Exploring the Contribution of Two Phosphorus-Based Groups to Polymer Flammability via Pyrolysis–Combustion Flow Calorimetry
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
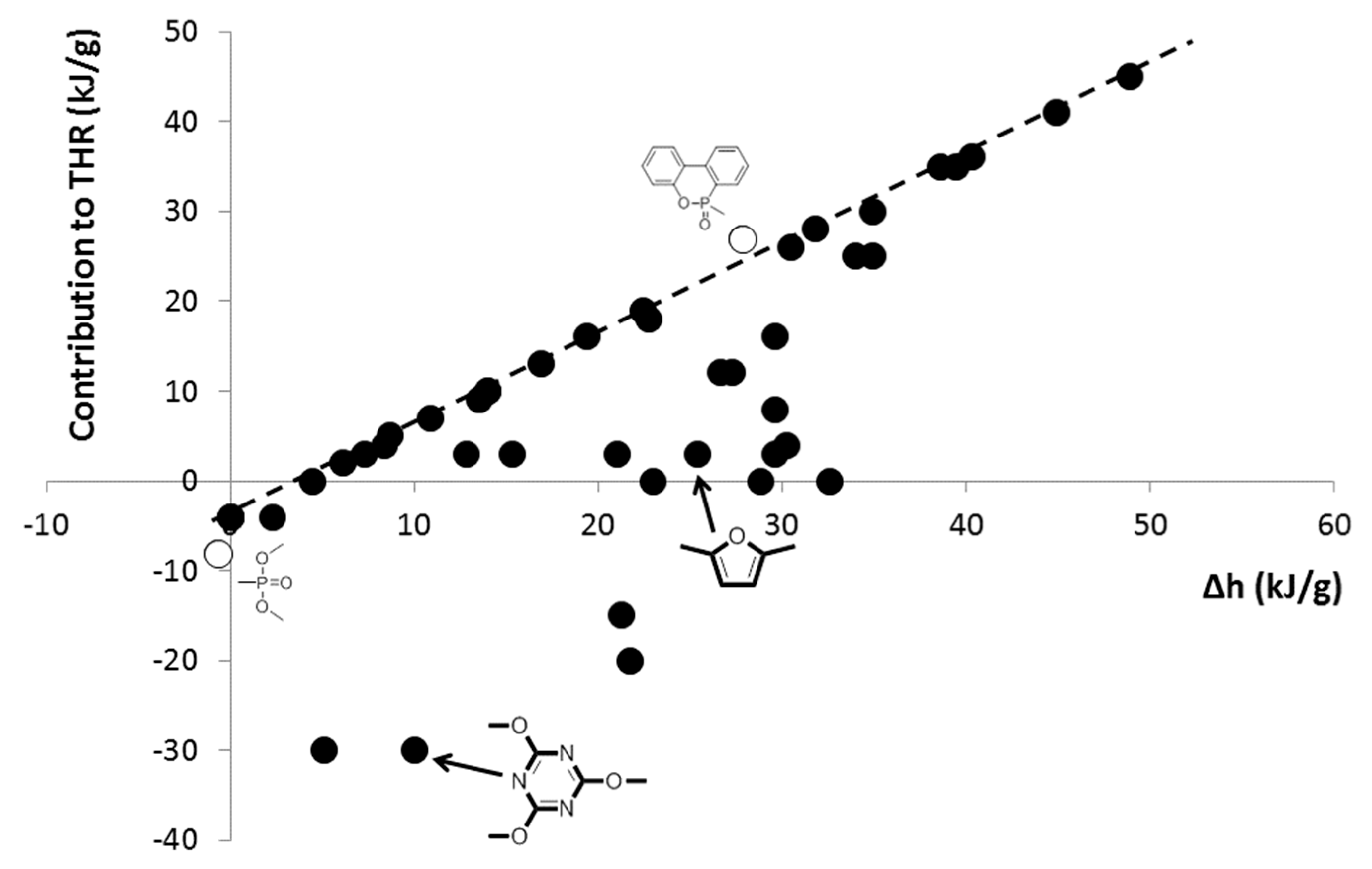
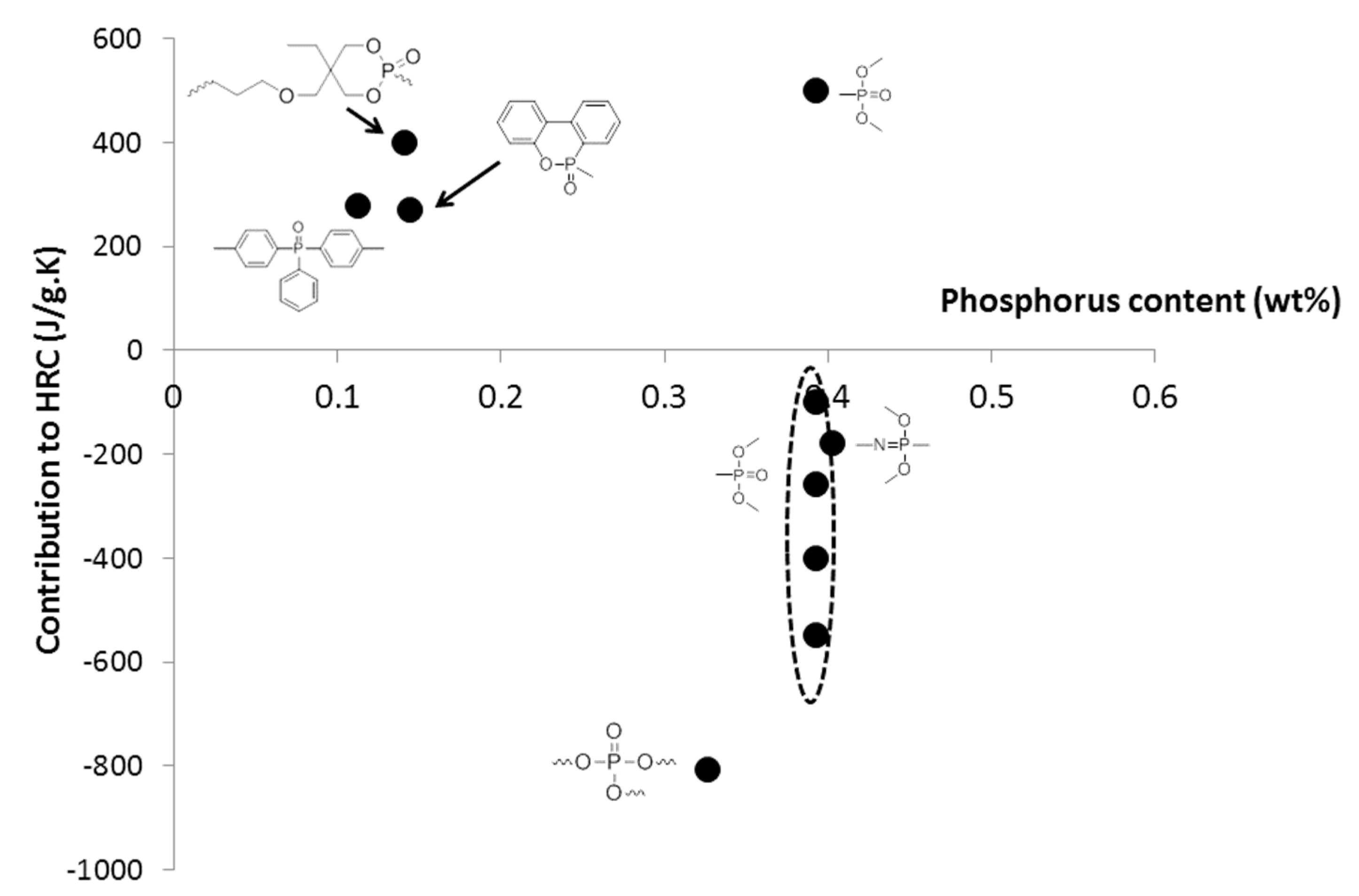
3.1. Cooperative Interactions
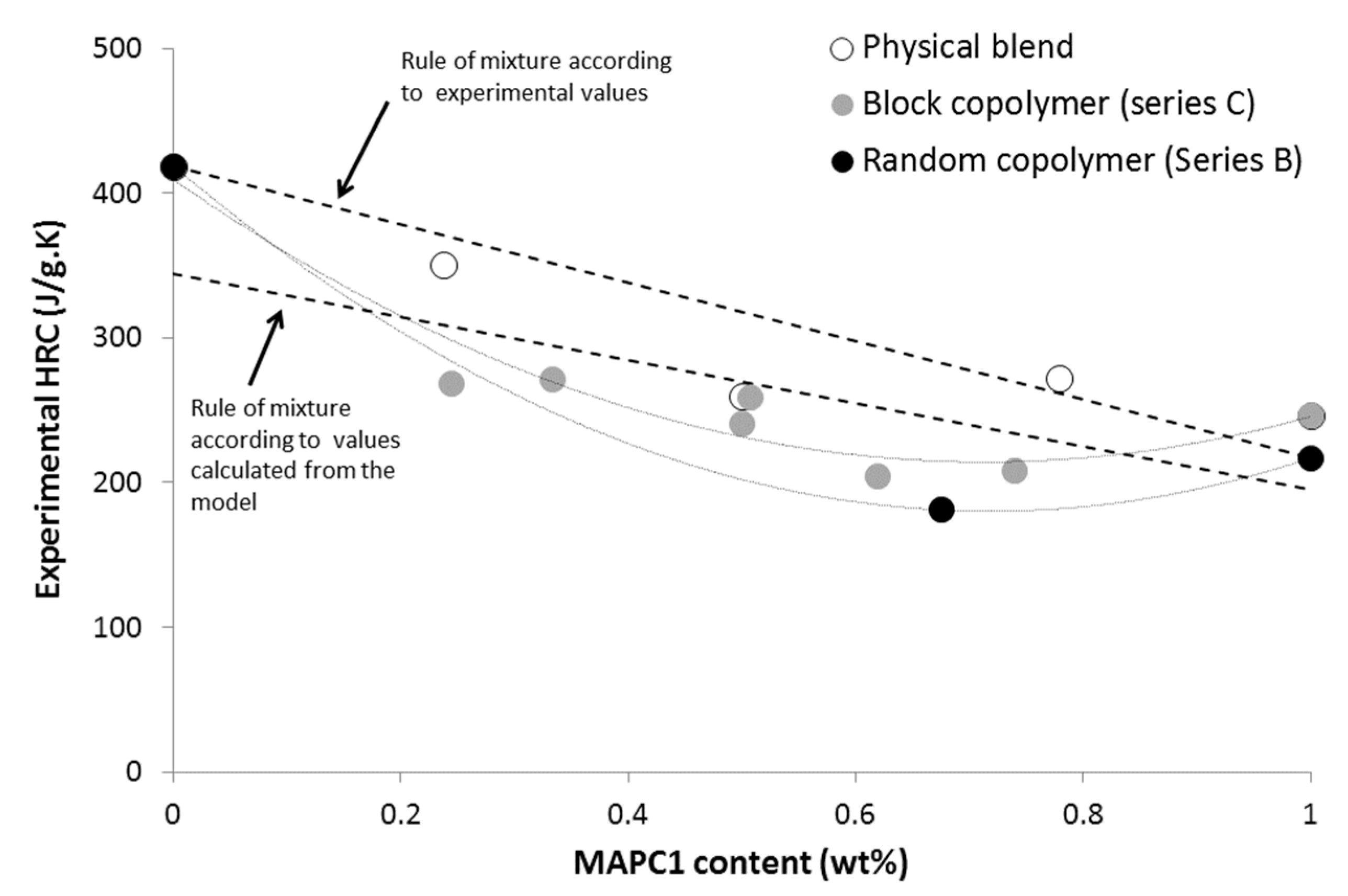
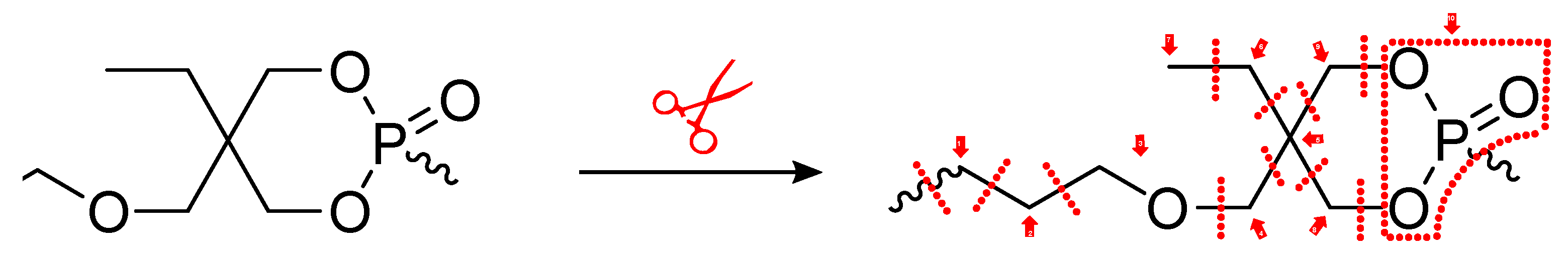
3.2. Influence of the Detailed Structure of Co-Polymers
3.3. Comparison of the Contributions Calculated from Other Works
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schartel, B. Phosphorus-based Flame Retardancy Mechanisms—Old Hat or a Starting Point for Future Development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Illy, N.; Fache, M.; Menard, R.; Negrell, C.; Caillol, S.; David, G. Phosphorylation of bio-based compounds: The state of the art. Polym. Chem. 2015, 35, 6257–6291. [Google Scholar] [CrossRef]
- Bauer, K.; Tee, H.; Velencoso, M.; Wurm, F. Main-chain poly(phosphoester)s: History, syntheses, degradation, bio-and flame-retardant applications. Prog. Polym. Sci. 2017, 73, 61–122. [Google Scholar] [CrossRef]
- Vahabi, H.; Sonnier, R.; Ferry, L. Effects of ageing on the fire behaviour of flame-retarded polymers: A review. Polym. Int. 2015, 64, 313–328. [Google Scholar] [CrossRef]
- Price, D.; Pyrah, K.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P.; Konkel, C.S. Flame retarding poly(methyl methacrylate) with phosphorus-containing compounds: Comparison of an additive with a reactive approach. Polym. Degrad. Stab. 2001, 74, 441–447. [Google Scholar] [CrossRef]
- Price, D.; Pyrah, K.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P. Flame retardance of poly(methyl methacrylate) modified with phosphorus-containing compounds. Polym. Degrad. Stab. 2002, 77, 227–233. [Google Scholar] [CrossRef]
- Price, D.; Cunliffe, L.K.; Bullett, K.J.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P. Thermal behavior of covalently bonded phosphonate flame-retarded poly(methyl methacrylate) systems. Polym. Adv. Technol. 2008, 19, 710–723. [Google Scholar] [CrossRef]
