Digital Light Processing of Freeze-cast Ceramic Layers for Macroporous Calcium Phosphate Scaffolds with Tailored Microporous Frameworks
Abstract
1. Introduction
2. Materials and Methods
2.1. Compositions of CaP Suspensions
2.2. Preparation of CaP Suspensions
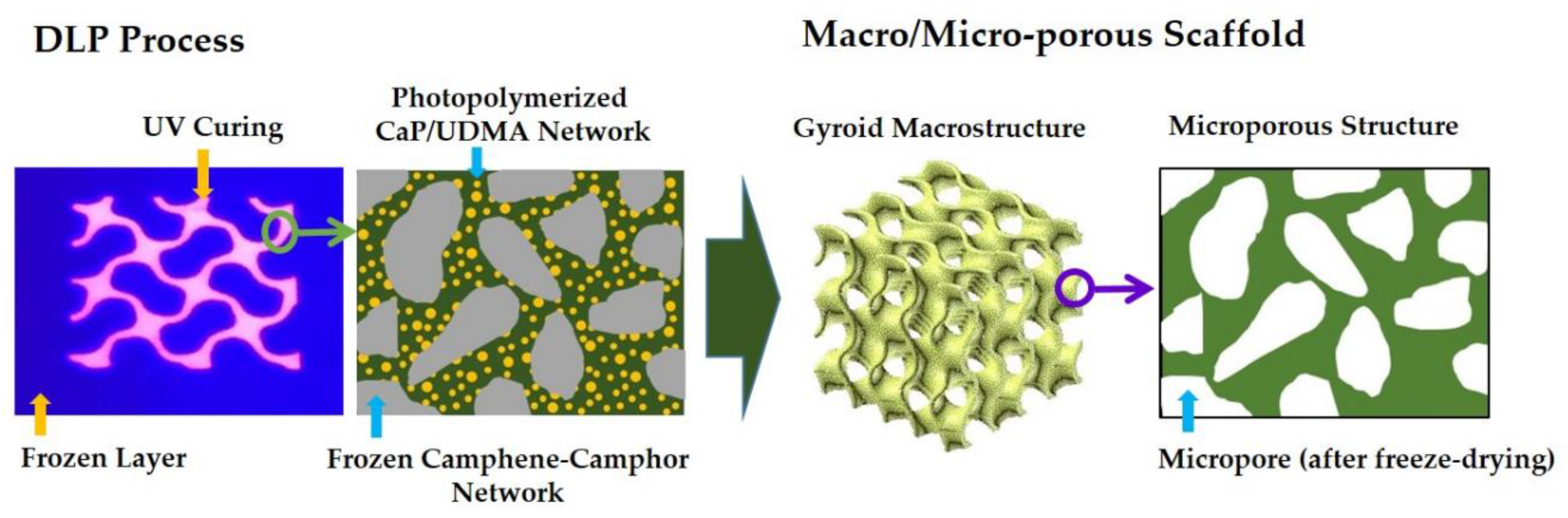
2.3. Manufacturing of Hierarchically Porous CaP Scaffolds
2.4. Characterization of Macrostructure and Microporous Structures
2.5. Measurement of Compressive Strengths and Modulus
2.6. Evaluation of Water Penetration Ability
2.7. Evaluation of In Vitro Cytocompatibility
2.8. Evaluation of In Vitro Apatite-Forming Ability
2.9. Statistical Analysis
3. Results and Discussion
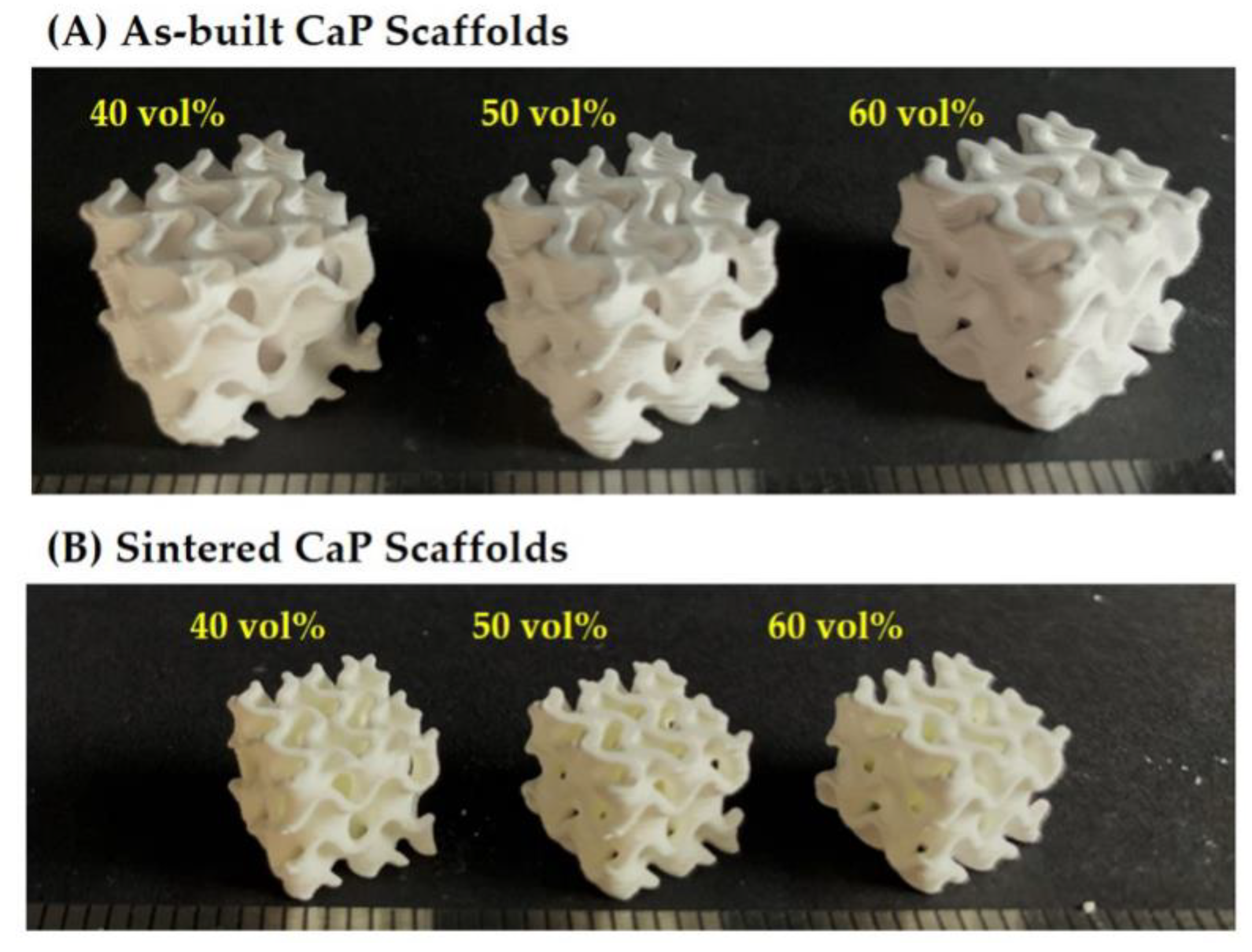
3.1. 3D Macrostructures of Hierarchically Porous CaP Scaffolds
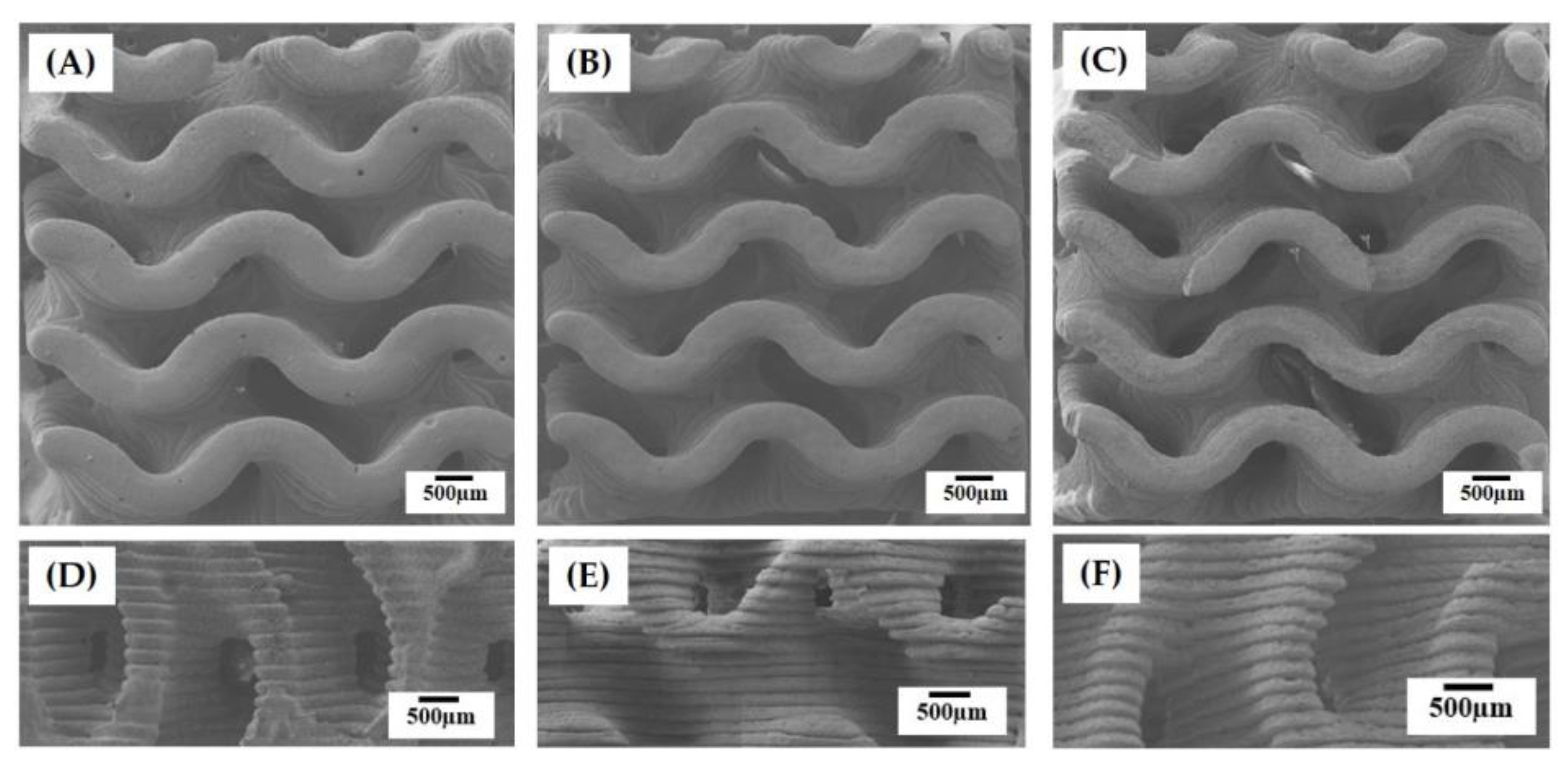
3.2. Internal Macroporous Structures
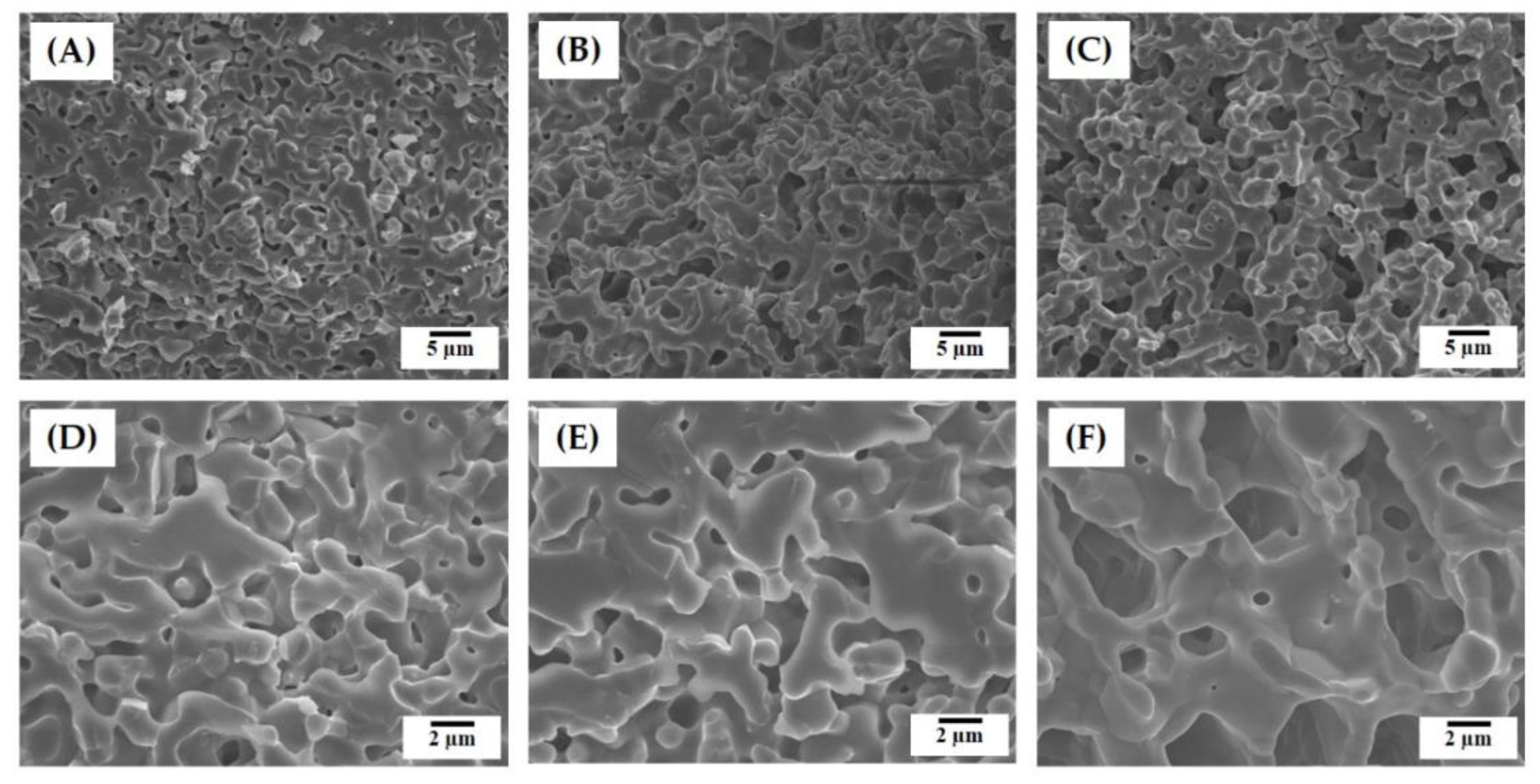
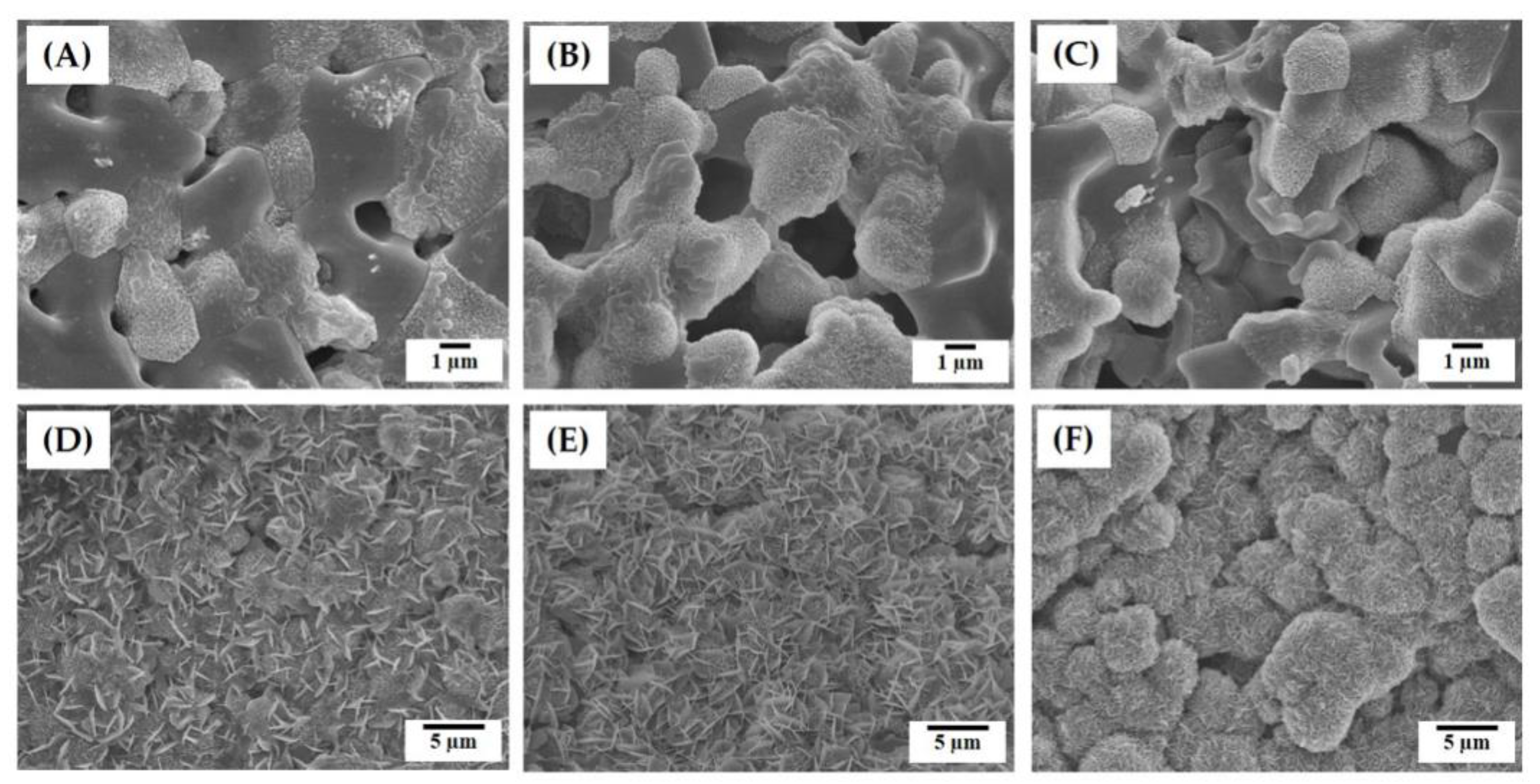
3.3. Hierarchical Macrostructure and Microporous Structures
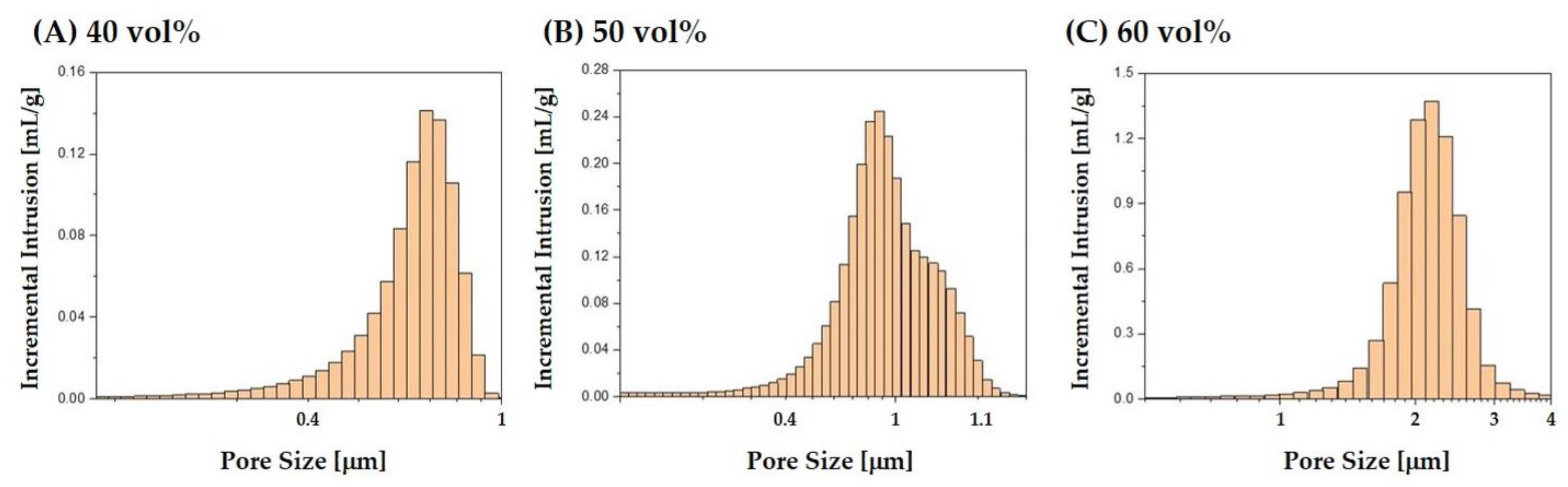
3.4. Size Distributions of Micropores
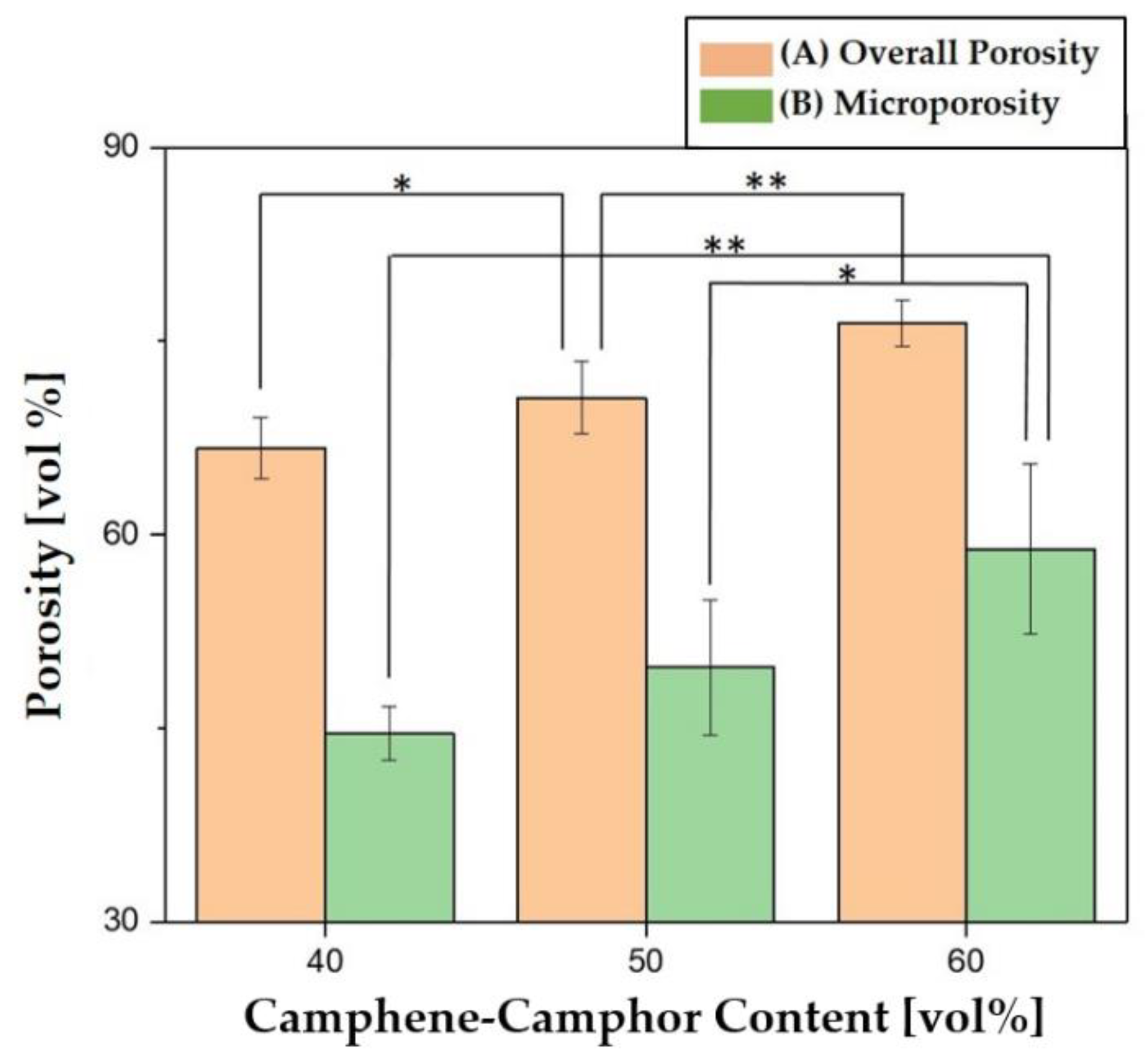
3.5. Overall Porosity and Microporosity
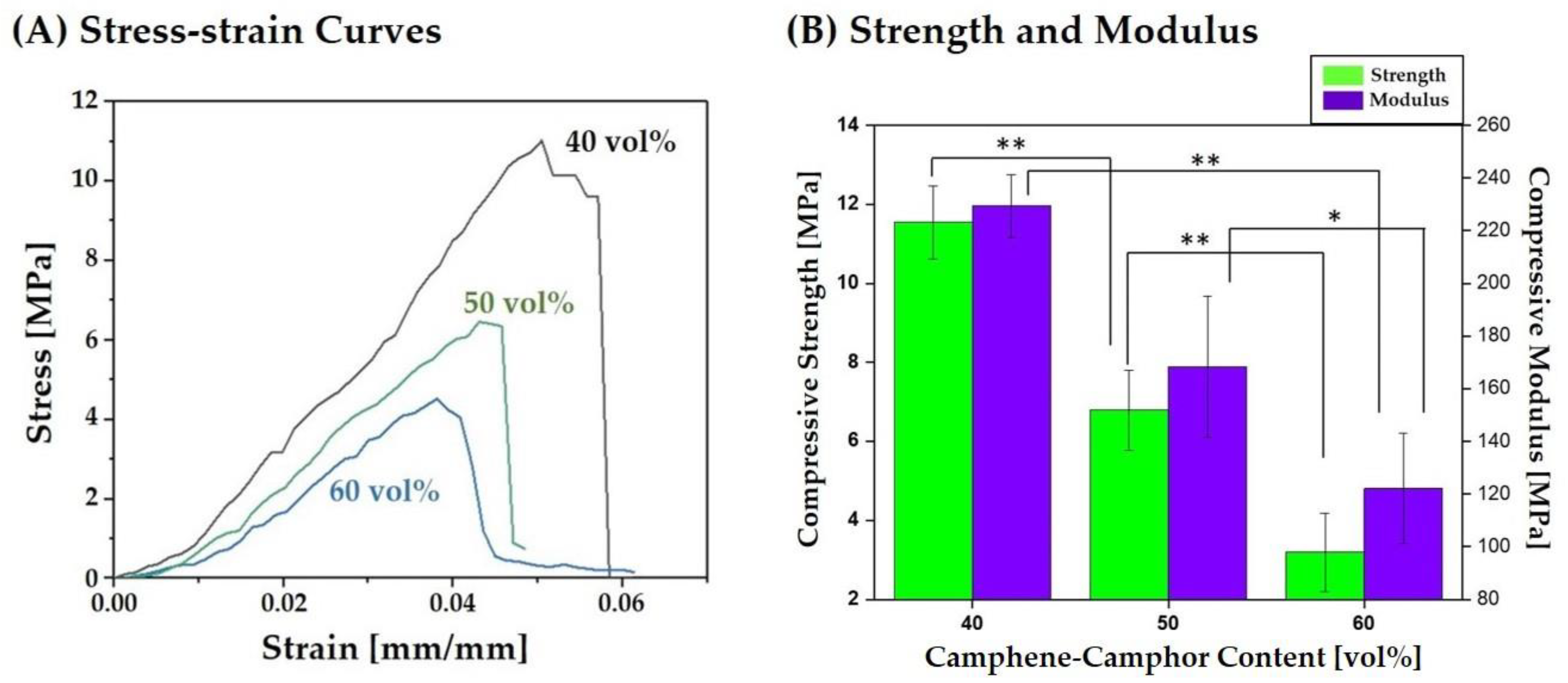
3.6. Fracture Behavior, Compressive Strengths, and Modulus
3.7. Crystalline Phases
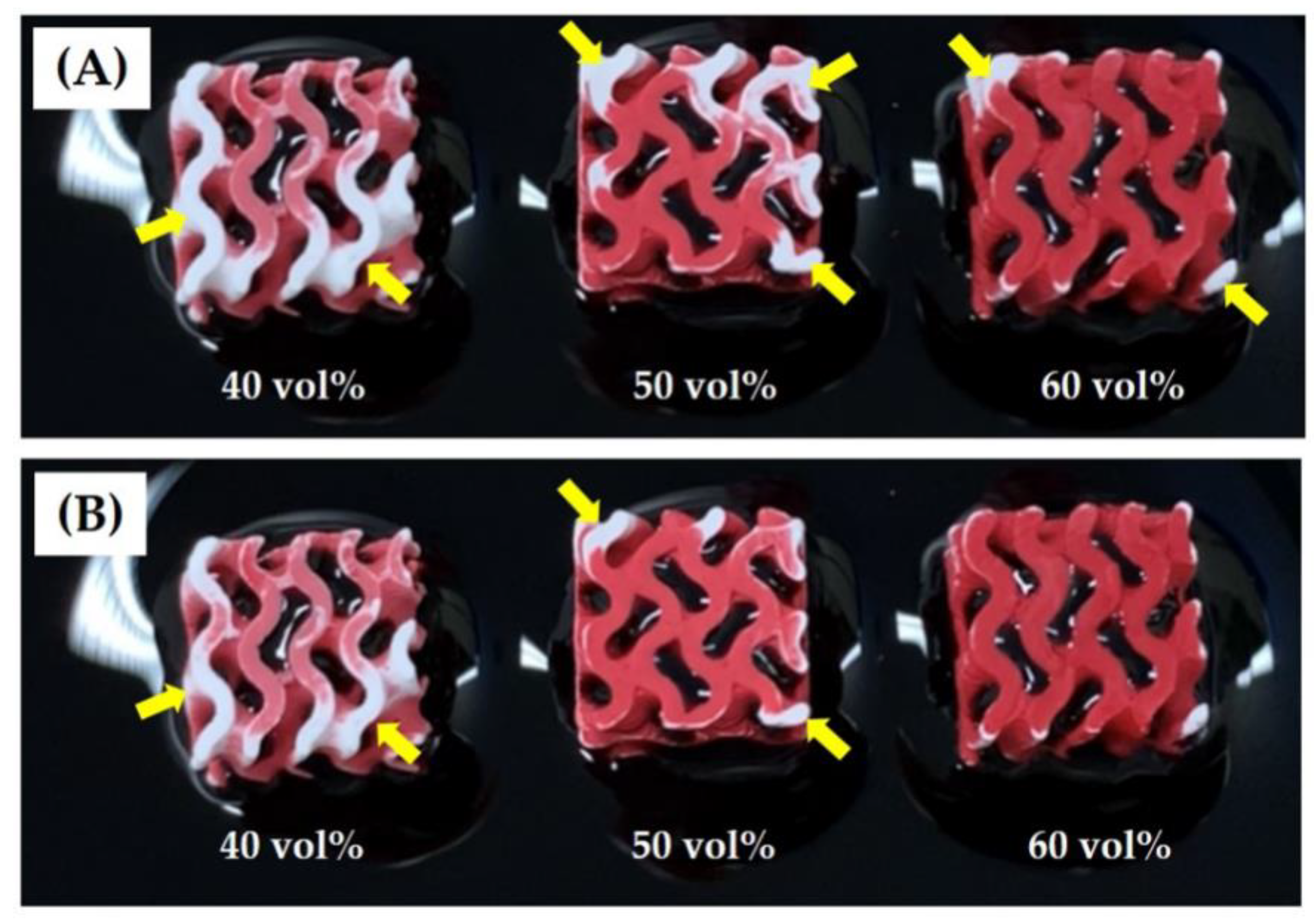
3.8. Water Penetration Capability
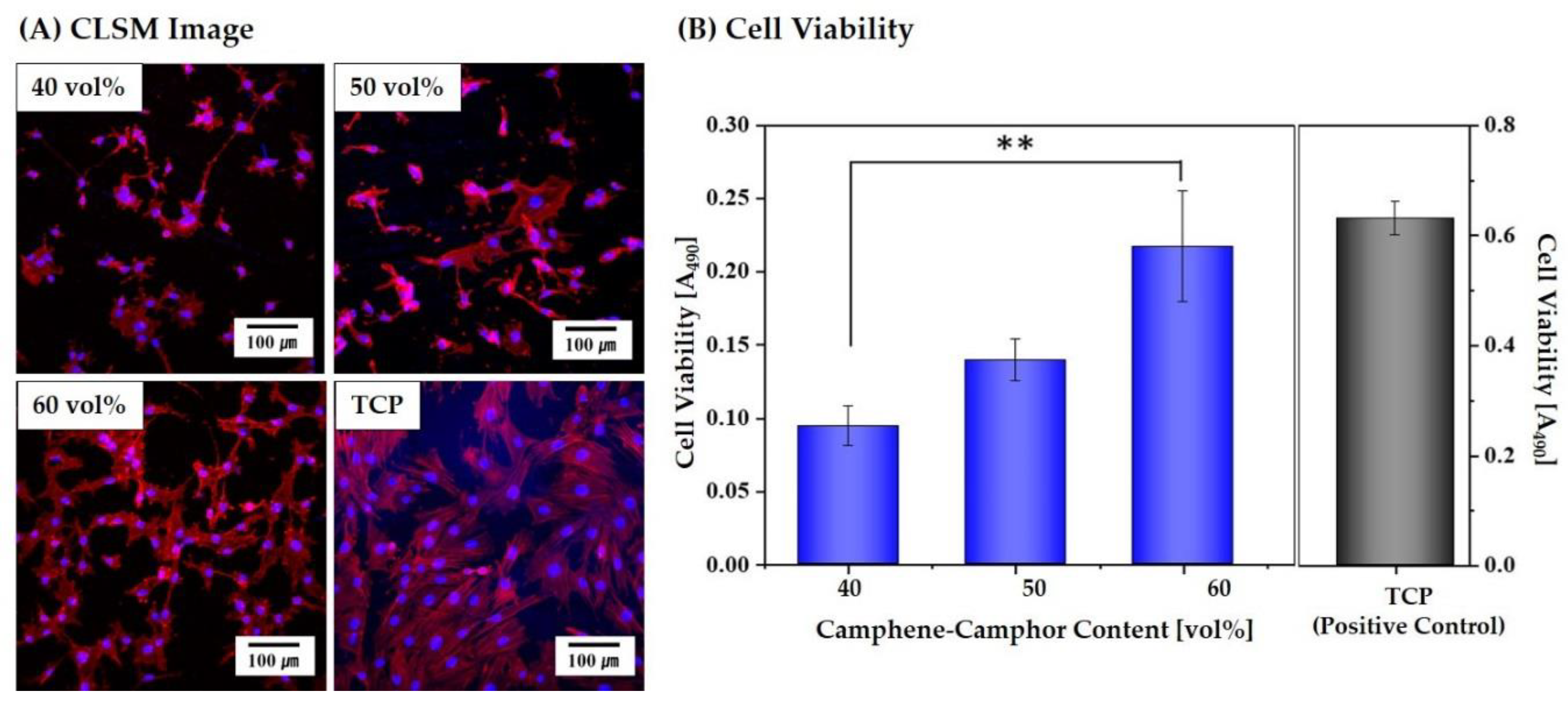
