NiCo2S4 Nanotrees Directly Grown on the Nickel NP-Doped Reduced Graphene Oxides for Efficient Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GO
2.3. Preparation of Ni-Doped rGOs (Ni-rGO)
2.4. Growth of NiCo2S4 Nanotrees the Ni Doped-rGO (NiCo2S4/Ni-rGO)
2.5. Characterization
2.6. Electrochemical Measurements
3. Results and Discussion
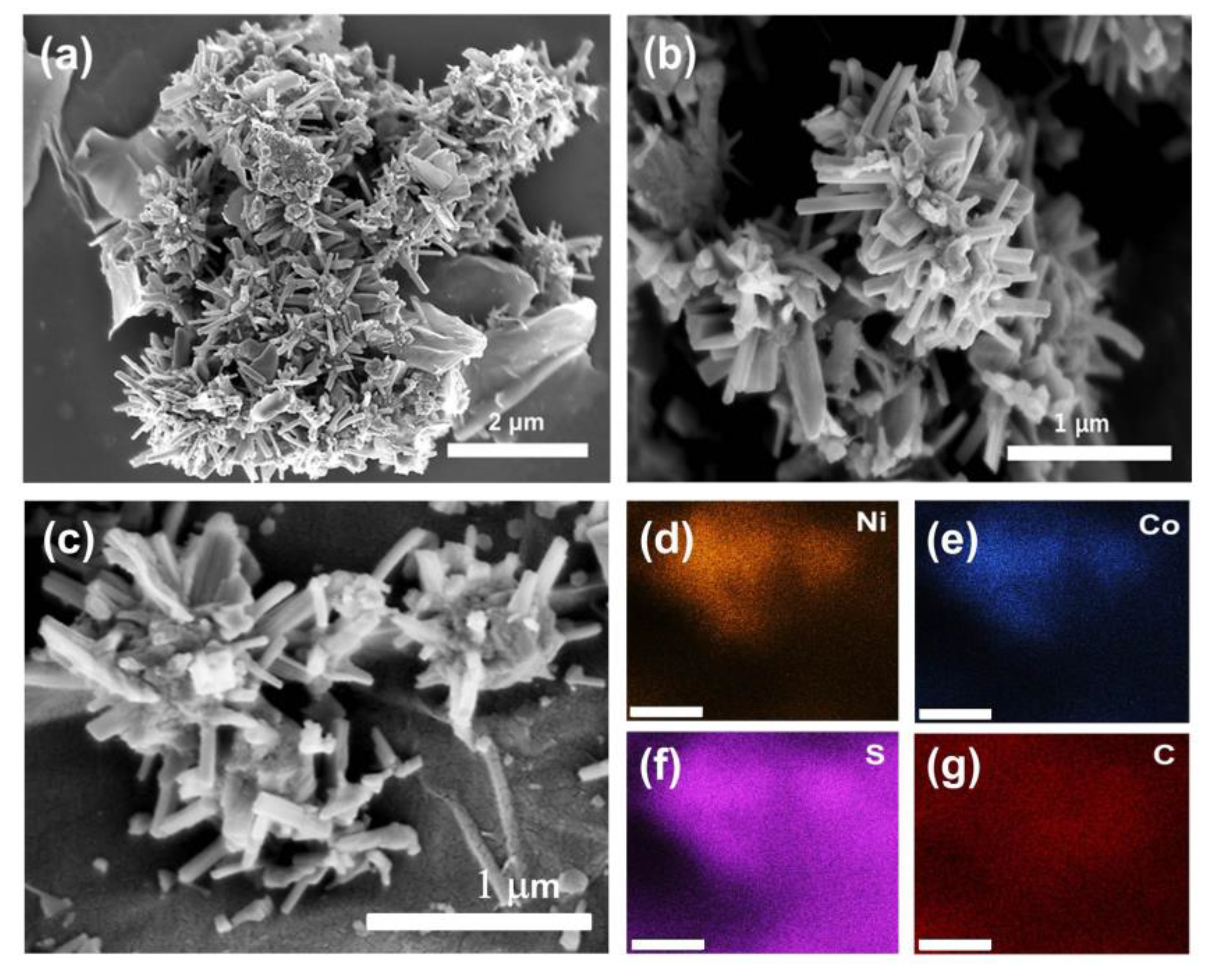
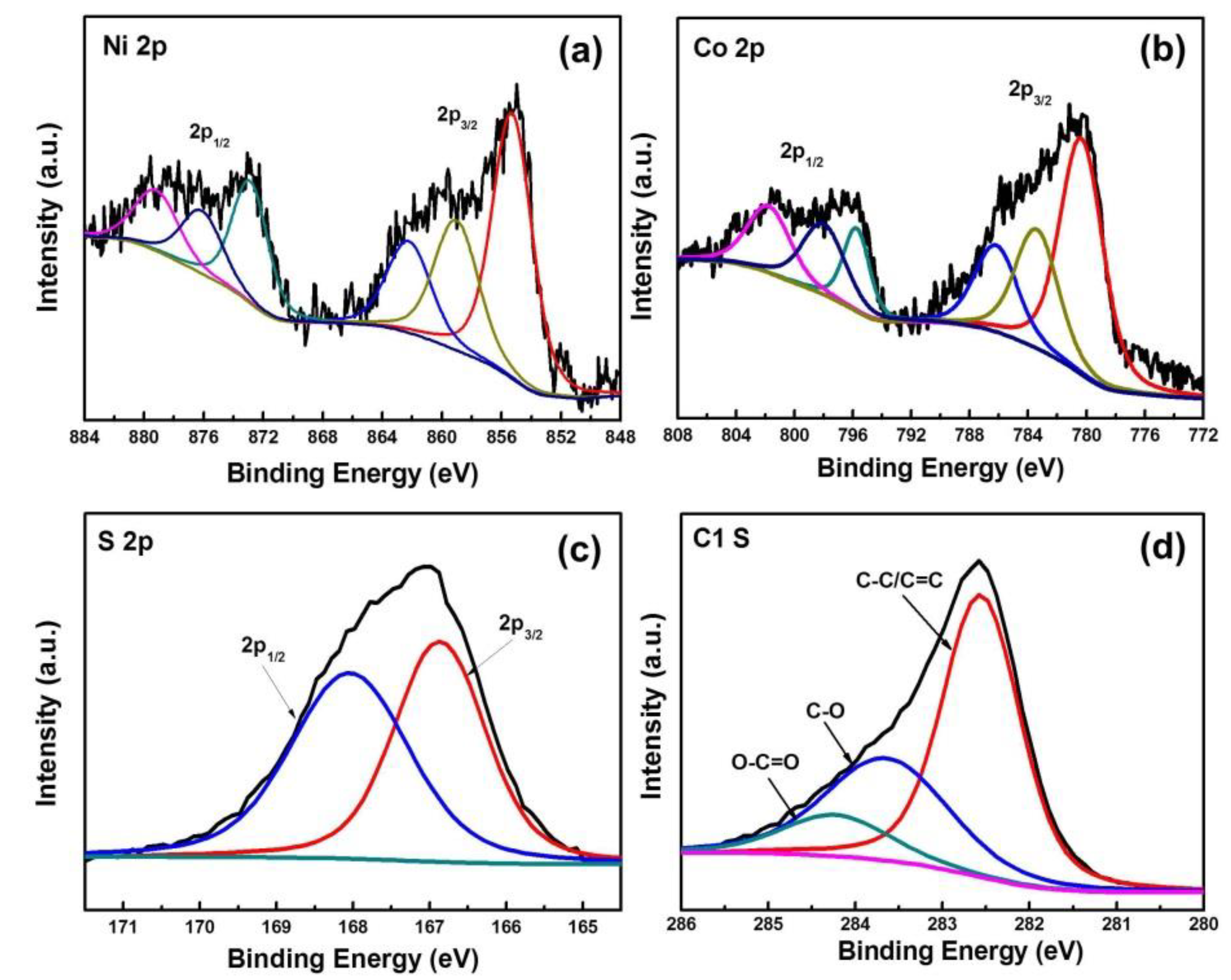
3.1. Materials Characterization
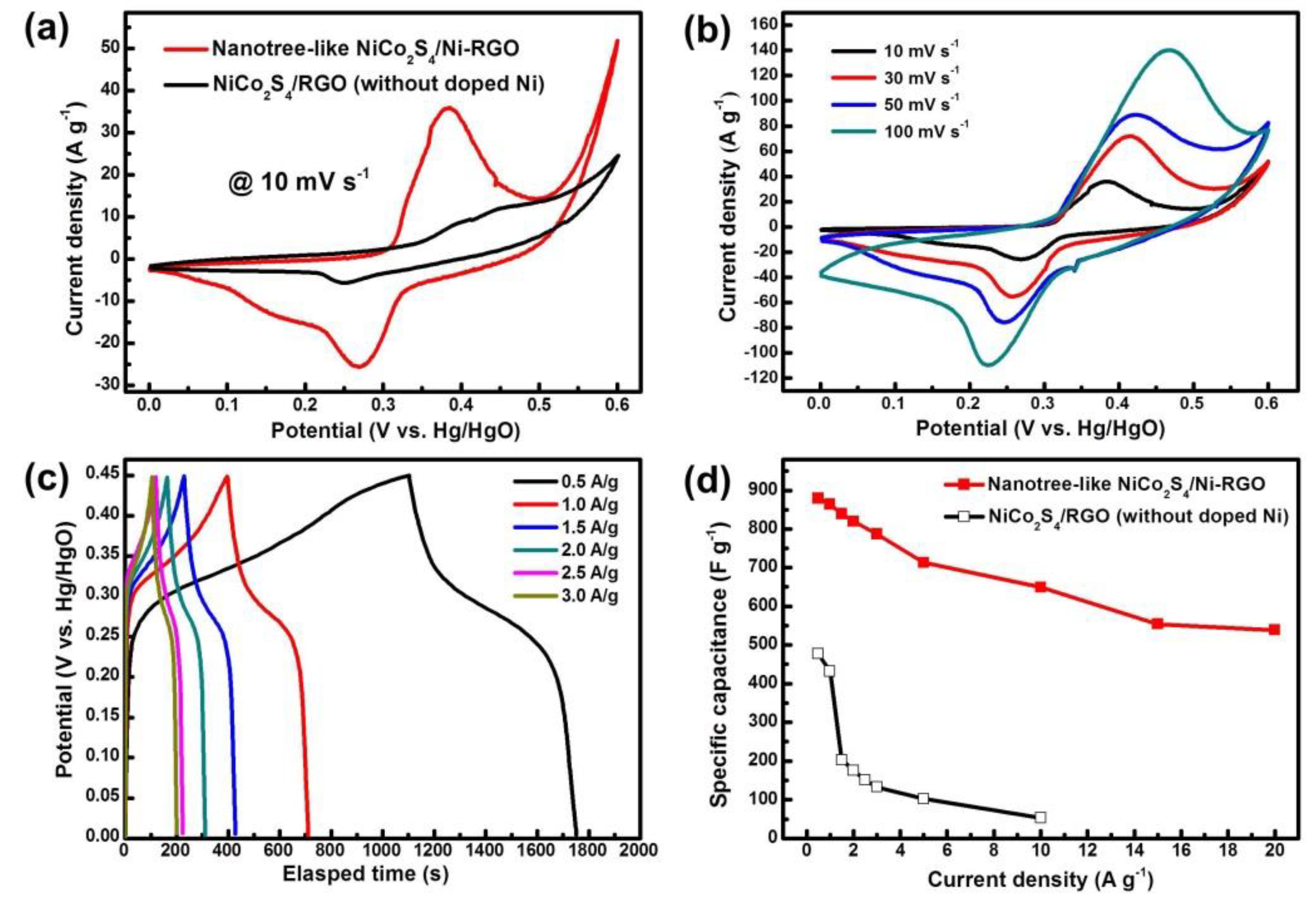
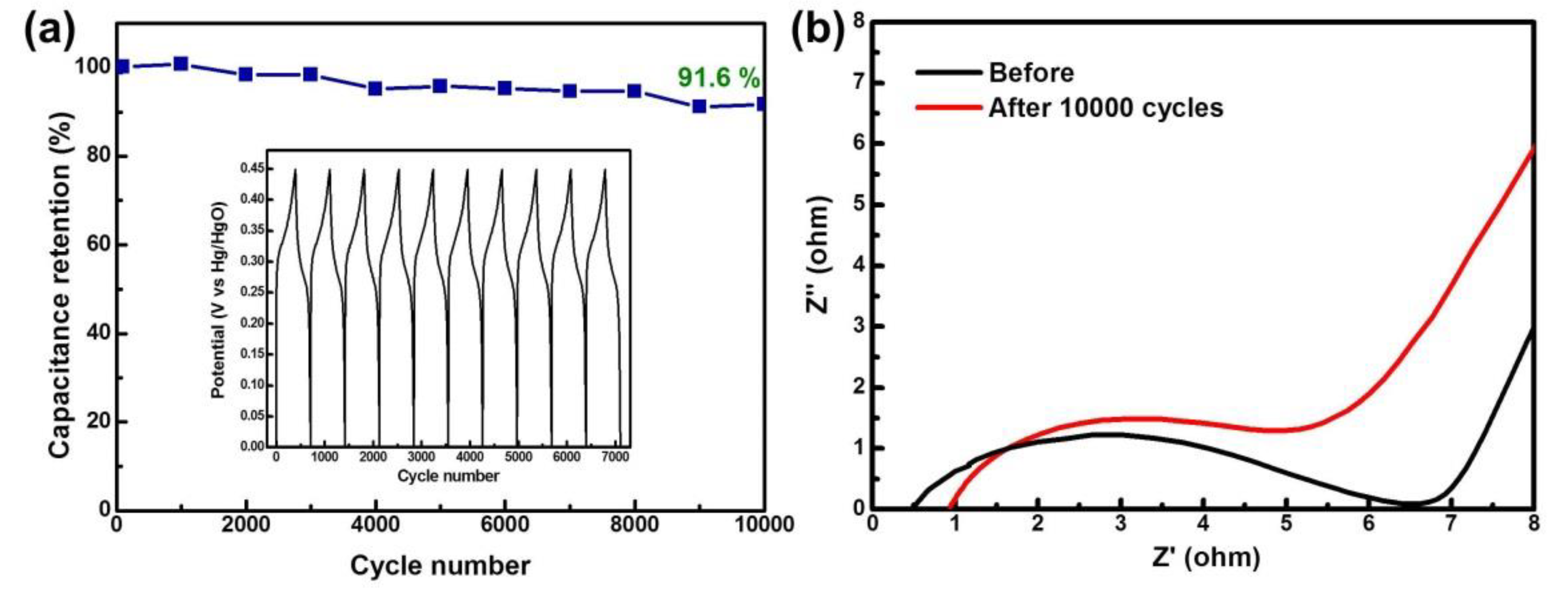
3.2. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, J.; Jiang, J.; Sun, Z.; Luo, J.; Fan, Z.; Huang, X.; Zhang, H.; Yu, T. 3D carbon/cobalt-nickel mixed-oxide hybrid nanostructured arrays for asymmetric supercapacitors. Small 2014, 10, 2937–2945. [Google Scholar] [CrossRef]
- Beka, L.; Li, X.; Liu, W. Nickel Cobalt Sulfide core/shell structure on 3D Graphene for supercapacitor application. Sci. Rep. 2017, 7, 2105. [Google Scholar] [CrossRef]