- Schartel, B.; Braun, U.; Balabanovich, A.I.; Artner, J.; Ciesielski, M.; Doring, M.; Perez, R.M.; Sandler, J.K.W.; Altstadt, V. Pyrolysis and fire behaviour of epoxy systems containing a novel 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide-(DOPO)-based diamino hardener. Eur. Polym. J. 2008, 44, 704–715. [Google Scholar] [CrossRef]
- Menard, R.; Negrell, C.; Ferry, L.; Sonnier, R.; David, G. Synthesis of biobased phosphorus-containing flame retardants for epoxy thermosets comparison of additive and reactive approaches. Polym. Degrad. Stab. 2015, 120, 300–312. [Google Scholar] [CrossRef]
- Sonnier, R.; Ferry, L.; Lopez-Cuesta, J.M. Flame Retardancy of Phosphorus-Containing Polymers. In Phosphorus-Based Polymers: From Synthesis to Applications; Royal Society of Chemistry: London, UK, 2014. [Google Scholar]
- Hergenrother, P.M.; Thompson, C.M.; Smith, J.G., Jr.; Connell, J.W.; Hinkley, J.A.; Lyon, R.E.; Moulton, R. Flame retardant aircraft epoxy resins containing phosphorus. Polymer 2005, 46, 5012–5024. [Google Scholar] [CrossRef]
- Braun, U.; Balabanovich, A.I.; Schartel, B.; Knoll, U.; Artner, J.; Ciesielski, M.; Döring, M.; Perez, R.; Sandler, J.K.; Altstädt, V. Influence of the oxidation state of phosphorus on the decomposition and fire behaviour of flame-retarded epoxy resin composites. Polymer 2006, 47, 8495–8508. [Google Scholar] [CrossRef]
- Lorenzetti, A.; Modesti, M.; Besco, S.; Hrelja, D.; Donadi, S. Influence of phosphorus valency on thermal behaviour of flame retarded polyurethane foams. Polym. Degrad. Stab. 2011, 96, 1455–1461. [Google Scholar] [CrossRef]
- Mariappan, T.; Zhou, Y.; Hao, J.; Wilkie, C.A. Influence of oxidation state of phosphorus on the thermal and flammability of polyurea and epoxy resin. Eur. Polym. J. 2013, 49, 3171–3180. [Google Scholar] [CrossRef]