3.9. In Vitro Cytocompatibility
3.10. In Vitro Apatite-forming Ability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- LeGeos, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates as bioceramics: State of the art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Felfel, R.M.; Neel, E.A.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate-based glasses and ceramics and their biomedical applications: A review. J. Tissue. Eng. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A. Bioceramic bone graft substitutes: Influence of porosity and chemistry. Int. J. Appl. Ceram. Technol. 2005, 2, 184–199. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar]
- Tamaddon, M.; Czernuszka, J.T. The need for hierarchical scaffolds in bone tissue engineering. Hard Tissue 2013, 2, 37. [Google Scholar] [CrossRef]
- Zhang, K.; Fan, Y.; Dunne, N.; Li, X. Effect of microporosity on scaffolds for bone tissue engineering. Regen. Biomater. 2018, 5, 115–124. [Google Scholar] [CrossRef]
- Habibovic, P.; Sees, T.M.; van den Doel, M.A.; van Blitterswijk, C.A.; de Groot, K. Osteoinduction by biomaterials-physicochemical and structural influences. J. Biomed. Mater. Res. A 2006, 77, 747–762. [Google Scholar] [CrossRef]
- Lan Levengood, S.K.; Polak, S.J.; Wheeler, M.B.; Maki, A.J.; Clark, S.G.; Jamison, R.D.; Wagoner Johnson, A.J.W. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials 2010, 31, 3552–3563. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Jia, J.; Wu, F.; Wei, S.; Zhou, H.; Zhang, H.; Shin, J.W.; Liu, C. Hierarchically microporous/macroporous scaffold of magnesium-calcium phosphate for bone tissue regeneration. Biomaterials 2010, 31, 1260–1269. [Google Scholar] [CrossRef] [PubMed]
- Lapczyna, H.; Galea, L.; Wüst, S.; Bohner, M.; Jerban, S.; Sweedy, A.; Doebelin, N.; van Garderen, N.; Hofmann, S.; Baroud, G. Effect of grain size and microporosity on the in vivo behaviour of β-tricalcium phosphate scaffolds. Eur. Cells Mater. 2014, 28, 299–319. [Google Scholar] [CrossRef]
- Rustom, L.E.; Boudou, T.; Lou, S.; Pignot-Paintrand, I.; Nemke, B.W.; Lu, Y.; Markel, M.D.; Picart, C.; Wagoner Johnson, A.J. Micropore-induced capillarity enhances bone distribution in vivo in biphasic calcium phosphate scaffolds. Acta Biomater. 2016, 44, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhu, K.; Li, J.; Wang, C.S.; Wang, S.B.; Chen, A.Z. Fabrication of arbitrary 3D components in cardiac surgery: From macro-, micro- to nanoscale. Biofabrication 2017, 9, 032002. [Google Scholar] [CrossRef]
- Kankala, R.K.; Xu, X.M.; Liu, C.G.; Chen, A.Z.; Shi-Bin Wang, S.B. 3D-printing of microfibrous porous scaffolds based on hybrid approaches for bone tissue engineering. Polymers 2018, 10, 807. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Yang, S.; Yang, H.; Chi, X.; Evans, J.R.G.; Thompson, I.; Cook, R.J.; Robinson, P. Rapid prototyping of ceramic lattices for hard tissue scaffolds. Mater. Des. 2008, 29, 1802–1809. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Tomsia, A.P. Bioinspired strong and highly porous glass scaffolds. Adv. Funct. Mater. 2011, 21, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Doiphode, N.D.; Huang, T.S.; Leu, M.C.; Rahaman, M.N.; Day, D.E. Freeze extrusion fabrication of 13-93 bioactive glass scaffolds for bone repair. J. Mater. Sci. Mater. Med. 2011, 22, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.S.; Rahaman, M.N.; Doiphode, N.D.; Leu, M.C.; Bal, B.S.; Day, D.E.; Liu, X. Porous and strong bioactive glass (13-93) scaffolds fabricated by freeze extrusion technique. Mater. Sci. Eng. C 2011, 31, 1482–1489. [Google Scholar] [CrossRef]
- Tesavibul, P.; Felzmann, R.; Gruber, S.; Liska, R.; Thompson, I.; Boccaccini, A.R.; Stampfl, J. Processing of 45S5 bioglass (R) by lithography-based additive manufacturing. Mater. Lett. 2012, 74, 81–84. [Google Scholar] [CrossRef]
- Felzmann, R.; Gruber, S.; Mitteramskogler, G.; Tesavibul, P.; Boccaccini, A.R.; Liska, R.; Stampfl, J. Lithography-based additive manufacturing of cellular ceramic structures. Adv. Eng. Mater. 2012, 14, 1052–1058. [Google Scholar] [CrossRef]