- Wu, J.; Dou, S.; Shen, A.; Wang, X.; Ma, Z.; Ouyang, C.; Wang, S. One-step hydrothermal synthesis of NiCo2S4-rGO as an efficient electrocatalyst for the oxygen reduction reaction. J. Mater. Chem. A 2014, 2, 20990–20995. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Tang, S.; Deng, M.; Du, Y. NiCo2S4@rGO hybrid nanostructures on Ni foam as high-performance supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 5912–5919. [Google Scholar] [CrossRef]
- Zhao, F.; Huang, W.; Shi, Q.; Zhou, D.; Zhao, L.; Zhang, H. Low temperature fabrication of hydrangea-like NiCo2S4 as electrode materials for high performance supercapacitors. Mater. Lett. 2017, 186, 206–209. [Google Scholar] [CrossRef]
- Zhu, T.; Zhang, G.; Hu, T.; He, Z.; Lu, Y.; Wang, G.; Guo, H.; Luo, J.; Lin, C.; Chen, Y. Synthesis of NiCo2S4-based nanostructured electrodes supported on nickel foams with superior electrochemical performance. J. Mater. Sci. 2016, 51, 1903–1913. [Google Scholar] [CrossRef]
- Zou, R.; Zhang, Z.; Yuen, M.F.; Sun, M.; Hu, J.; Lee, C.S.; Zhang, W. Three-dimensional-networked NiCo2S4 nanosheet array/carbon cloth anodes for high-performance lithium-ion batteries. NPG Asia Mater. 2015, 7, e195. [Google Scholar] [CrossRef]
