Evaluation of Slag Reaction Efficiency in Slag-Cement Mortars under Different Curing Temperature
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Mix Proportions
2.2. Mortar Preparation and Curing
2.3. Experimental Method
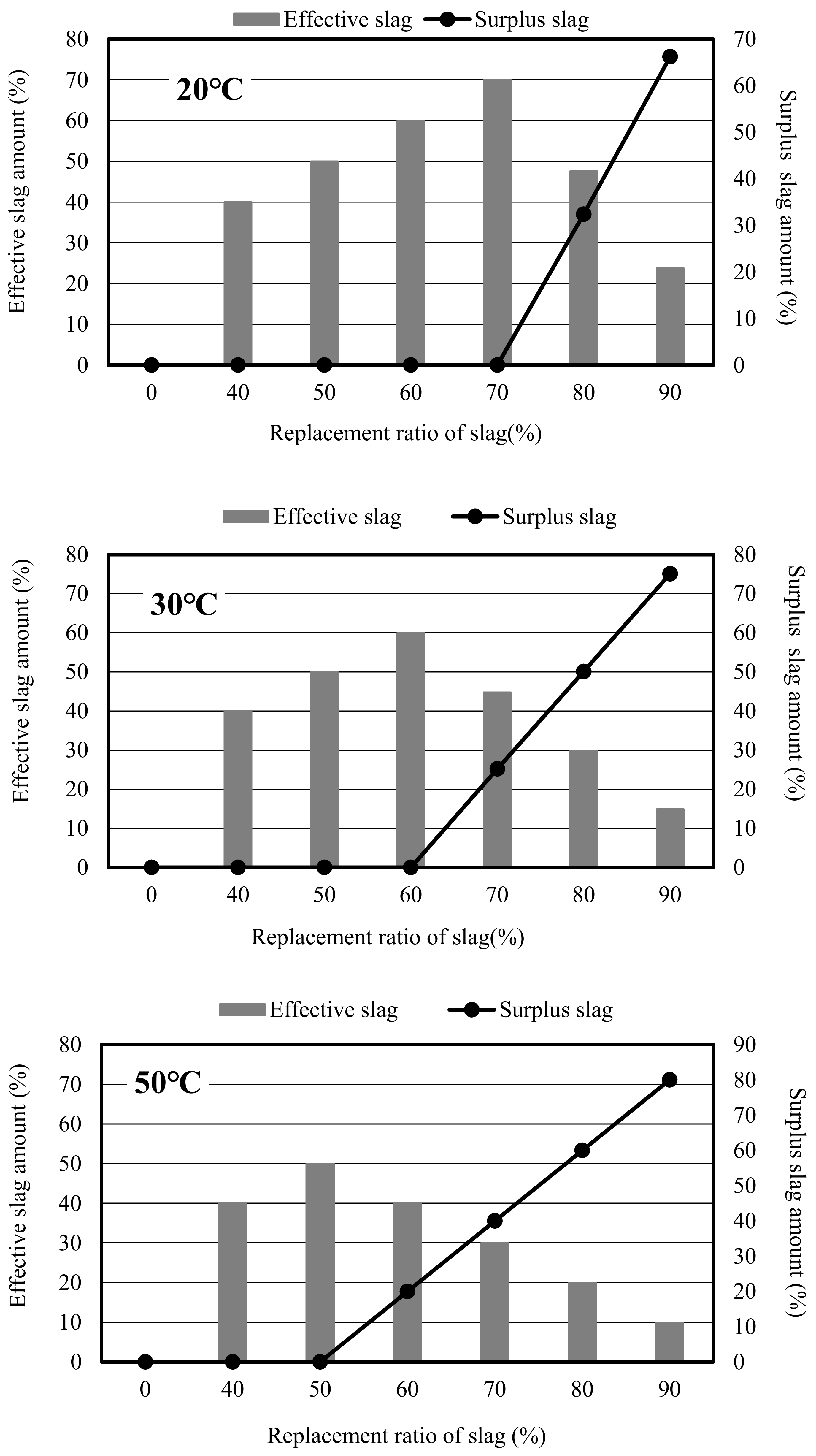
3. Results and Discussion
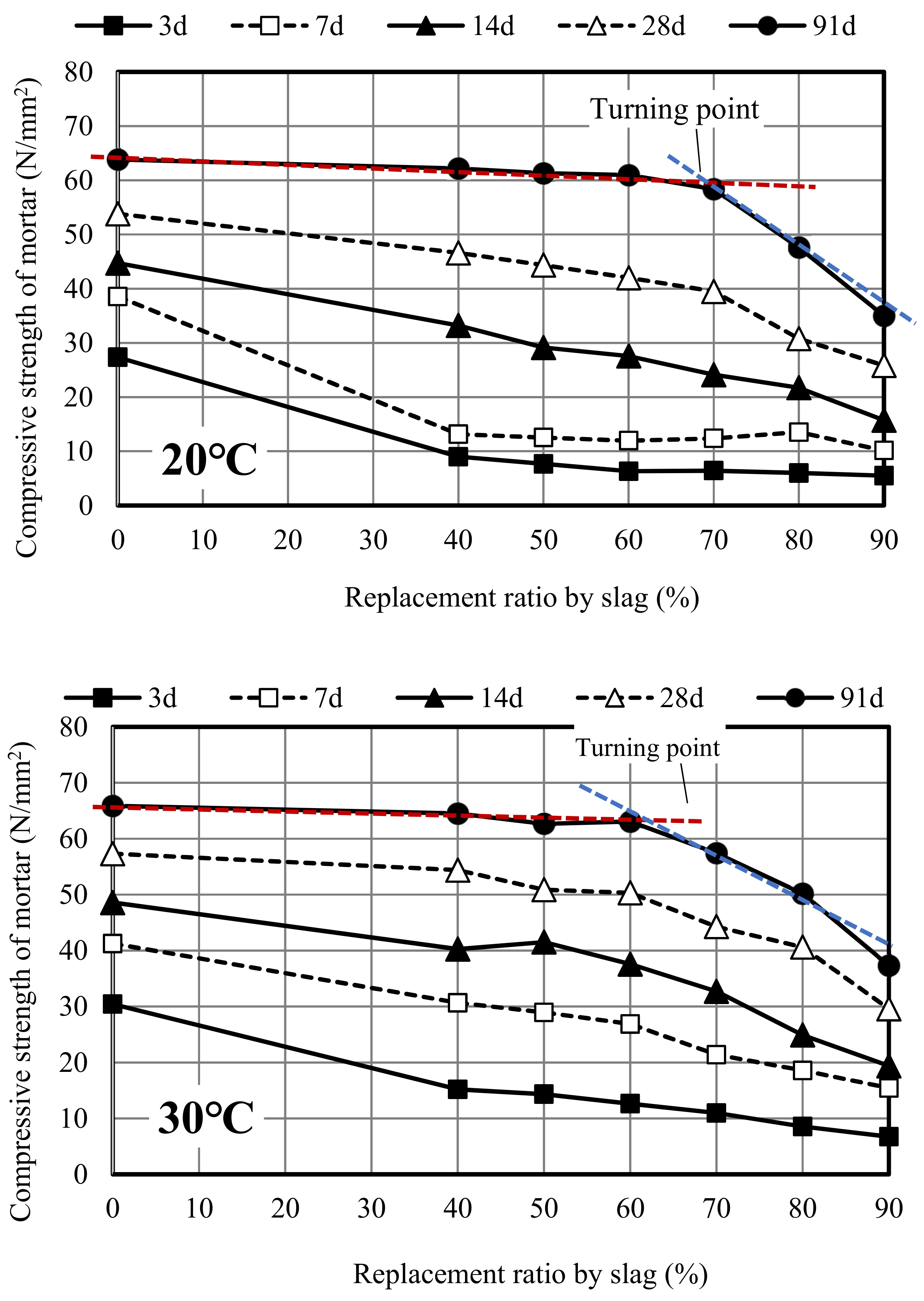
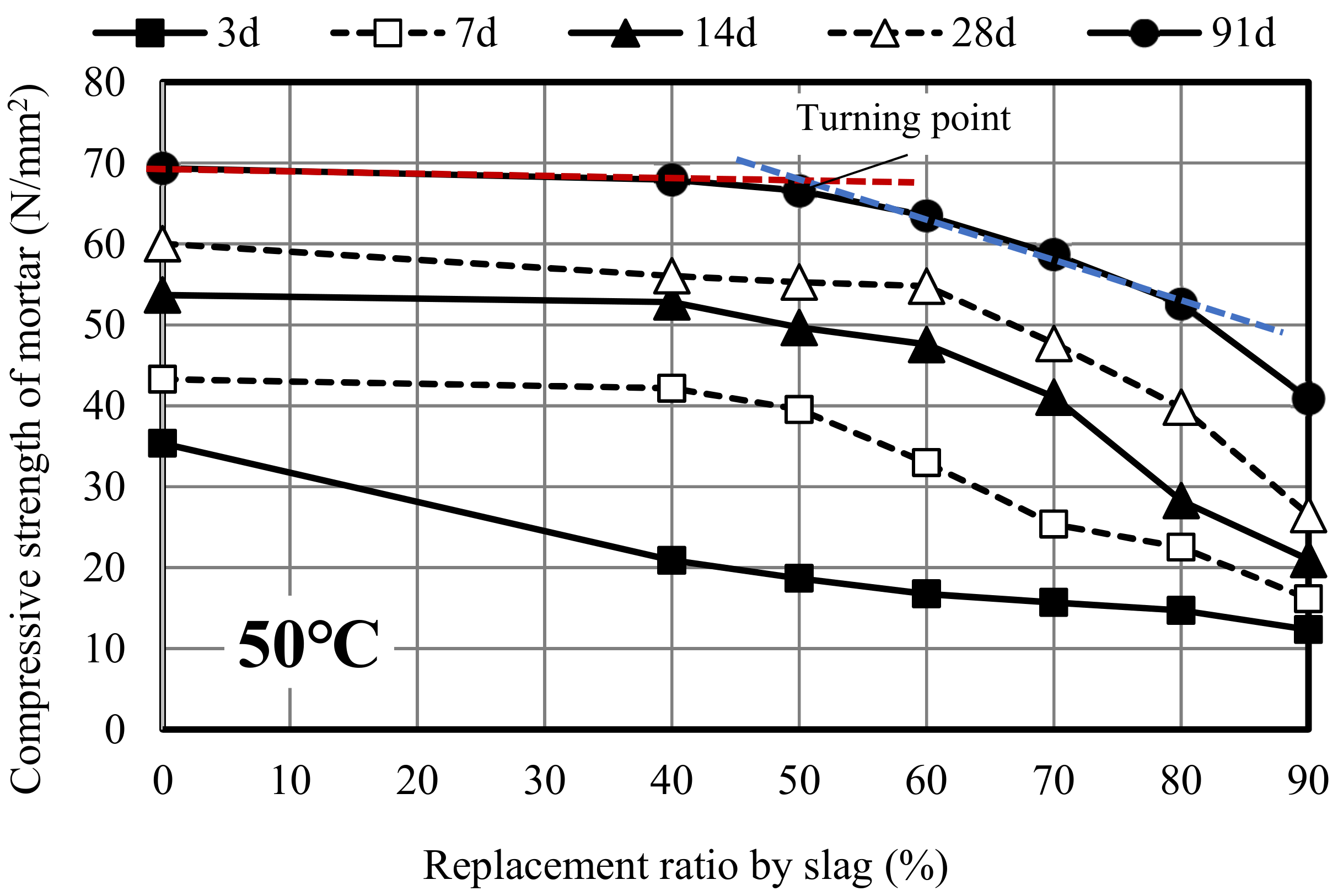