- Franks, G.V.; Tallon, C.; Studart, A.R.; Sesso, M.L.; Leo, S. Colloidal processing: Enabling complex shaped ceramics with unique multiscale structures. J. Am. Ceram. Soc. 2017, 100, 458–490. [Google Scholar] [CrossRef]
- Polak, S.J.; Levengood, S.K.L.; Wheeler, M.B.; Maki, A.J.; Clark, S.G.; Johnson, A.J.W. Analysis of the roles of microporosity and BMP-2 on multiple measures of bone regeneration and healing in calcium phosphate scaffolds. Acta Biomater. 2011, 7, 1760–1771. [Google Scholar] [CrossRef] [PubMed]
- Minas, C.; Carnelli, D.; Tervoort, E.; Studart, A.R. 3D Printing of emulsions and foams into hierarchically porous ceramics. Adv. Mater. 2016, 28, 9993–9999. [Google Scholar] [CrossRef]
- Muth, J.T.; Dixon, P.G.; Woish, L.; Gibson, L.J.; Lewis, J.A. Architected cellular ceramics with tailored stiffness via direct foam writing. Proc. Natl. Acad. Sci. USA 2017, 114, 1832–1837. [Google Scholar] [CrossRef]
- Alison, L.; Menasce, S.; Bouville, F.; Tervoort, E.; Mattich, I.; Ofner, A.; Studartm, A.R. 3D printing of sacrificial templates into hierarchically porous materials. Sci. Rep. 2019, 9, 409. [Google Scholar] [CrossRef]
- Moon, Y.W.; Shin, K.H.; Koh, Y.H.; Jung, H.D.; Kim, H.E. Three-dimensional ceramic/camphene-based co-extrusion for unidirectionally macrochanneled alumina ceramics with controlled porous walls. J. Am. Ceram. Soc. 2014, 97, 32–34. [Google Scholar] [CrossRef]
