Continuous Preparation and Properties of Silica/Rubber Composite Using Serial Modular Mixing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Formulas
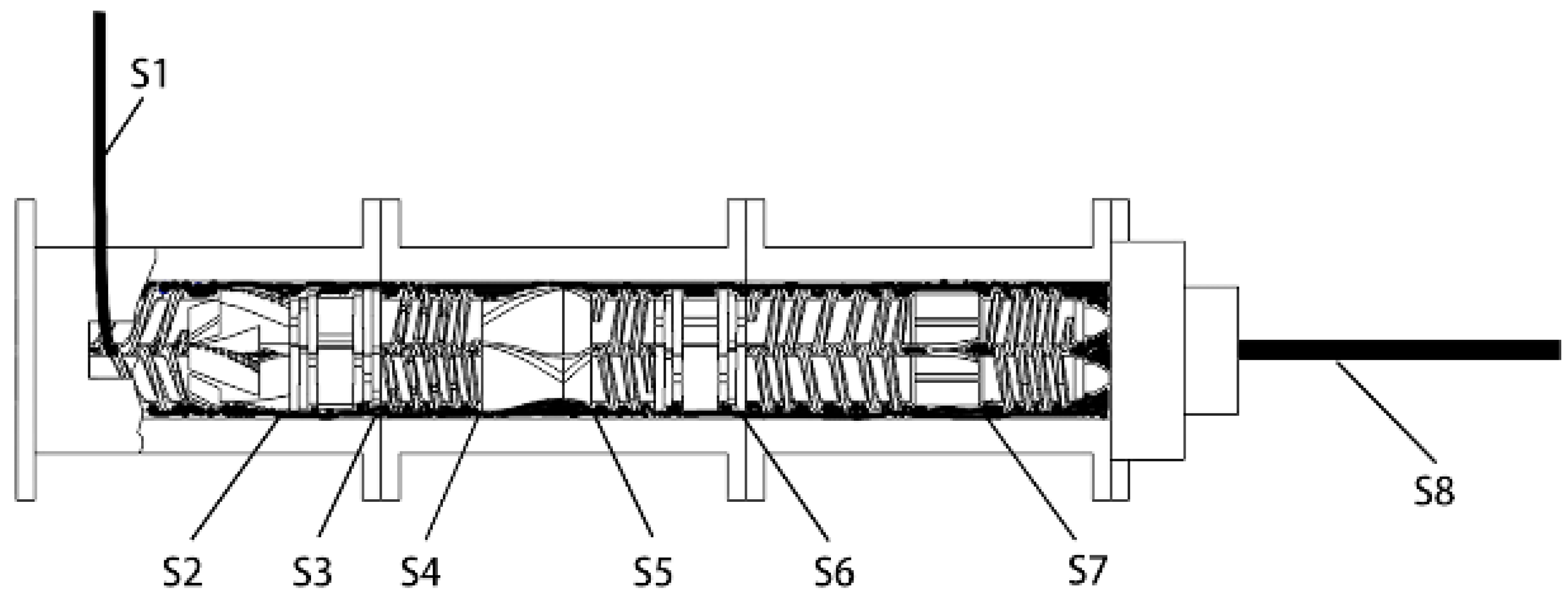
2.2. Equipment
2.3. Sample Preparation
2.3.1. Cooling Visualization Experiment
2.3.2. Comparison Experiment
- (i)
- Add rubber and mix for 30 s.
- (ii)
- Add carbon black, 1/2 silica, and the rest additives, then mix for 30 s.
- (iii)
- Add the rest of the silica, and mix to 110 °C.
- (iv)
- Sweep and add oil.
- (v)
- When the temperature reaches 125 °C and 140 °C, up and down the ram.
- (vi)
- Mix to 155 °C and then drop.