- Rabe, S.; Chuenban, Y.; Schartel, B. Exploring the modes of action of phosphorus-based flame retardants in polymeric systems. Materials 2017, 10, 455. [Google Scholar] [CrossRef]
- Lyon, R.E.; Speitel, L.; Walters, R.N.; Crowley, S. Fire-resistant elastomers. Fire Mater. 2003, 27, 195–208. [Google Scholar] [CrossRef]
- Dumitrascu, A.; Howell, B.A. Flame retardant polymeric materials achieved by incorporation of styrene monomers containing both nitrogen and phosphorus. Polym. Degrad. Stab. 2012, 97, 2611–2618. [Google Scholar] [CrossRef]
- Joseph, P.; Tretsiakova-McNally, S. Combustion behaviours of chemically modified polyacrylonitrile polymers containing phosphorylamino groups. Polym. Degrad. Stab. 2012, 97, 2531–2535. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Y.Z.; Ban, D.M.; Liu, X.H.; Zhou, Q. Burning behavior and pyrolysis products of flame-retardant PET containing sulfur-containing aryl polyphosphonate. J. Anal. Appl. Pyrolysis 2006, 76, 198–202. [Google Scholar] [CrossRef]
- Howell, B.; Daniel, Y. The impact of sulfur oxidation level on flame retardancy. J. Fire Sci. 2018, 36, 518–534. [Google Scholar] [CrossRef]
- Chang, T.; Chen, Y.; Ho, S.; Chiu, Y. The effect of silicon and phosphorus on the degradation of poly (methyl methacrylate). Polymer 1996, 37, 2963–2968. [Google Scholar] [CrossRef]
- Sponton, M.; Mercado, L.; Ronda, J.; Galia, M.; Cadiz, V. Preparation, thermal properties and flame retardancy of phosphorus-and silicon-containing epoxy resins. Polym. Degrad. Stab. 2008, 93, 2025–2031. [Google Scholar] [CrossRef]
- Cochez, M.; Ferriol, M.; Weber, J.; Chaudron, P.; Oget, N.; Mieloszynski, J. Thermal degradation of methyl methacrylate polymers functionalized by phosphorus-containing molecules I. TGA/FT–IR experiments on polymers with the monomeric formula CH2C (CH3) C (O) OCHRP (O)(OC2H5) 2 (R= H,(CH2) 4CH3, C6H5Br, C10H7). Polym. Degrad. Stab. 2000, 70, 455–462. [Google Scholar] [CrossRef]
