Erosion Behavior of a Cu-Ti3AlC2 Cathode by Multi-Electric Arc
Abstract
:1. Introduction
2. Materials and Methods
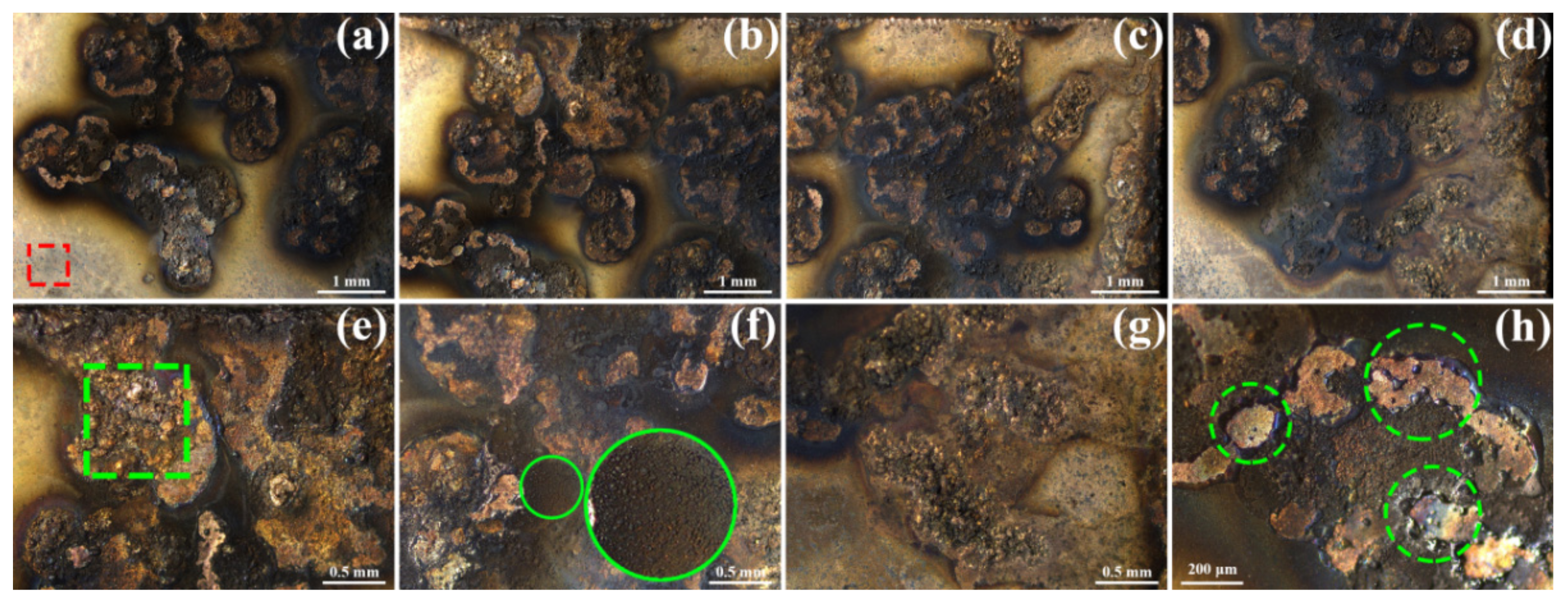
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Zheng, Z.; Ren, W.B. An experimental investigation of dynamic welding mechanism of contacts used in low current switching devices. In Proceedings of the 64th IEEE Holm Conference on Electrical Contacts and the 29th International Conference on Electrical Contacts, Albuquerque, NM, USA, 14–18 October 2018; pp. 488–495. [Google Scholar]
- Chen, Q.Y.; Liang, S.H.; Wang, F.; Zhuo, L.C. Microstructural investigation after vacuum electrical breakdown of the w-30 wt.% Cu contact material. Vacuum 2018, 149, 256–261. [Google Scholar]
- Dong, L.L.; Chen, W.G.; Deng, N.; Song, J.L.; Wang, J.J. Investigation on arc erosion behaviors and mechanism of W70Cu30 electrical contact materials adding graphene. J. Alloy. Compd. 2017, 696, 923–930. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y.; Yang, Z.; Chen, L.; Qiao, S. Cathode spot movement on a continuous carbon fiber reinforced cu matrix composite in vacuum. Vacuum 2013, 93, 45–49. [Google Scholar] [CrossRef]
- Wei, X.; Wang, J.; Yang, Z.; Sun, Z.; Yu, D.; Song, X.; Ding, B.; Yang, S. Liquid phase separation of Cu-Cr alloys during the vacuum breakdown. J. Alloy. Compd. 2011, 509, 7116–7120. [Google Scholar] [CrossRef]
- Zhu, S.; Liu, Y.; Tian, B.; Zhang, Y.; Song, K. Arc erosion behavior and mechanism of Cu/Cr20 electrical contact material. Vacuum 2017, 143, 129–137. [Google Scholar] [CrossRef]
- Zhang, X.H.; Zhang, Y.; Tian, B.H.; An, J.C.; Zhao, Z.; Volinsky, A.A.; Liu, Y.; Song, K.X. Arc erosion behavior of the Al2o3-Cu/(W, Cr) electrical contacts. Compos. Part B Eng. 2019, 160, 110–118. [Google Scholar] [CrossRef]
- Barsoum, M.W. The Mn+1AXN phases: A new class of solids: Thermodynamically stable nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. [Google Scholar] [CrossRef]
- Radovic, M.; Barsoum, M.W. Max phases: Bridging the gap between metals and ceramics. Am. Ceram. Soc. Bull. 2013, 92, 20–27. [Google Scholar]
- Sun, Z.M. Progress in research and development on max phases: A family of layered ternary compounds. Int. Mater. Rev. 2011, 56, 143–166. [Google Scholar] [CrossRef]
- Bentzel, G.W.; Sokol, M.; Griggs, J.; Lang, A.C.; Barsoum, M.W. On the interactions of Ti2ALC, Ti3ALC2, Ti3SIC2 and Cr2ALC with palladium at 900 °C. J. Alloy. Compd. 2019, 771, 1103–1110. [Google Scholar] [CrossRef]
- Wang, H.; Han, H.; Yin, G.; Wang, C.-Y.; Hou, Y.-Y.; Tang, J.; Dai, J.-X.; Ren, C.-L.; Zhang, W.; Huai, P. First-principles study of vacancies in Ti3SIC2 and Ti3ALC2. Materials 2017, 10, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; Jiang, Y. Pore formation process of porous Ti3SIC2 fabricated by reactive sintering. Materials 2017, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Bei, G. Toughening mechanisms in nanolayered max phase ceramics—A review. Materials 2017, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Scheibe, B.; Kupka, V.; Peplińska, B.; Jarek, M.; Tadyszak, K. The influence of oxygen concentration during max phases (Ti3ALC2) preparation on the α-Al2O3 microparticles content and specific surface area of multilayered mxenes (Ti3C2Tx). Materials 2019, 12, 353. [Google Scholar] [CrossRef]
