of zirconia and yttria-stabilized zirconia microspheres. J. Colloid Interface Sci. 2015, 448, 582–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, K.; Ogihara, T.; Ikemoto, T.; Mizutani, N.; Kato, M. Preparation of monodispersed Y-doped ZrO2 powders. J. Mater. Sci. 1987, 22, 4343–4347. [Google Scholar] [CrossRef]
- Mahendran, R.; Babu, S.P.; Natarajan, S.; Vallimanalan, A.; Manivannan, S. Thermal kinetics and phase stability of 8 mol% samaria doped zirconia nanopowders prepared via reverse coprecipitation. Ceram. Int. 2017, 43, 8051–8056. [Google Scholar] [CrossRef]
- Lücke, K.; Rixen, R.; Rosenbaum, F.W. On the Theory of Grain Boundary Motion. In The Nature and Behavior of Grain Boundaries; Hu, H., Ed.; Springer: New York, NY, USA, 1972; pp. 245–283. [Google Scholar] [CrossRef]
- Chen, I.W. Mobility control of ceramic grain boundaries and interfaces. Mater. Sci. Eng. A 1993, 166, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Rösler, J.; Bäker, M.; Harders, H. Mechanical behaviour of ceramics. In Mechanical Behaviour of Engineering Materials: Metals, Ceramics, Polymers, and Composites; Springer: Berlin/Heidelberg, Germany, 2007; pp. 227–255. [Google Scholar] [CrossRef]
- Rajendran, S.; Drennan, J.; Badwal, S.P.S. Effect of alumina additions on the grain boundary and volume resistivity of tetragonal zirconia polycrystals. J. Mater. Sci. Lett. 1987, 6, 1431–1434. [Google Scholar] [CrossRef]
- Nazeri, A.; Qadri, S.B. Alumina-stabilized zirconia coatings for high-temperature protection of turbine blades. Surf. Coatings Technol. 1996, 86–87, 166–169. [Google Scholar] [CrossRef]
- Kandaswamy, R. Optimizing the Mechanical Properties of Partially Yttria Stabilized Zirconia with Alumina Additions. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2010. [Google Scholar]
- Keyvani, A.; Saremi, M.; Sohi, M.H. Oxidation resistance of YSZ-alumina composites compared to normal YSZ TBC coatings at 1100 C. J. Alloys Compd. 2011, 509, 8370–8377. [Google Scholar] [CrossRef]
- Zhu, C.; Javed, A.; Li, P.; Yang, F.; Liang, G.Y.; Xiao, P. A study of the microstructure and oxidation behavior of alumina/yttria-stabilized zirconia (Al2O3/YSZ) thermal barrier coatings. Surf. Coatings Technol. 2012, 212, 214–222. [Google Scholar] [CrossRef]
- Tsukuma, K.; Ueda, K.; Shimada, M. Strength and Fracture Toughness of Isostatically Hot-Pressed Composites of Al2O3 and Y2O3-Partially-Stabilized ZrO2. J. Am. Ceram. Soc. 1985, 68, C-4–C-5. [Google Scholar] [CrossRef]
- Rajendran, S. Production of reactive single- and multi-component ceramic oxide powders and fabrication of high-strength ceramics. J. Mater. Sci. 1992, 27, 433–440. [Google Scholar] [CrossRef]
- Ishida, K.; Hirota, K.; Yamaguchi, O.; Kume, H.; Inamura, S.; Miyamoto, H. Formation of Zirconia Solid Solutions Containing Alumina Prepared by New Preparation Method. J. Am. Ceram. Soc. 1994, 77, 1391–1395. [Google Scholar] [CrossRef]
- Srdić, V.V.; Winterer, M.; Hahn, H. Sintering Behavior of Nanocrystalline Zirconia Doped with Alumina Prepared by Chemical Vapor Synthesis. J. Am. Ceram. Soc. 2000, 83, 1853–1860. [Google Scholar] [CrossRef]
- Stough, M.A.; Hellmann, J.R. Solid Solubility and Precipitation in a Single-Crystal Alumina-Zirconia System. J. Am. Ceram. Soc. 2002, 85, 2895–2902. [Google Scholar] [CrossRef]
- Shang, G.; Maiwald, L.; Renner, H.; Jalas, D.; Dosta, M.; Heinrich, S.; Petrov, A.; Eich, M. Photonic glass for high contrast structural color. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Shukla, S.; Seal, S.; Vij, R.; Bandyopadhyay, S. Reduced activation energy for grain growth in nanocrystalline yttria-stabilized zirconia. Nano Lett. 2003, 3, 397–401. [Google Scholar] [CrossRef]