- Troev, K.; Kisiova, T.; Grozeva, A.; Borisov, G. Phosphorus-and metal-containing poly (ethylene terephthalate)—1. Phosphorus-and calcium-containing polymer. Eur. Polym. J. 1993, 29, 1205–1209. [Google Scholar] [CrossRef]
- Troev, K.; Kisiova, T.; Grozeva, A.; Borisov, G. Phosphorus-and metal-containing poly (ethylene terephthalate)—2. Phosphorus-, calcium-and chlorine-containing polymer. Eur. Polym. J. 1993, 29, 1211–1215. [Google Scholar] [CrossRef]
- Troev, K.; Kisiova, T.; Grozeva, A.; Borisov, G. Synthesis of phosphorus-, zinc-, and chlorine-containing poly (ethylene terephthalate). J. Appl. Polym. Sci. 1993, 49, 777–784. [Google Scholar] [CrossRef]
- Liu, Y.L. Flame-retardant epoxy resins from novel phosphorus-containing novolac. Polymer 2001, 42, 3445–3454. [Google Scholar] [CrossRef]
- Liu, Y.L.; Tsai, S.H. Synthesis and properties of new organosoluble aromatic polyamides with cyclic bulky groups containing phosphorus. Polymer 2002, 43, 5757–5762. [Google Scholar] [CrossRef]
- Sablong, R.; Duchateau, R.; Koning, C.E.; Pospiech, D.; Korwitz, A.; Komber, H.; Starke, S.; Häußler, L.; Jehnichen, D.; der Landwehr, M.A. Incorporation of a flame retardancy enhancing phosphorus-containing diol into poly (butylene terephthalate) via solid state polycondensation: A comparative study. Polym. Degrad. Stab. 2011, 96, 334–341. [Google Scholar] [CrossRef]
- Petreus, O.; Avram, E.; Serbezeanu, D. Synthesis and characterization of phosphorus-containing polysulfone. Polym. Eng. Sci. 2010, 50, 48–56. [Google Scholar] [CrossRef]
- Negrell, C.; Frénéhard, O.; Sonnier, R.; Dumazert, L.; Briffaud, T.; Flat, J.J. Self-extinguishing bio-based polyamides. Polym. Degrad. Stab. 2016, 134, 10–18. [Google Scholar] [CrossRef]
- Chang, S.J.; Sheen, Y.C.; Chang, R.S.; Chang, F.C. The thermal degradation of phosphorus-containing copolyesters. Polym. Degrad. Stab. 1996, 54, 365–371. [Google Scholar] [CrossRef]