- Zhang, P.; Ngai, T.L.; Wang, A.D.; Ye, Z.Y. Arc erosion behavior of Cu-Ti3SIC2 cathode and anode. Vacuum 2017, 141, 235–242. [Google Scholar] [CrossRef]
- Huang, X.C.; Feng, Y.; Qian, G.; Zhao, H.; Song, Z.K.; Zhang, J.C.; Zhang, X.B. Arc corrosion behavior of Cu-Ti3ALC2 composites in air atmosphere. Sci. China Technol. Sci. 2018, 61, 551–557. [Google Scholar] [CrossRef]
- Huang, X.C.; Feng, Y.; Qian, G.; Zhao, H.; Zhang, J.C.; Zhang, X.B. Physical, mechanical, and ablation properties of Cu-Ti3ALC2 composites with various Ti3ALC2 contents. Mater. Sci. Technol. 2018, 34, 757–762. [Google Scholar] [CrossRef]
- Ding, J.; Tian, W.B.; Zhang, P.; Zhang, M.; Zhang, Y.M.; Sun, Z.M. Arc erosion behavior of Ag/Ti3ALC2 electrical contact materials. J. Alloy. Compd. 2018, 740, 669–676. [Google Scholar] [CrossRef]
- Huang, X.C.; Feng, Y.; Qian, G.; Liu, K. Erosion behavior of Ti3ALC2 cathode under atmosphere air arc. J. Alloy. Compd. 2017, 727, 419–427. [Google Scholar] [CrossRef]
- Huang, X.C.; Feng, Y.; Qian, G.; Zhang, J.C.; Zhang, X.B. Influence of breakdown voltages on arc erosion of a Ti3ALC2 cathode in an air atmosphere. Ceram. Int. 2017, 43, 10601–10605. [Google Scholar] [CrossRef]
- Ding, J.; Tian, W.; Wang, D.; Zhang, P.; Chen, J.; Zhang, Y.; Sun, Z. Microstructure evolution, oxidation behavior and corrosion mechanism of Ag/Ti2SNC composite during dynamic electric arc discharging. J. Alloy. Compd. 2019, 785, 1086–1096. [Google Scholar] [CrossRef]
- Ding, J.; Tian, W.; Zhang, P.; Zhang, M.; Chen, J.; Zhang, Y.; Sun, Z. Preparation and arc erosion properties of Ag/Ti2SNC composites under electric arc discharging. J. Adv. Ceram. 2019, 8, 90–101. [Google Scholar] [CrossRef]
- Zhang, M.; Tian, W.-B.; Zhang, P.; Ding, J.; Zhang, Y.; Sun, Z. Microstructure and properties of Ag-Ti3SIC2 contact materials prepared by pressureless sintering. Int. J. Miner. Metall. Mater. 2018, 25, 810–816. [Google Scholar] [CrossRef]
- Ding, J.; Tian, W.; Wang, D.; Zhang, P.; Chen, J.; Zhang, Y.; Sun, Z. Corrosion and degradation mechanism of Ag/Ti3ALC2 composites under dynamic electric arc discharge. Corros. Sci. 2019, 156, 147–160. [Google Scholar] [CrossRef]
- Wang, D.; Tian, W.; Ma, A.; Ding, J.; Wang, C.; You, Y.; Zhang, P.; Chen, J.; Zhang, Y.; Sun, Z. Anisotropic properties of Ag/Ti3ALC2 electrical contact materials prepared by equal channel angular pressing. J. Alloy. Compd. 2019, 784, 431–438. [Google Scholar] [CrossRef]
- Hai, T.; Yi, F.; Xiaochen, H.; Yakun, D.; Dongdong, D.; Meng, X.; Pei, T.; Gang, Q.; Xuebin, Z. Reactive synthesis of polycrystalline Ti3ALC2 and its sintering behavior. Rare Met. Mater. Eng. 2017, 46, 2108–2113. [Google Scholar] [CrossRef]
- Presser, V.; Naguib, M.; Chaput, L.; Togo, A.; Hug, G.; Barsoum, M.W. First-order raman scattering of the max phases: Ti2ALN, Ti2ALC0.5N0.5, Ti2ALC, (Ti0.5V0.5)2ALC, V2ALC, Ti3ALC2, and Ti3GEC2. J. Raman Spectrosc. 2012, 43, 168–172. [Google Scholar]