- Leib, E.W.; Pasquarelli, R.M.; Do Rosário, J.J.; Dyachenko, P.N.; Döring, S.; Puchert, A.; Petrov, A.Y.; Eich, M.; Schneider, G.A.; Janssen, R.; et al. Yttria-stabilized zirconia microspheres: Novel building blocks for high-temperature photonics. J. Mater. Chem. C 2016, 4, 62–74. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable Alumina Polymorphs: Crystal Structures and Transition Sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Chraska, T.; Neufuss, K.; Dubský, J.; Ctibor, P.; Rohan, P. Fabrication of bulk nanocrystalline alumina-zirconia materials. Ceram. Int. 2008, 34, 1229–1236. [Google Scholar] [CrossRef]
- Bennison, S.J. Grain Growth. In Engineered Materials Handbook, Vol. 4: Ceramics and Glasses; ASM International: Metals Park, OH, USA, 1991; pp. 304–312. [Google Scholar]
- Matsui, K.; Ohmichi, N.; Ohgai, M.; Yoshida, H.; Ikuhara, Y. Effect of alumina-doping on grain boundary segregation-induced phase transformation in yttria-stabilized tetragonal zirconia polycrystal. J. Mater. Res. 2006, 21, 2278–2289. [Google Scholar] [CrossRef]
- Navrotsky, A. Thermochemical insights into refractory ceramic materials based on oxides with large tetravalent cations. J. Mater. Chem. 2005, 15, 1883–1890. [Google Scholar] [CrossRef]
- Leib, E.W.; Pasquarelli, R.M.; Blankenburg, M.; Müller, M.; Schreyer, A.; Janssen, R.; Weller, H.; Vossmeyer, T. High-Temperature Stable Zirconia Particles Doped with Yttrium, Lanthanum, and Gadolinium. Part. Part. Syst. Charact. 2016, 33, 645–655. [Google Scholar] [CrossRef]
- Allemann, J.A.; Michel, B.; Märki, H.B.; Gauckler, L.J.; Moser, E.M. Grain growth of differently doped zirconia. J. Eur. Ceram. Soc. 1995, 15, 951–958. [Google Scholar] [CrossRef]
- Prokse, O. Analyse der Metalle, 2nd ed.; Springer: Berlin/Göttingen/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- Lutterotti, L.; Scardi, P. Simultaneous structure and size-strain refinement by the rietveld method. J. Appl. Crystallogr. 1990, 23, 246–252. [Google Scholar] [CrossRef]
- Howard, C.J.; Hill, R.J.; Reichert, B.E. Structures of ZrO2 polymorphs at room temperature by high-resolution neutron powder diffraction. Acta Crystallogr. Sect. B 1988, 44, 116–120. [Google Scholar] [CrossRef]
- Schwenger, J.; Romeis, S.; Herre, P.; Yokosawa, T.; Finsel, M.; Leib, E.W.; Weller, H.; Vossmeyer, T.; Spiecker, E.; Peukert, W. Pressure induced local phase transformation in nanocrystalline tetragonal zirconia microparticles. Scr. Mater. 2019, 163, 86–90. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahl, G.T.; Döring, S.; Krekeler, T.; Janssen, R.; Ritter, M.; Weller, H.; Vossmeyer, T. Alumina-Doped Zirconia Submicro-Particles: Synthesis, Thermal Stability, and Microstructural Characterization. Materials 2019, 12, 2856. https://doi.org/10.3390/ma12182856
Dahl GT, Döring S, Krekeler T, Janssen R, Ritter M, Weller H, Vossmeyer T. Alumina-Doped Zirconia Submicro-Particles: Synthesis, Thermal Stability, and Microstructural Characterization. Materials. 2019; 12(18):2856. https://doi.org/10.3390/ma12182856
Chicago/Turabian StyleDahl, Gregor Thomas, Sebastian Döring, Tobias Krekeler, Rolf Janssen, Martin Ritter, Horst Weller, and Tobias Vossmeyer. 2019. "Alumina-Doped Zirconia Submicro-Particles: Synthesis, Thermal Stability, and Microstructural Characterization" Materials 12, no. 18: 2856. https://doi.org/10.3390/ma12182856