- Liu, Y.L.; Hsiue, G.H.; Lan, C.W.; Chiu, Y.S. Flame-retardant polyurethanes from phosphorus-containing isocyanates. J. Polym. Sci. Part A Polym. Chem. 1997, 35, 1769–1780. [Google Scholar] [CrossRef]
- Tibiletti, L.; Ferry, L.; Longuet, C.; Mas, A.; Robin, J.J.; Lopez-Cuesta, J.M. Thermal degradation and fire behavior of thermoset resins modified with phosphorus containing styrene. Polym. Degrad. Stab. 2012, 97, 2602–2610. [Google Scholar] [CrossRef]
- Vahabi, H.; Ferry, L.; Longuet, C.; Sonnier, R.; Negrell-Guirao, C.; David, G.; Lopez-Cuesta, J.M. Theoretical and empirical approaches to understanding the effect of phosphonate groups on the thermal degradation for two chemically modified PMMA. Eur. Polym. J. 2012, 48, 604–612. [Google Scholar] [CrossRef]
- Ménard, R.; Negrell, C.; Fache, M.; Ferry, L.; Sonnier, R.; David, G. From a bio-based phosphorus-containing epoxy monomer to fully bio-based flame-retardant thermosets. RSC Adv. 2015, 5, 70856–70867. [Google Scholar] [CrossRef]
- Dumitrascu, A.; Howell, B.A. Flame-retarding vinyl polymers using phosphorus-functionalized styrene monomers. Polym. Degrad. Stab. 2011, 96, 342–349. [Google Scholar] [CrossRef]
- Sonnier, R.; Otazaghine, B.; Iftene, F.; Negrell, C.; David, G.; Howell, B.A. Predicting the flammability of polymers from their chemical structure: An improved model based on group contributions. Polymer 2016, 86, 42–55. [Google Scholar] [CrossRef]
- Sonnier, R.; Otazaghine, B.; Dumazert, L.; Ménard, R.; Viretto, A.; Dumas, L.; Bonnaud, L.; Dubois, P.; Safronava, N.; Walters, R. Prediction of thermosets flammability using a model based on group contributions. Polymer 2017, 127, 203–213. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 1990; pp. 627–653. [Google Scholar]
- Lyon, R.E.; Takemori, M.T.; Safronava, N.; Stoliarov, S.I.; Walters, R.N. A molecular basis for polymer flammability. Polymer 2009, 50, 2608–2617. [Google Scholar] [CrossRef]
- Walters, R.N.; Lyon, R.E. Molar group contributions to polymer flammability. J. Appl. Polym. Sci. 2003, 87, 548–563. [Google Scholar] [CrossRef]