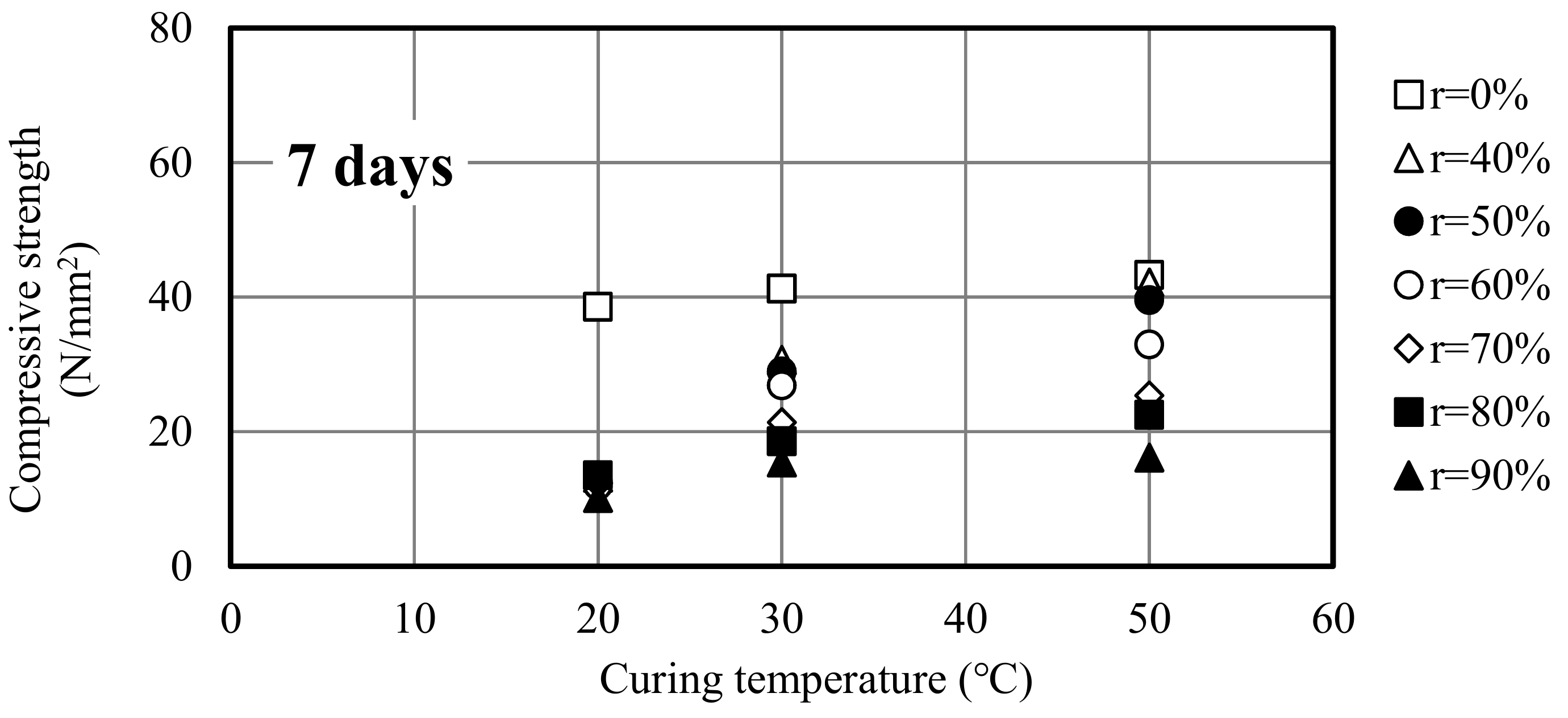
3.1. Compressive Strength Development
3.2. Relationship Between Curing Temperature and Compressive Strength
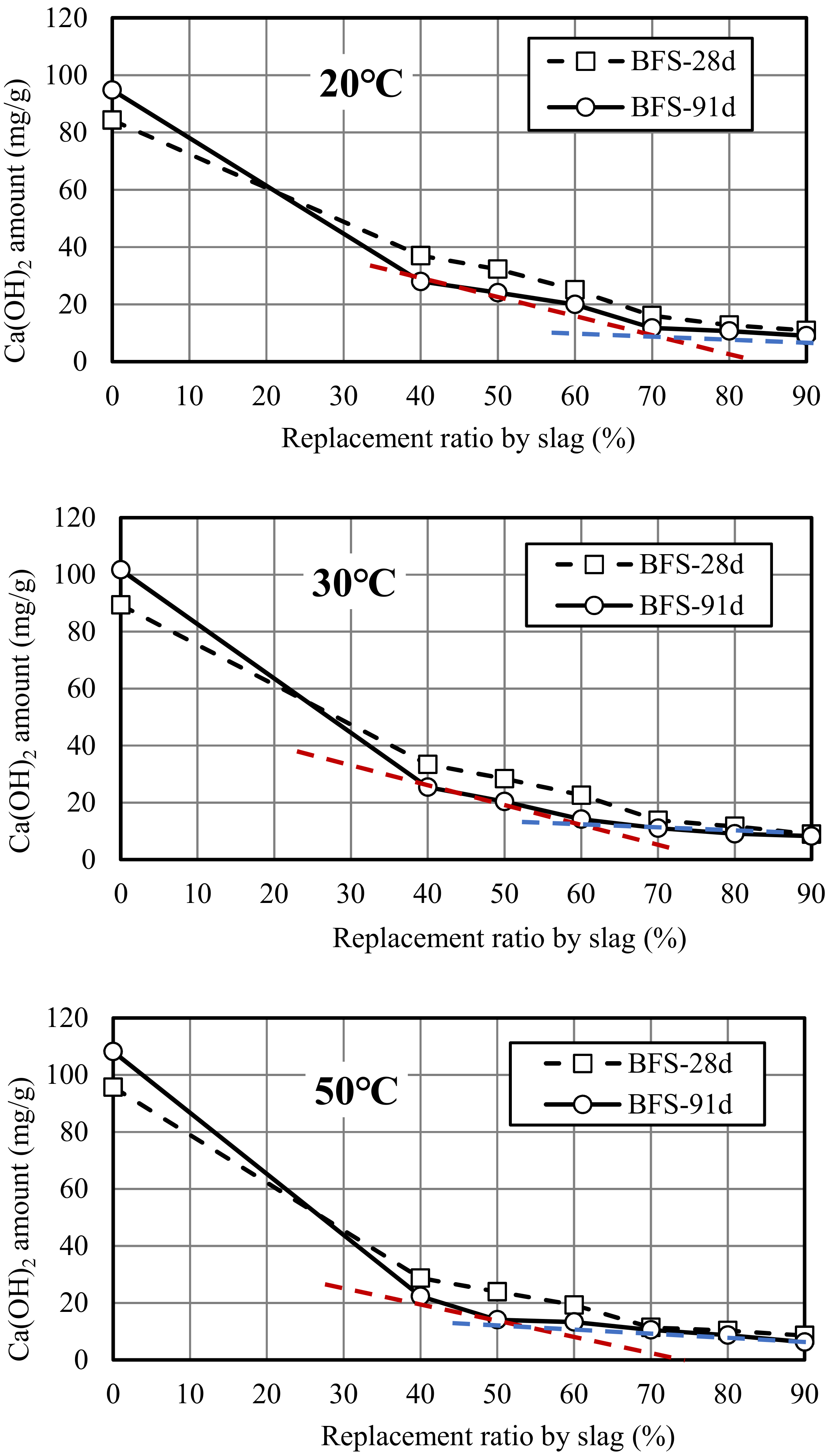
3.3. Changes in Ca(OH)2 Amount with Replacement Ratio by Slag
- CHA: Consumption of Ca(OH)2 by 1 g slag (g),
- CHPC: Ca(OH)2 amount in ordinary mortar (g),