- Wang, T.; Le, Q.; Zhang, G.; Zhu, S.; Guan, B.; Zhang, J.; Xing, S.; Zhang, Y. Facile preparation and sulfidation analysis for activated multiporous carbon@NiCo2S4 nanostructure with enhanced supercapacitive properties. Electrochim. Acta 2016, 211, 627–635. [Google Scholar] [CrossRef]
- Fan, Y.M.; Liu, Y.; Liu, X.; Liu, Y.; Fan, L.Z. Hierarchical porous NiCo2S4-rGO composites for high-performance supercapacitors. Electrochim. Acta 2017, 249, 1–8. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Hussain, I.; Shim, J.J. One-step synthesis of hollow C-NiCo2S4 nanostructures for high-performance supercapacitor electrodes. Nanoscale 2018, 10, 6620–6628. [Google Scholar] [CrossRef]
- Xu, J.; Yang, Y.; Chu, H.; Tang, J.; Ge, Y.; Shen, J.; Ye, M. Novel NiCo2S4@reduced graphene oxide@carbon nanotube nanocomposites for high performance supercapacitors. RSC Adv. 2016, 6, 100504–100510. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Shen, L.; Wang, J.; Xu, G.; Li, H.; Dou, H.; Zhang, X. NiCo2S4 nanosheets grown on nitrogen-doped carbon foams as an advanced electrode for supercapacitors. Adv. Energy Mater. 2015, 5, 1400977. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.; Guo, X.; Wang, R.; Zhang, H. Facile Growth of Caterpillar-like NiCo2S4 Nanocrystal Arrays on Nickel Foam for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 18774–18781. [Google Scholar] [CrossRef]