- Moon, Y.W.; Shin, K.H.; Koh, Y.H.; Jung, H.D.; Kim, H.E. Macroporous alumina scaffolds consisting of highly microporous hollow filaments using three-dimensional ceramic/camphene-based co-extrusion. J. Eur. Ceram. Soc. 2015, 35, 4623–4627. [Google Scholar] [CrossRef]
- Moon, Y.W.; Choi, I.J.; Koh, Y.H.; Kim, H.W. Porous alumina ceramic scaffolds with biomimetic macro/micro-porous structure using three-dimensional (3-D) ceramic/camphene-based extrusion. Ceram. Int. 2015, 41, 12371–12377. [Google Scholar] [CrossRef]
- Lee, J.B.; Maeng, W.Y.; Koh, Y.H.; Kim, H.E. Novel additive manufacturing of photocurable ceramic slurry containing freezing vehicle as porogen for hierarchically porous structure. Ceram. Int 2019. [Google Scholar] [CrossRef]
- Tomeckova, V.; Halloran, J.W. Macroporous polyacrylates from terpene-acrylate thermoreversible photopolymerizable vehicle. J. Mater. Sci. 2012, 47, 6166–6178. [Google Scholar] [CrossRef]
- Tomeckova, V.; Halloran, J.W. Porous ceramics by photopolymerization with terpene-acrylate vehicles. J. Am. Ceram. Soc. 2012, 95, 3763–3768. [Google Scholar] [CrossRef]
- Prins, H.J.; Braat, A.K.; Gawlitta, D.; Dhert, W.J.; Egan, D.A.; Tijssen-Slump, E.; Yuan, H.; Coffer, P.J.; Rozemuller, H.; Martens, A.C. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem. Cell Res. 2014, 12, 428–440. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C. 2014, 35, 134–143. [Google Scholar] [CrossRef]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef]