- CHSL: Amount of Ca(OH)2 in slag mortar at the replacement ratio of r (g),
- r: Replacement ratio of slag
3.4. Evaluation Method of Reactivity of Slag Mortar
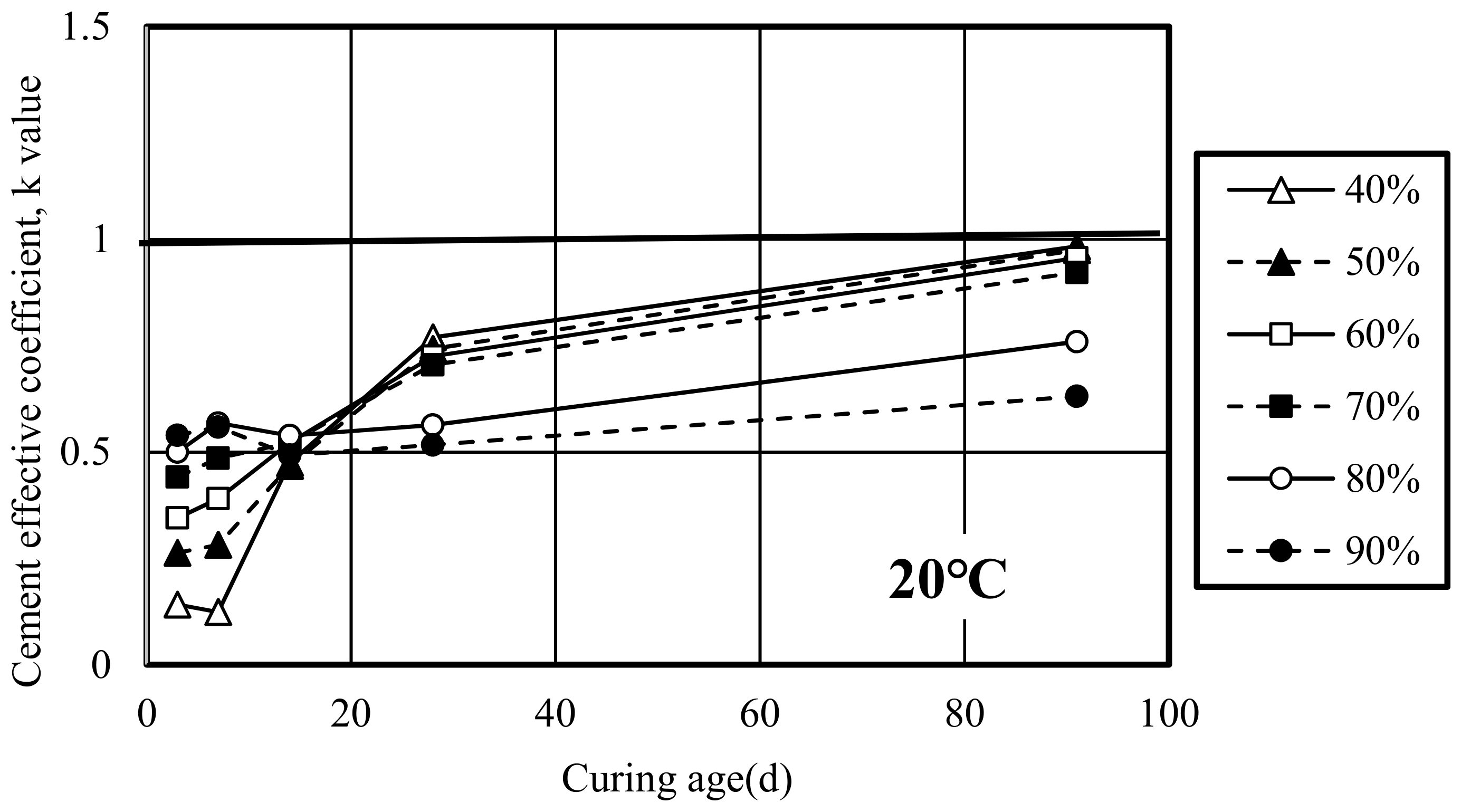
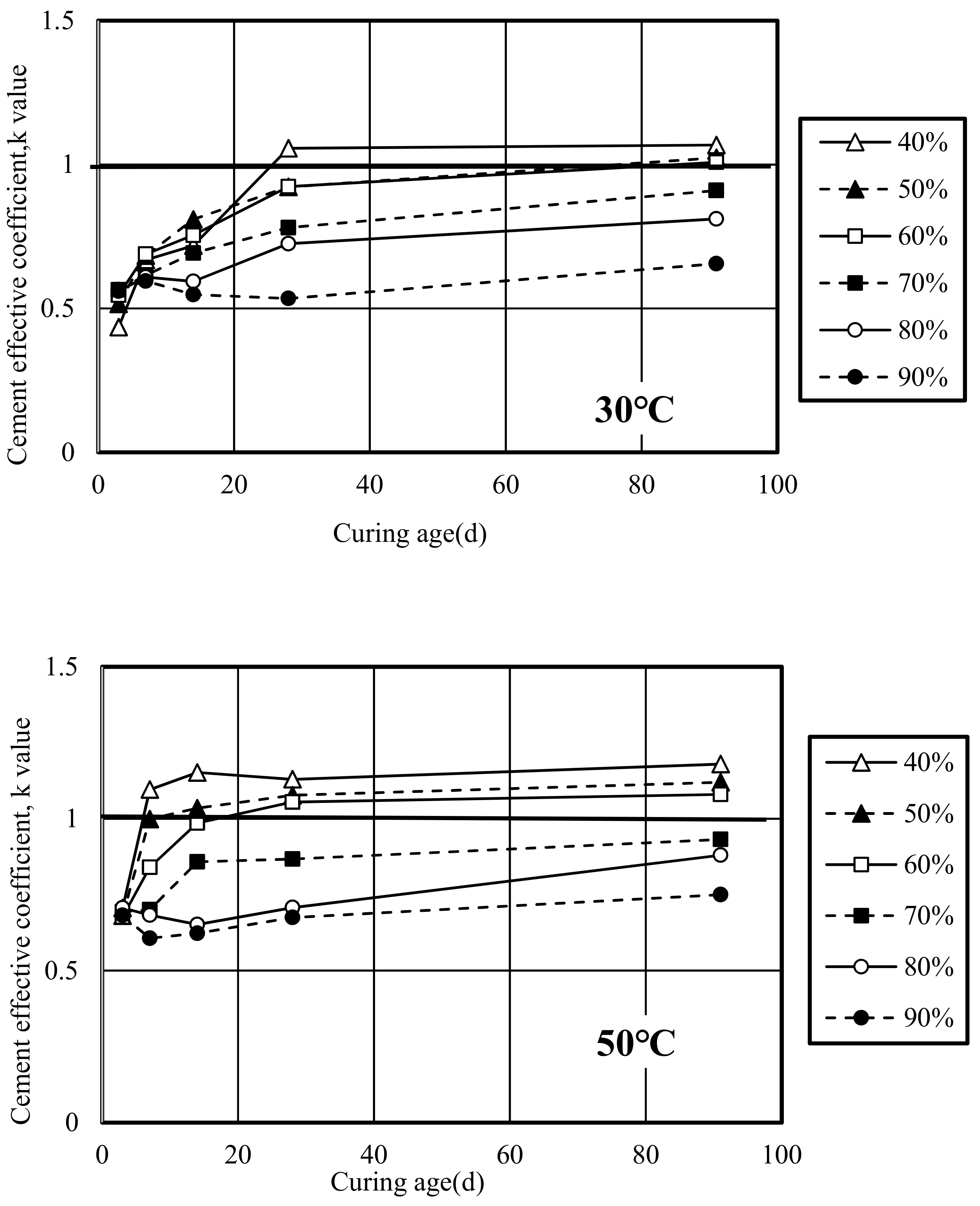
3.4.1. Cement Effective Coefficient (k Value)
- W: Unit Water Content (kg/m3)
- C: Unit Cement Content (kg/m3)
- k: Cement effective coefficient
- SL: Unit slag Content (kg/m3)
- (C/W)eq: Equivalent Water Cement Ratio
3.4.2. Relationship between Compressive Strength and Basicity of Mortar
3.4.3. Calculation of Reaction Efficiency Coefficient of Slag in Mortar
- ET: Reaction efficiency coefficient of slag at the age of 91 days at (t °C), (%).
- : Threshold value of effective replacement ratio by slag in mortar at the age of 91 days at (t °C), (%).
- E: Reaction efficiency coefficient of slag.
- T: Curing temperature (°C).
- : Reaction efficiency coefficient of slag under the condition of standard curing at (t °C), %.
- ET: Reaction efficiency coefficient of slag under the condition of sealed curing at (t °C), %.