- Furlan, A.; Lu, J.; Hultman, L.; Jansson, U.; Magnuson, M. Crystallization characteristics and chemical bonding properties of nickel carbide thin film nanocomposites. J. Phys. 2014, 26, 415501–415511. [Google Scholar] [CrossRef]
- He, G.; Qiao, M.; Li, W.; Lu, Y.; Zhao, T.; Zou, R.; Li, B.; Darr, J.; Hu, J.; Titirici, M.; et al. N-Co-doped graphene-nickel cobalt sulfide aerogel: Improved energy storage and electrocatalytic performance. Adv. Sci. 2017, 4, 1600214. [Google Scholar] [CrossRef]
- Li, W.; Zhang, B.; Lin, R.; Ho-kimura, S.; He, G.; Zhou, X.; Hu, J.; Parkin, I.A. Dendritic nickel cobalt sulfide nanostructure for alkaline battery electrodes. Adv. Funct. Mater. 2018, 28, 1705937. [Google Scholar] [CrossRef]
- Ouyang, Y.; Ye, H.; Xia, X.; Jiao, X.; Li, G.; Mutahir, S.; Wang, L.; Mandler, D.; Lei, W.; Hao, Q. Hierarchical electrodes of NiCo2S4 nanosheets-anchored sulfur-doped Co3O4 nanoneedles with advanced performance for battery-supercapacitor hybrid devices. J. Mater. Chem. A 2019, 7, 3228–3237. [Google Scholar] [CrossRef]