- Ruiz-Cantu, L.; Gleadall, A.; Faris, C.; Segal, J.; Shakesheff, K.; Yang, J. Characterisation of the surface structure of 3D printed scaffolds for cell infiltration and surgical suturing. Biofabrication 2016, 8, 15016. [Google Scholar] [CrossRef]
- Xin, R.; Leng, Y.; Chen, J.; Zhang, Q. A comparative study of calcium phosphate formation on bioceramics in vitro and in vivo. Biomaterials 2005, 26, 6477–6486. [Google Scholar] [CrossRef] [PubMed]
- Uchino, T.; Yamaguchi, K.; Suzuki, I.; Kamitakahara, M.; Otsuka, M.; Ohtsuki, C. Hydroxyapatite formation on porous ceramics of alpha- tricalcium phosphate in a simulated body fluid. J. Mater. Sci. Mater. Med. 2010, 21, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Sureshbabu, S.; Komath, M.; Varma, H.K. In situ formation of hydroxyapatite-alpha tricalcium phosphate biphasic ceramics with higher strength and bioactivity. J. Am. Ceram. Soc. 2012, 95, 915–924. [Google Scholar]
- Ahn, M.K.; Moon, Y.W.; Koh, Y.H.; Kim, H.E. Production of highly porous triphasic calcium phosphate scaffolds with excellent in vitro bioactivity using vacuum-assisted foaming of ceramic suspension (VFC) technique. Ceram. Int. 2013, 39, 5879–5885. [Google Scholar] [CrossRef]


| Role | Ceramic Powder | Photocurable Monomer | Freezing Vehicle | Dispersant | Photoinitiator |
|---|---|---|---|---|---|
| Material | CaP | UDMA | 2 Camphene + 1 Camphor | KD4 | PPO |