- : Strength of ordinary cement mortar at 91 days under the condition of standard curing in water (MPa).
- : Strength of ordinary cement mortar at 91 days under the condition of sealed curing in water (MPa).
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Market Development Research and Investment Prospects Report of Slag Powder Industry in China. Available online: http://www.qianinfo.com/index/60/4605544.html (accessed on 13 April 2019).
- Jiang, L.; Li, C.; Wang, C.; Xu, N.; Chu, H. Utilization of flue gas desulfurization gypsum as an activation agent for high-volume slag concrete. J. Clean. Prod. 2018, 205, 589–598. [Google Scholar] [CrossRef]
- Ishida, T.; Luan, Y.; Sagawa, T.; Nawa, T. Modeling of early age behavior of blast furnace slag concrete based on micro-physical properties. Cem. Concr. Res. 2011, 41, 1357–1367. [Google Scholar] [CrossRef]
- Ahmed, F.A. Development of greener alkali-activated cement: Utilization of sodium carbonate for activating slag and slag mixtures. Clean. Prod. 2016, 113, 66–75. [Google Scholar]
- Rashad, A.M. An overview on rheology, mechanical properties and durability of high-volume slag used as a cement replacement in paste, mortar and concrete. Constr. Build. Mater. 2018, 187, 89–117. [Google Scholar] [CrossRef]
- Shen, D.J.; Liu, K.Q.; Wen, C.Y.; Shen, Y.Q.; Jiang, G.Q. Early-age cracking resistance of ground granulated blast furnace slag concrete. Constr. Build. Mater. 2019, 222, 278–287. [Google Scholar] [CrossRef]
- Han-Seung, L.; Wang, X.-Y. Evaluation of compressive strength development and carbonation depth of high volume slag-blended concrete. Constr. Build. Mater. 2016, 124, 45–54. [Google Scholar] [CrossRef]
- Kali, P.S.; Dinakar, P.; Umesh, C.S. Utilization of high volume of industrial slag in self compacting concrete. Clean. Prod. 2016, 112, 581–587. [Google Scholar]
- Salvador, R.P.; Rambo, D.A.; Bueno, R.M.; Silva, K.T.; De Figueiredo, A.D. On the use of blast-furnace slag in sprayed concrete applications. Constr. Build. Mater. 2019, 218, 543–555. [Google Scholar] [CrossRef]
- Hosokawa, D. Study on evaluation of thermal crack using mechanical properties in consideration of temperature and humidity of concrete with blast furnace slag. Hosei Univ. Repos. 2012, 1, 127–135. [Google Scholar]
- Saito, T.; Uchida, T.; Lee, Y.S.; Otsuki, N. The Influence of Curing Temperature on Hydrated Products of Blast Furnace Slag Cement and Its Porosity at the Early Stage of Hydration. J. Soc. Mater. Sci. Jpn. 2009, 58, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Nagao, Y.; Suzuki, K. Basic properties and utilization of steam-cured concrete using ground granulated blast-furnace slag. J. Nippon. Steel 2014, 399, 127–131. [Google Scholar]