3. Results and Discussion
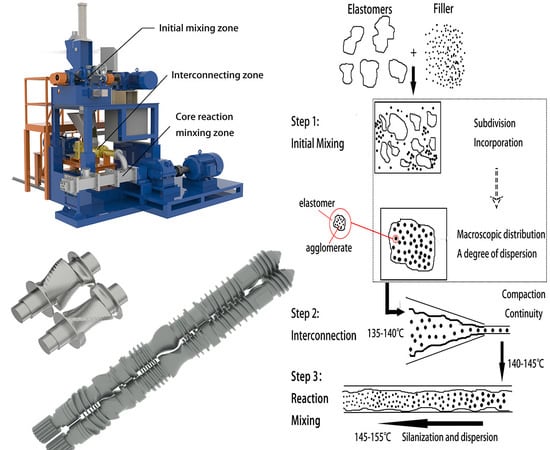
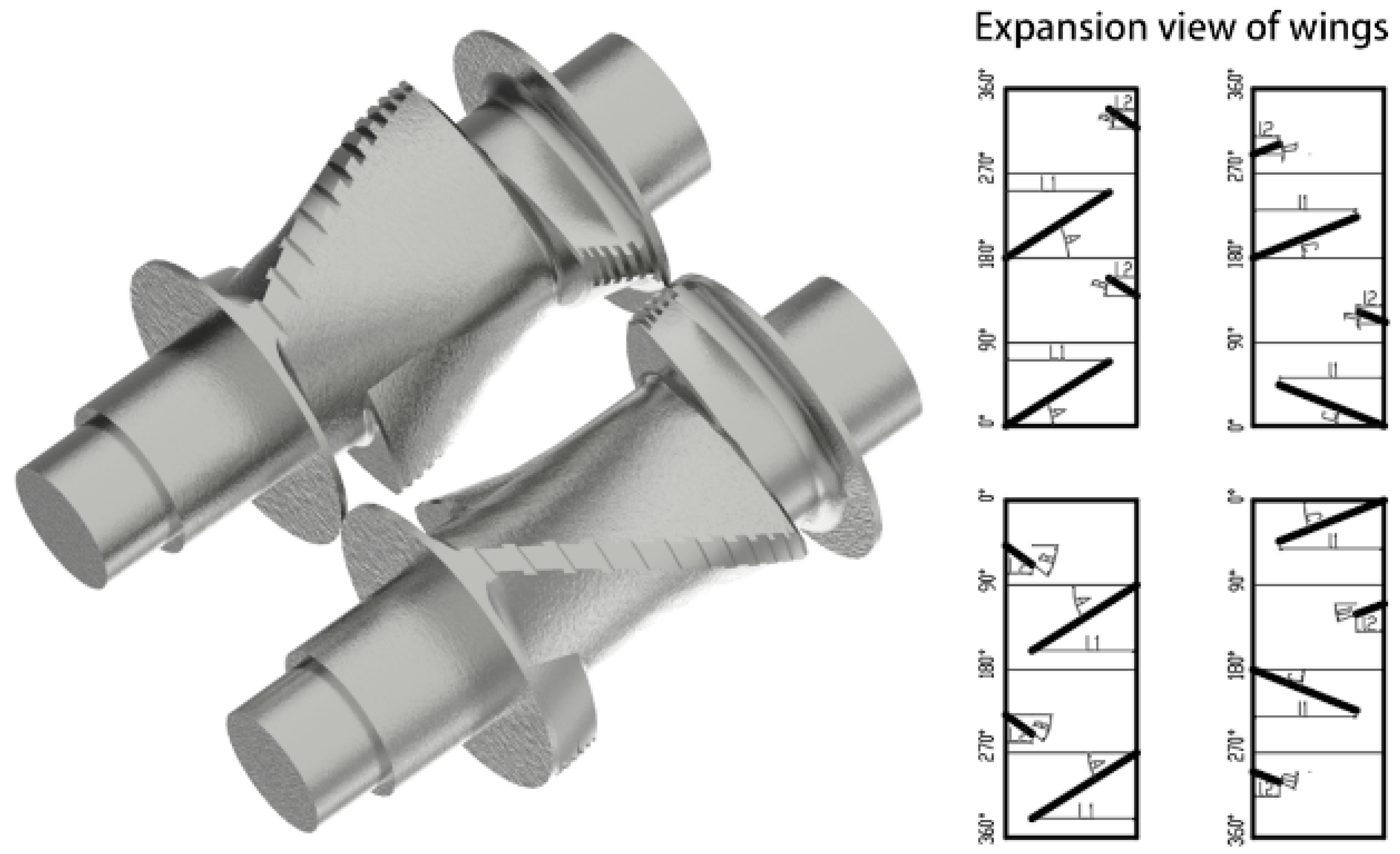