- Sonnier, R.; Negrell-Guirao, C.; Vahabi, H.; Otazaghine, B.; David, G.; Lopez-Cuesta, J.M. Relationships between the molecular structure and the flammability of polymers: Study of phosphonate functions using microscale combustion calorimeter. Polymer 2012, 53, 1258–1266. [Google Scholar] [CrossRef]
- Bier, F.; Six, J.L.; Durand, A. DOPO-Based Phosphorus-Containing Methacrylic (Co)Polymers: Glass Transition Temperature Investigation. Macromol. Mater. Eng. 2019, 304, 1800645–1800655. [Google Scholar] [CrossRef]
- Atherton, F.; Openshaw, H.; Todd, A. Studies on phosphorylation. 2. the reaction of dialkyl phosphites with polyhalogen compounds in presence of bases-a new method for the phosphorylation of amines. J. Chem. Soc. 1945, 660–663. [Google Scholar] [CrossRef]
- Atherton, F.; Todd, A. 129. Studies on phosphorylation. Part III. Further observations on the reaction of phosphites with polyhalogen compounds in presence of bases and its application to the phosphorylation of alcohols. J. Chem. Soc. 1947, 674–678. [Google Scholar] [CrossRef]
- Canniccioni, B. Synthèse et caractérisation de copolymères à blocs amphiphiles et doubles hydrophiles à base de phosphore par polymérisation radicalaire contrôlée RAFT. Ph.D. Thesis, Montpellier University 2, Montpellier, France, 2013. [Google Scholar]
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Walters, R.N.; Safronava, N.; Lyon, R.E. A microscale combustion calorimeter study of gas phase combustion of polymers. Combust. Flame 2015, 162, 855–863. [Google Scholar] [CrossRef]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Correa, S.M. A review of NOx formation under gas-turbine combustion conditions. Combust. Sci. Technol. 1993, 87, 329–362. [Google Scholar] [CrossRef]
- Walters, R.N.; Hackett, S.M.; Lyon, R.E. Heats of combustion of high temperature polymers. Fire Mater. 2000, 24, 245–252. [Google Scholar] [CrossRef]