- Bougara, A.; Lynsdale, C.; Milestone, N.B. The influence of slag properties, mix parameters and curing temperature on hydration and strength development of slag/cement blends. Constr. Build. Mater. 2018, 187, 339–347. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Hussin, M.W. Influence of different curing temperatures and alkali activators on properties of GBFS geopolymer mortars containing fly ash and palm-oil fuel ash. Constr. Build. Mater. 2016, 125, 1229–1240. [Google Scholar] [CrossRef]
- Ogirigbo, O.R.; Black, L. Influence of slag composition and temperature on the hydration and microstructure of slag blended cements. Constr. Build. Mater. 2016, 126, 496–507. [Google Scholar] [CrossRef] [Green Version]
- Han, F.H.; He, X.J.; Zhang, Z.Q.; Liu, J.H. Hydration heat of slag or fly ash in the composite binder at different temperatures. Thermochim. Acta. 2017, 655, 202–210. [Google Scholar] [CrossRef]
- Rashad, A.M. An investigation on very high volume slag pastes subjected to elevated temperatures. Constr. Build. Mater. 2015, 74, 249–258. [Google Scholar] [CrossRef]
- Ribeiro, A.B.; Santos, T.; Gonçalves, A. Performance of concrete exposed to natural carbonation: Use of the k-value concept. Constr. Build. Mater. 2018, 175, 360–370. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Raj, A.S.; Srinivasan, S.; Babu, K.G. Quantification of hydrated cement products of blended cements in low and medium strength concrete using TG and DTA technique. Thermochim. Acta 2003, 407, 49–60. [Google Scholar] [CrossRef]
- Xu, G.D.; Tian, Q.; Miao, J.X.; Liu, J.P. Early-age hydration and mechanical properties of high volume slag and slag concrete at different curing temperatures. Constr. Build. Mater. 2017, 149, 367–377. [Google Scholar] [CrossRef]
- Shaikh, F.U.A.; Hosan, A. Effect of nano silica on compressive strength and microstructures of high volume blast furnace slag and high volume blast furnace slag-fly ash blended pastes. Sustain. Mater. Technol. 2019, 20, e00111. [Google Scholar] [CrossRef]
- Abdelli, K.; Tahlaiti, M.; Belarbi, R.; Oudjit, M.N. Influence of the pozzolanic reactivity of the Blast Furnace Slag (BFS) and metakaolin on mortars. Energy Procedia 2017, 139, 224–229. [Google Scholar] [CrossRef]
- Kaya, A.; Ustabaş, I.; Ustabaş, I. Comparing the pozzolanic activity properties of obsidian to those of fly ash and blast furnace slag. Constr. Build. Mater. 2018, 164, 297–307. [Google Scholar]
- Ogirigbo, O.R.; Black, L. Chloride binding and diffusion in slag blends: Influence of slag composition and temperature. Constr. Build. Mater. 2017, 149, 816–825. [Google Scholar] [CrossRef]
- Huang, H.; Huang, T.; Yuan, Q.; Zhou, D.; Deng, D.; Zhang, L. Temperature dependence of structural build-up and its relation with hydration kinetics of cement paste. Constr. Build. Mater. 2019, 201, 553–562. [Google Scholar] [CrossRef]
- Kokubu, K.; Murata, Y.; Takahashi, S. Studies on adiabatic temperature rise and hydration ofportland cement concrete incorporating groundgranulated blast furnace slag. Doboku Gakkai Ronbunshu 1988, 9, 39–48. [Google Scholar] [CrossRef]