| Amount (g) | 47.6 | 15 | 18.14–40.8 (1) | 1.47 (2) | 0.3 (3) |
| Freezing Conditions | Photopolymerization Conditions | |||
|---|---|---|---|---|
| Temperature of CaP Slurry | Temperature of Building Platform | Layer Thickness | UV Power | Exposure Time |
| 70 °C | ~33–38 °C † | ~220 μm | 60 W/m2 | 20 s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-W.; Lee, J.-B.; Koh, Y.-H.; Kim, H.-E. Digital Light Processing of Freeze-cast Ceramic Layers for Macroporous Calcium Phosphate Scaffolds with Tailored Microporous Frameworks. Materials 2019, 12, 2893. https://doi.org/10.3390/ma12182893
Kim J-W, Lee J-B, Koh Y-H, Kim H-E. Digital Light Processing of Freeze-cast Ceramic Layers for Macroporous Calcium Phosphate Scaffolds with Tailored Microporous Frameworks. Materials. 2019; 12(18):2893. https://doi.org/10.3390/ma12182893
Chicago/Turabian StyleKim, Jong-Woo, Jung-Bin Lee, Young-Hag Koh, and Hyoun-Ee Kim. 2019. "Digital Light Processing of Freeze-cast Ceramic Layers for Macroporous Calcium Phosphate Scaffolds with Tailored Microporous Frameworks" Materials 12, no. 18: 2893. https://doi.org/10.3390/ma12182893
APA StyleKim, J.-W., Lee, J.-B., Koh, Y.-H., & Kim, H.-E. (2019). Digital Light Processing of Freeze-cast Ceramic Layers for Macroporous Calcium Phosphate Scaffolds with Tailored Microporous Frameworks. Materials, 12(18), 2893. https://doi.org/10.3390/ma12182893