- Sonnier, R.; Otazaghine, B.; Ferry, L.; Lopez-Cuesta, J.M. Study of the combustion efficiency of polymers using a pyrolysis–combustion flow calorimeter. Combust. Flame 2013, 160, 2182–2193. [Google Scholar] [CrossRef]
- Sonnier, R.; Dorez, G.; Vahabi, H.; Longuet, C.; Ferry, L. FTIR–PCFC coupling: A new method for studying the combustion of polymers. Combust. Flame 2014, 161, 1398–1407. [Google Scholar] [CrossRef]
- Lewin, M. Synergism and catalysis in flame retardancy of polymers. Polym. Adv. Technol. 2001, 12, 215–222. [Google Scholar] [CrossRef]
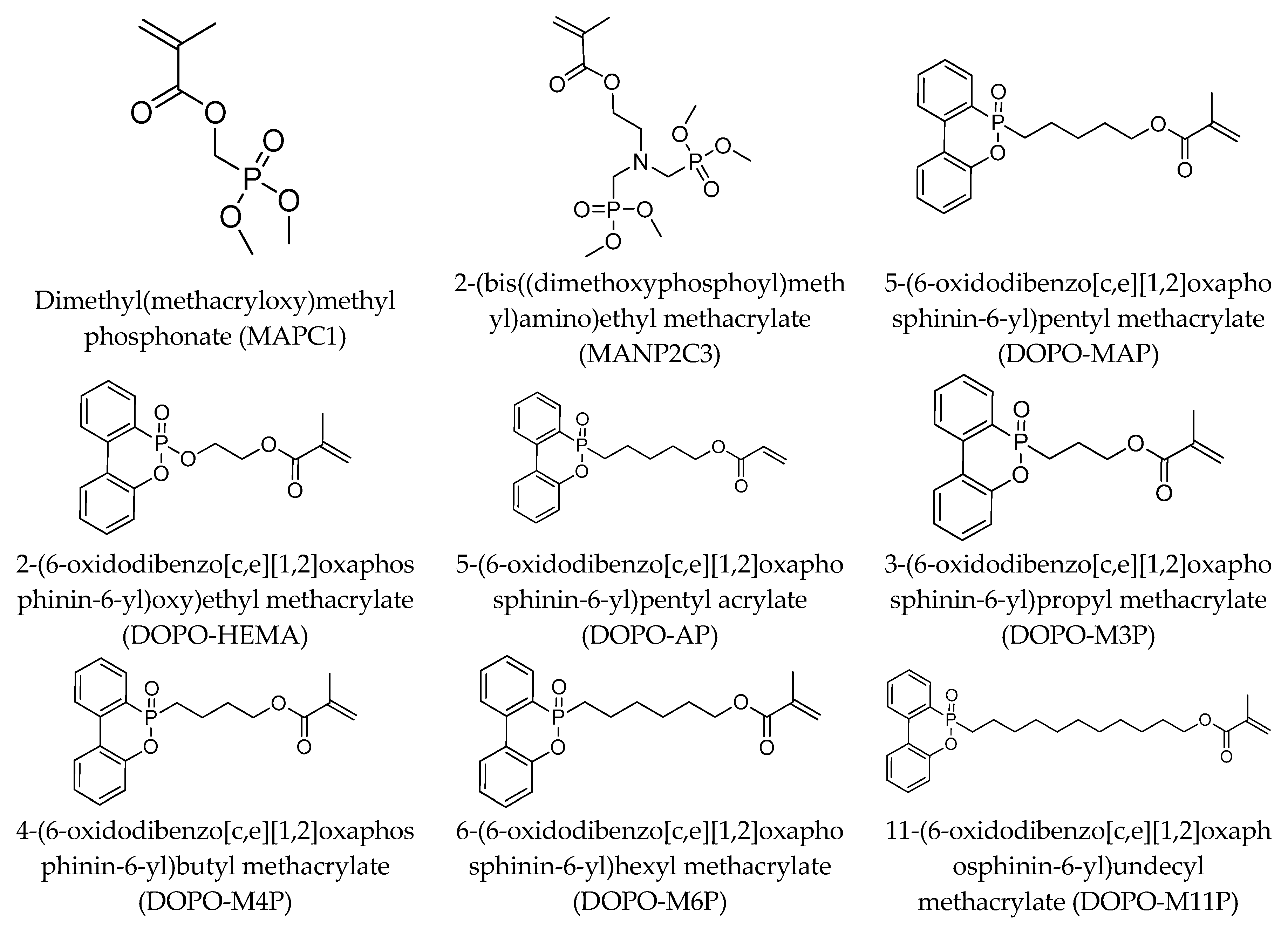
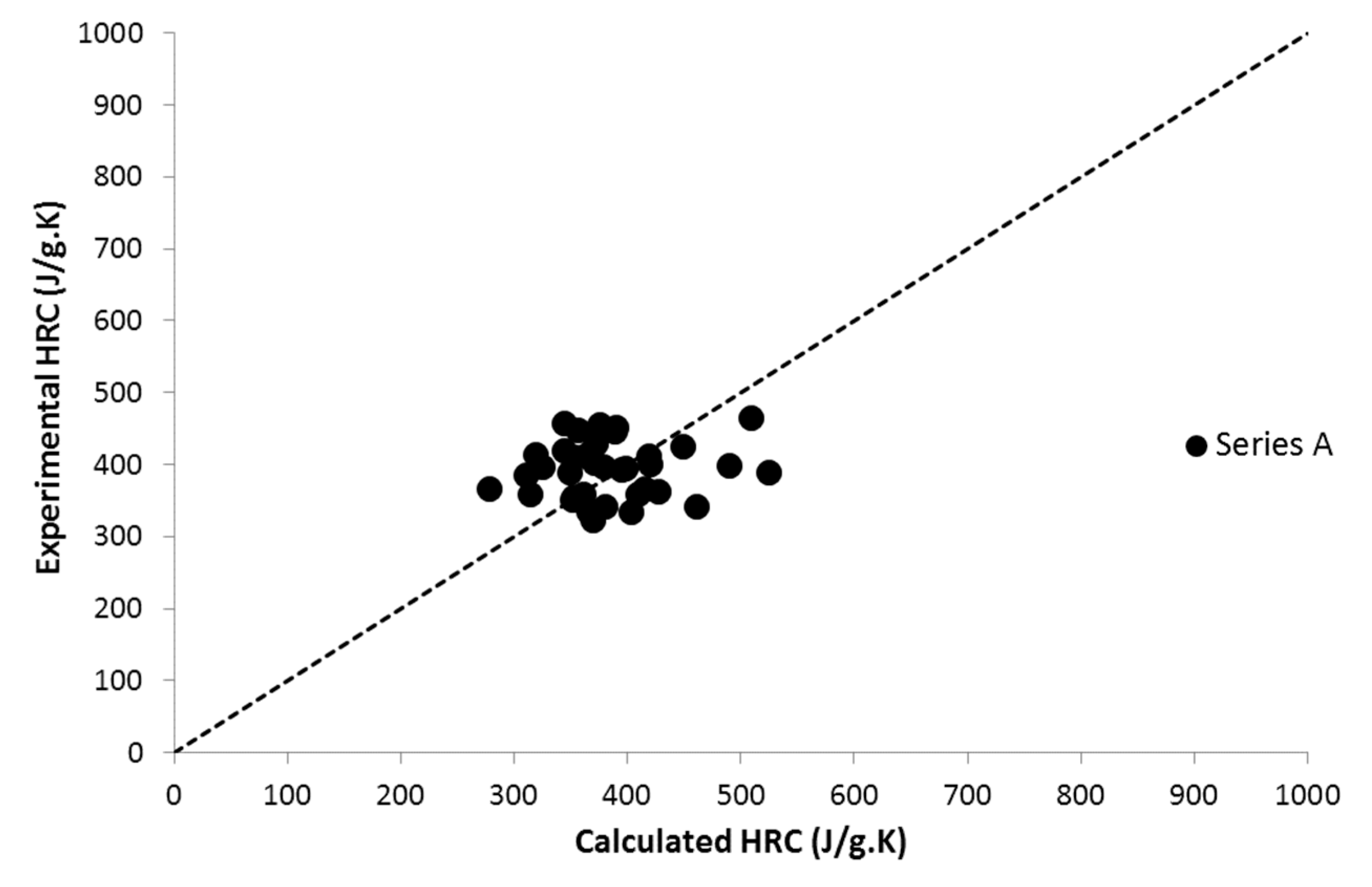

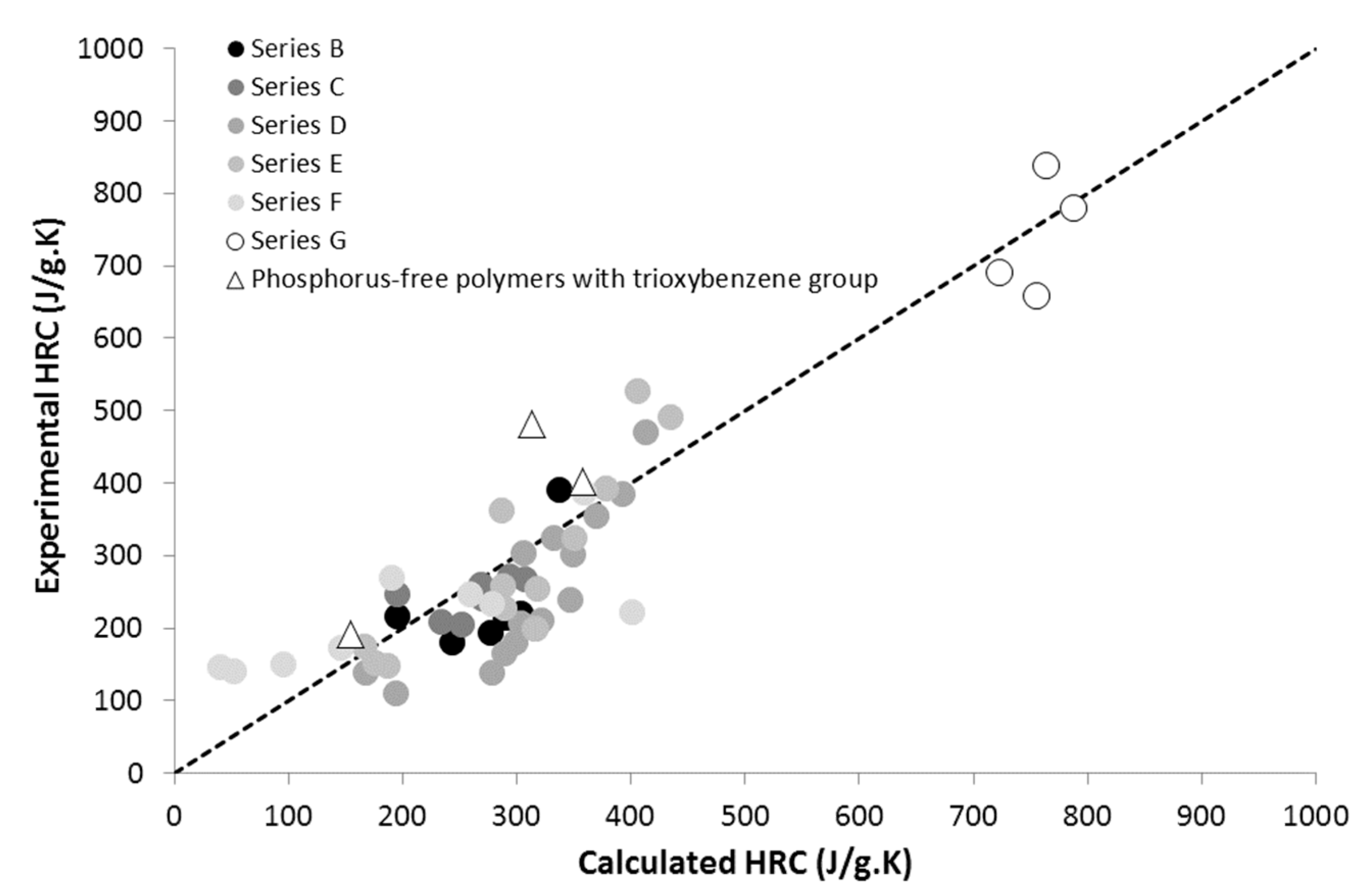

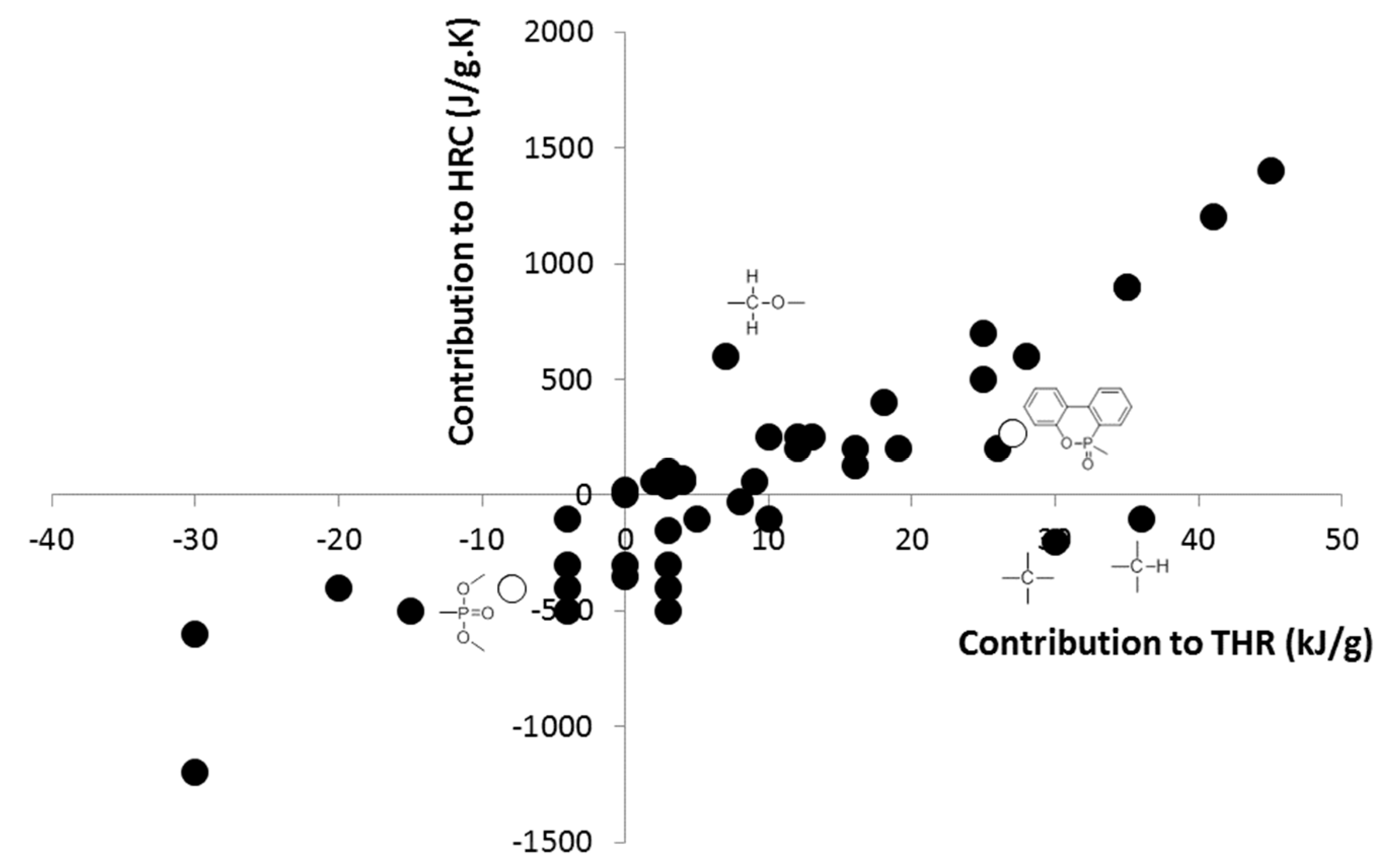



| Series | Description | Number of Tested Polymers | Structure and Content (wt.%) of the Phosphorus Group | Reference |
|---|---|---|---|---|
| A | DOPO-containing acrylate and methacrylate co-polymers | 35 | DOPO Up to 63 wt.% | This work |
| B | MAPC1 and MANP2C3-containing methacrylate random co-polymers | 6 | PO3 Up to 41 wt.% | [35] |
| C | MAPC1-containing methacrylate block co-polymers | 7 | PO3 Up to 38 wt.% | [47] |
| D | Phosphonate-containing epoxy thermosets (including trioxybenzene group **) | 17 | PO3 Up to 14 wt.% | [36] |
| E | Phosphonate and sulfur-containing epoxy thermosets (including trioxybenzene and methylene sulfide groups **) | 12 | PO3 Up to 8 wt.% | [9] |
| F | Phosphonate-containing co-polymers | 9 | PO3 Up to 60 wt.% | [38] |
| G | Polymers from phosphorus-modified styrene monomers | 7 | PO3 Up to 35 wt.% | [17,37] |
| H * | Polymers from phosphorus-modified styrene monomers | 4 | NHPO and NHPO3 Up to 39 wt.% | [17] |
| Group | Number of Polymers | Molar Mass (g/mol) | Contribution to | |||
|---|---|---|---|---|---|---|
| THR (kJ/g) | HRC (J/g∙K) | Δh (kJ/g) | Char (g/g) | |||
 | 35 | 215 | 27 | 270 | 27.8 | 0.02 |
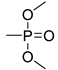 | 57 | 79 | -8 | −400 | -0.7 | 0.20 |