- Gruyaert, E.; Maes, M.; De Belie, N. Performance of BFS concrete: k-Value concept versus equivalent performance concept. Constr. Build. Mater. 2013, 47, 441–455. [Google Scholar] [CrossRef]
- Ogawa, Y.; Uji, K.; Ueno, A. Influence of curing temperature change to performance of fly ash as cementitious material. J. Jpn. Soc. Civ. Eng. Ser. E2 (Mater. Concr. Struct.) 2011, 67, 482–492. [Google Scholar] [CrossRef]
- Wang, W.L.; Xue, L.W.; Zhang, T.S.; Zhou, L.; Pan, Z. Thermodynamic corrosion behavior of Al2O3, ZrO2 and MgO refractories in contact with high basicity refining slag. Ceram. Int. 2019, 45, 20664–20673. [Google Scholar] [CrossRef]
- Kocaba, V.; Gallucci, E.; Scrivener, K.L. Methods for determination of degree of reaction of slag in blended cement pastes. Cem. Concr. Res. 2012, 42, 511–525. [Google Scholar] [CrossRef]
- Han, F.H.; Liu, J.L.; Yan, P.Y. Comparative study of reaction degree of mineral admixture by selective dissolution and image analysis. Constr. Build. Mater. 2016, 114, 946–955. [Google Scholar] [CrossRef]
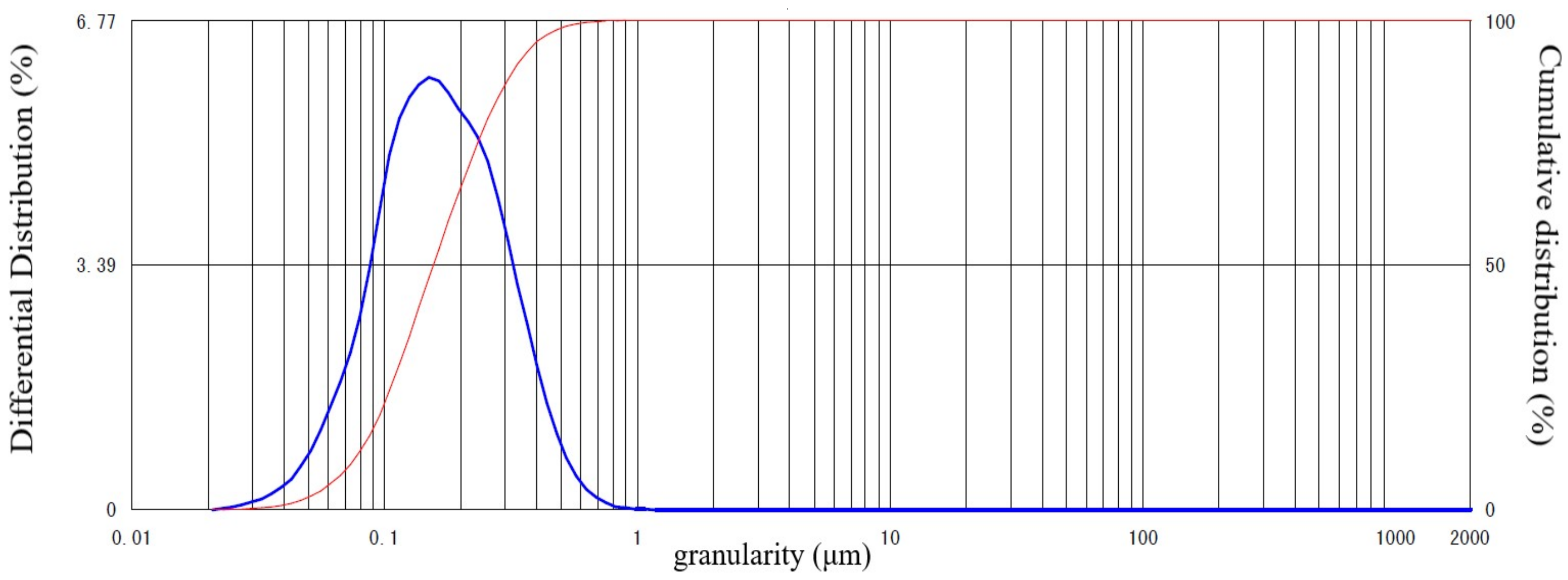
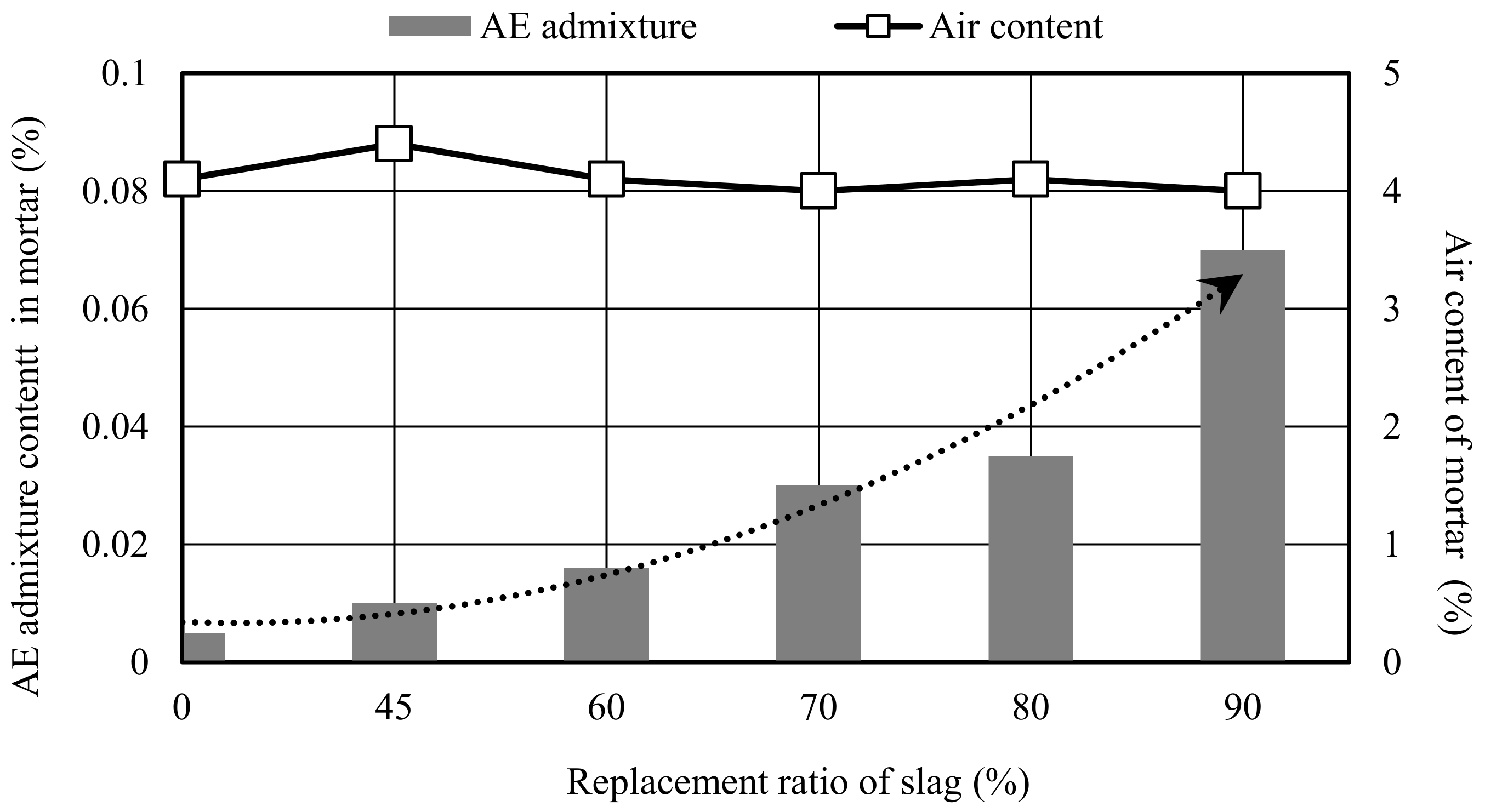



| Density (g/cm3) | Specific Surface Area (cm2/g) | Setting Time | Compressive Strength (N/mm2) | ||||
|---|---|---|---|---|---|---|---|
| Water (%) | Initial (h-m) | Final (h-m) | 3d | 7d | 28d | ||
| 3.16 | 3350 | 28.8 | 2-00 | 3-12 | 27.0 | 45.4 | 65.0 |
| Type | Ig.Loss (%) | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O | TiO2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 0.7 | 19.54 | 4.68 | 3.17 | 65.66 | 1.77 | 2.99 | 0.28 | 0.57 | 0.28 |
| Slag | 0.1 | 34.11 | 14.56 | 0.28 | 43.51 | 5.54 | -- | 0.24 | 0.34 | 0.54 |
| Mineral Admixture | W/(SL + C) (%) | Replacement Ratio SL/(SL + C) (%) | Unit Content (kg/m3) | |||
|---|---|---|---|---|---|---|
| W | C | SL | S | |||
| Blast furnace slag | 50 | 0 | 306 | 612 | 0 | 1310 |
| 40 | 304 | 365 | 243 | 1310 | ||
| 50 | 302 | 302 | 302 | 1310 | ||
| 60 | 300 | 240 | 360 | 1310 | ||
| 70 | 298 | 179 | 417 | 1310 | ||
| 80 | 298 | 119 | 476 | 1310 | ||
| 90 | 295 | 59 | 531 | 1310 | ||
| Cement | 40 | 0 | 279 | 698 | 0 | 1310 |
| 60 | 0 | 327 | 546 | 0 | 1310 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Quan, H.; Li, Q. Evaluation of Slag Reaction Efficiency in Slag-Cement Mortars under Different Curing Temperature. Materials 2019, 12, 2875. https://doi.org/10.3390/ma12182875
Wang L, Quan H, Li Q. Evaluation of Slag Reaction Efficiency in Slag-Cement Mortars under Different Curing Temperature. Materials. 2019; 12(18):2875. https://doi.org/10.3390/ma12182875
Chicago/Turabian StyleWang, Liang, Hongzhu Quan, and Qiuyi Li. 2019. "Evaluation of Slag Reaction Efficiency in Slag-Cement Mortars under Different Curing Temperature" Materials 12, no. 18: 2875. https://doi.org/10.3390/ma12182875