- Peng, T.; Yi, H.; Sun, P.; Jing, Y.; Wang, R.; Wang, H.; Wang, X. In situ growth of binder-free CNTs@Ni-Co-S nanosheets core/shell hybrids on Ni mesh for high energy density asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 8888–8897. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Attia, S.Y.; Hassan, H.H. Spinel-structured FeCo2O4 mesoporous nanosheets as efficient electrode for supercapacitor applications. Microporous Mesoporous Mater. 2017, 251, 26–33. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Jayaseelan, S.S.; Seo, M.K.; Kim, H.Y.; Kim, B.S. NiCo2S4 nanosheet-decorated 3D, porous Ni film@Ni wire electrode materials for all solid-state asymmetric supercapacitor applicaions. Nanoscale 2017, 9, 18819–18834. [Google Scholar] [CrossRef]
- Mai, L.Q.; Minhas-Khan, A.; Tian, X.; Hercule, K.M.; Zhao, Y.L.; Lin, X.; Xu, X. Synergistic interaction between redox-active electrolyte and binder-free functionalized carbon for ultrahigh supercapacitor performance. Nat. Commun. 2013, 4, 2923–2929. [Google Scholar] [CrossRef]
- Chen, Y.M.; Yu, B.Z.; Miao, Y.Q.; Gao, F.; Jing, G.Y.; Fan, H.M. Pushing the cycling stability limit of hierarchical metal oxide core/shell nanoarrays pseudocapacitor electrodes by nanoscale interface optimization. Nanoscale 2018, 10, 14352–14358. [Google Scholar] [CrossRef]
- Wang, N.; Yao, M.; Zhao, P.; Hu, W.; Komarneni, S. Remarkable electrochemical properties of novel LaNi0.5Co0.5O3/0.333Co3O4 hollow spheres with a mesoporous shell. J. Mater. Chem. A 2017, 5, 5838–5845. [Google Scholar]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, W.; Choi, W.S.; Lee, Y.-S.; Koo, H.Y. NiCo2S4 Nanotrees Directly Grown on the Nickel NP-Doped Reduced Graphene Oxides for Efficient Supercapacitors. Materials 2019, 12, 2865. https://doi.org/10.3390/ma12182865
Jang W, Choi WS, Lee Y-S, Koo HY. NiCo2S4 Nanotrees Directly Grown on the Nickel NP-Doped Reduced Graphene Oxides for Efficient Supercapacitors. Materials. 2019; 12(18):2865. https://doi.org/10.3390/ma12182865
Chicago/Turabian StyleJang, Wooree, Won San Choi, Youn-Sik Lee, and Hye Young Koo. 2019. "NiCo2S4 Nanotrees Directly Grown on the Nickel NP-Doped Reduced Graphene Oxides for Efficient Supercapacitors" Materials 12, no. 18: 2865. https://doi.org/10.3390/ma12182865