- Zhao, H.; Feng, Y.; Qian, G.; Huang, X.; Guo, S.; Sun, X. Effect of Ti3ALC2 content on electrical friction and wear behaviors of Cu-Ti3ALC2 composites. Tribol. Lett. 2019, 67, 96. [Google Scholar] [CrossRef]
- Huang, X.; Feng, Y.; Qian, G.; Zhou, Z. Arc ablation properties of Ti3SIC2 material. Ceram. Int. 2019, 45, 20297–20306. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.C. Microstructure, mechanical, and electrical properties of Cu-Ti3ALC2 and in situ Cu-TiCx composites. J. Mater. Res. 2011, 23, 924–932. [Google Scholar] [CrossRef]
- Parker, J.C.; Siegel, R.W. Calibration of the raman spectrum to the oxygen stoichiometry of nanophase TiO2. Appl. Phys. Lett. 1990, 57, 943–945. [Google Scholar] [CrossRef]
- Parker, J.C.; Siegel, R.W. Raman microprobe study of nanophase TiO2 and oxidation-induced spectral changes. J. Mater. Res. 1990, 5, 1246–1252. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, A.; Li, Z.; Wang, L. Corrosion behavior of Ti3ALC2 in molten KOH at 700 °C. J. Adv. Ceram. 2013, 2, 313–317. [Google Scholar] [CrossRef]
- Kumar, H.V.; Woltornist, S.J.; Adamson, D.H. Fractionation and characterization of graphene oxide by oxidation extent through emulsion stabilization. Carbon 2016, 98, 491–495. [Google Scholar] [CrossRef]
- Li, X.; Magnuson, C.W.; Venugopal, A.; Tromp, R.M.; Hannon, J.B.; Vogel, E.M.; Colombo, L.; Ruoff, R.S. Large-area graphene single crystals grown by low-pressure chemical vapor deposition of methane on copper. J. Am. Chem. Soc. 2011, 133, 2816–2819. [Google Scholar] [CrossRef] [PubMed]
- Freedy, K.M.; Beechem, T.E.; Litwin, P.M.; Sales, M.G.; Huang, M.; Ruoff, R.S.; McDonnell, S.J. Unraveling chemical interactions between titanium and graphene for electrical contact applications. ACS Appl. Nano Mater. 2018, 1, 4828–4835. [Google Scholar] [CrossRef]
- Wang, J.; Kang, Y.; Wang, C. Microstructure and vacuum arc characteristics of CuO skeletal structure Ag-CuO contact materials. J. Alloy. Compd. 2016, 686, 702–707. [Google Scholar] [CrossRef]
- Wang, H.; Wen, P.; Liang, L.; Zhu, Y. Effect of wettability on properties of AgSnO2TiO2 contact material. Electron. Compon. Mater. 2016, 35, 80–83. [Google Scholar]
- Li, G.J.; Cui, H.J.; Chen, J.; Fang, X.Q.; Feng, W.J.; Liu, J.X. Formation and effects of CuO nanoparticles on Ag/SnO2 electrical contact materials. J. Alloy. Compd. 2017, 696, 1228–1234. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Feng, Y.; Li, L.; Li, Z. Erosion Behavior of a Cu-Ti3AlC2 Cathode by Multi-Electric Arc. Materials 2019, 12, 2947. https://doi.org/10.3390/ma12182947
Huang X, Feng Y, Li L, Li Z. Erosion Behavior of a Cu-Ti3AlC2 Cathode by Multi-Electric Arc. Materials. 2019; 12(18):2947. https://doi.org/10.3390/ma12182947
Chicago/Turabian StyleHuang, Xiaochen, Yi Feng, Liang Li, and Zongqun Li. 2019. "Erosion Behavior of a Cu-Ti3AlC2 Cathode by Multi-Electric Arc" Materials 12, no. 18: 2947. https://doi.org/10.3390/ma12182947