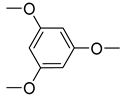 | 29 | 123 | 0 | −350 | 23 | 0.62 |
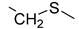 | 12 | 46 | 5 | −300 | 22.8 | 0.48 |
| Contribution to THR (kJ/g) | Contribution to HRC (J/g∙K) | Reference | |
|---|---|---|---|
 | −8 | −400 | This work |
| −5 | −100 | [38] | |
| −0.2 * | −258 | [35] | |
| 1.4 * | −549 | [35] | |
| 0.5 | 500 | [43] | |
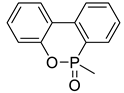 | 27 | 270 | This work |
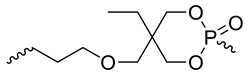 | 20 | 400 | [43] |
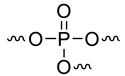 | / | -807 | [42] |
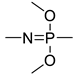 | / | −179 | [42] |
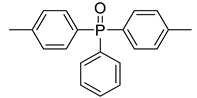 | / | 279 | [42] |
 | −17 | / | This work |
 | −10 | / | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonnier, R.; Otazaghine, B.; Vagner, C.; Bier, F.; Six, J.-L.; Durand, A.; Vahabi, H. Exploring the Contribution of Two Phosphorus-Based Groups to Polymer Flammability via Pyrolysis–Combustion Flow Calorimetry. Materials 2019, 12, 2961. https://doi.org/10.3390/ma12182961
Sonnier R, Otazaghine B, Vagner C, Bier F, Six J-L, Durand A, Vahabi H. Exploring the Contribution of Two Phosphorus-Based Groups to Polymer Flammability via Pyrolysis–Combustion Flow Calorimetry. Materials. 2019; 12(18):2961. https://doi.org/10.3390/ma12182961
Chicago/Turabian StyleSonnier, Rodolphe, Belkacem Otazaghine, Christelle Vagner, Frédéric Bier, Jean-Luc Six, Alain Durand, and Henri Vahabi. 2019. "Exploring the Contribution of Two Phosphorus-Based Groups to Polymer Flammability via Pyrolysis–Combustion Flow Calorimetry" Materials 12, no. 18: 2961. https://doi.org/10.3390/ma12182961
APA StyleSonnier, R., Otazaghine, B., Vagner, C., Bier, F., Six, J. -L., Durand, A., & Vahabi, H. (2019). Exploring the Contribution of Two Phosphorus-Based Groups to Polymer Flammability via Pyrolysis–Combustion Flow Calorimetry. Materials, 12(18), 2961. https://doi.org/10.3390/ma12182961