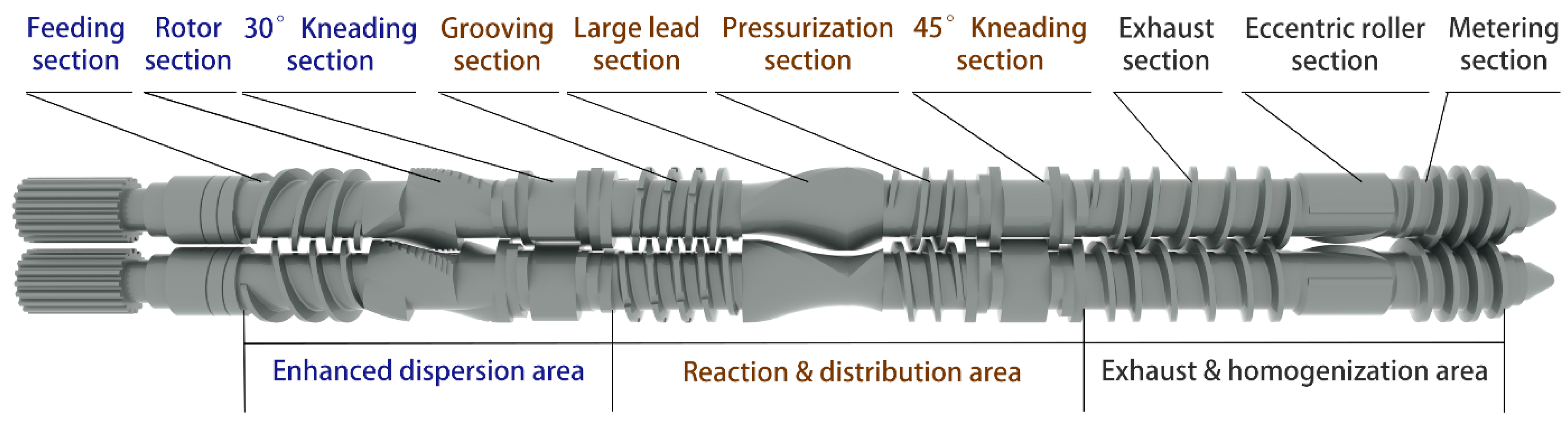
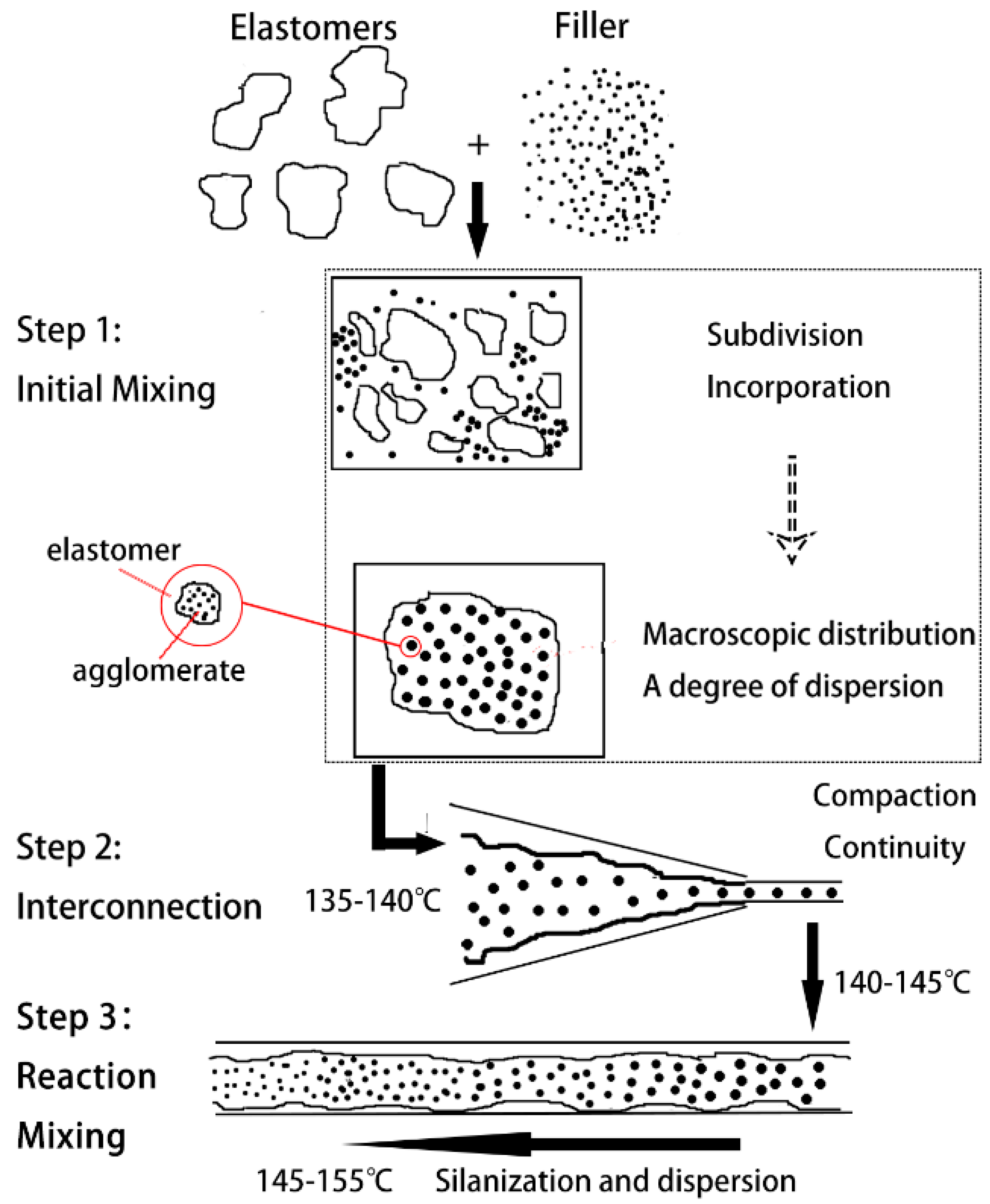
3.1. Mechanism of Serial Modular Continuous Mixing
3.2. Mixing Effect of Core Reaction Mixing Zone
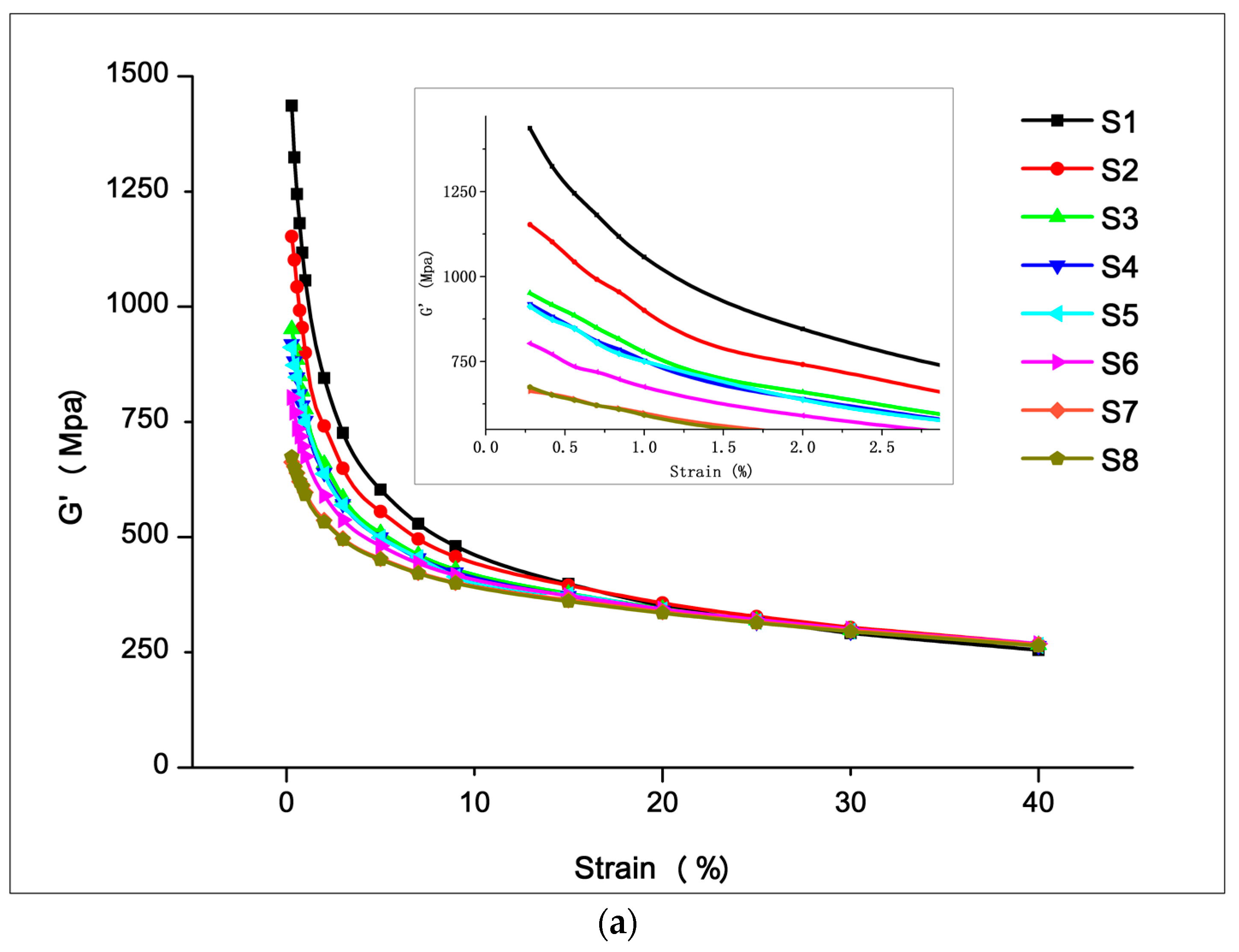
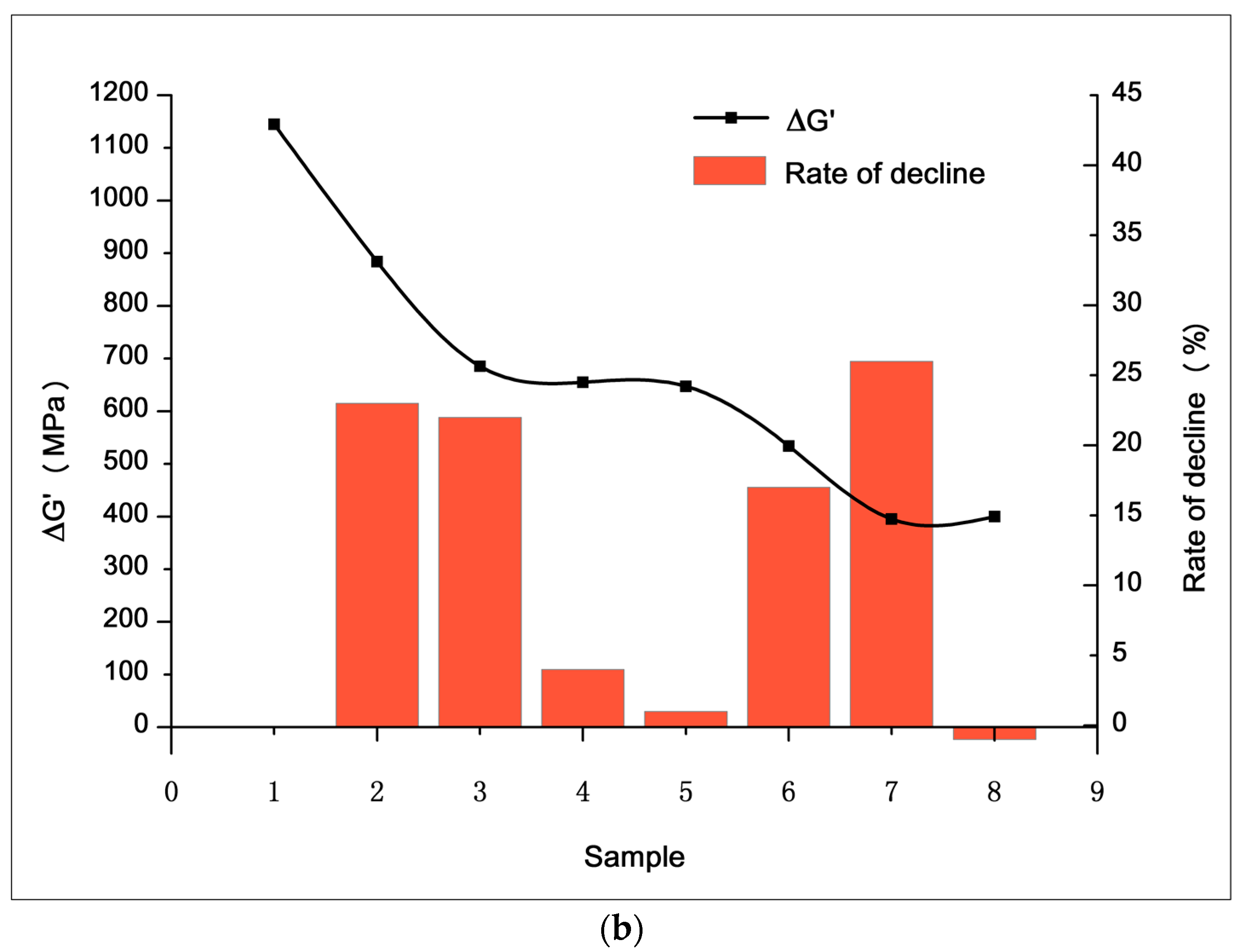
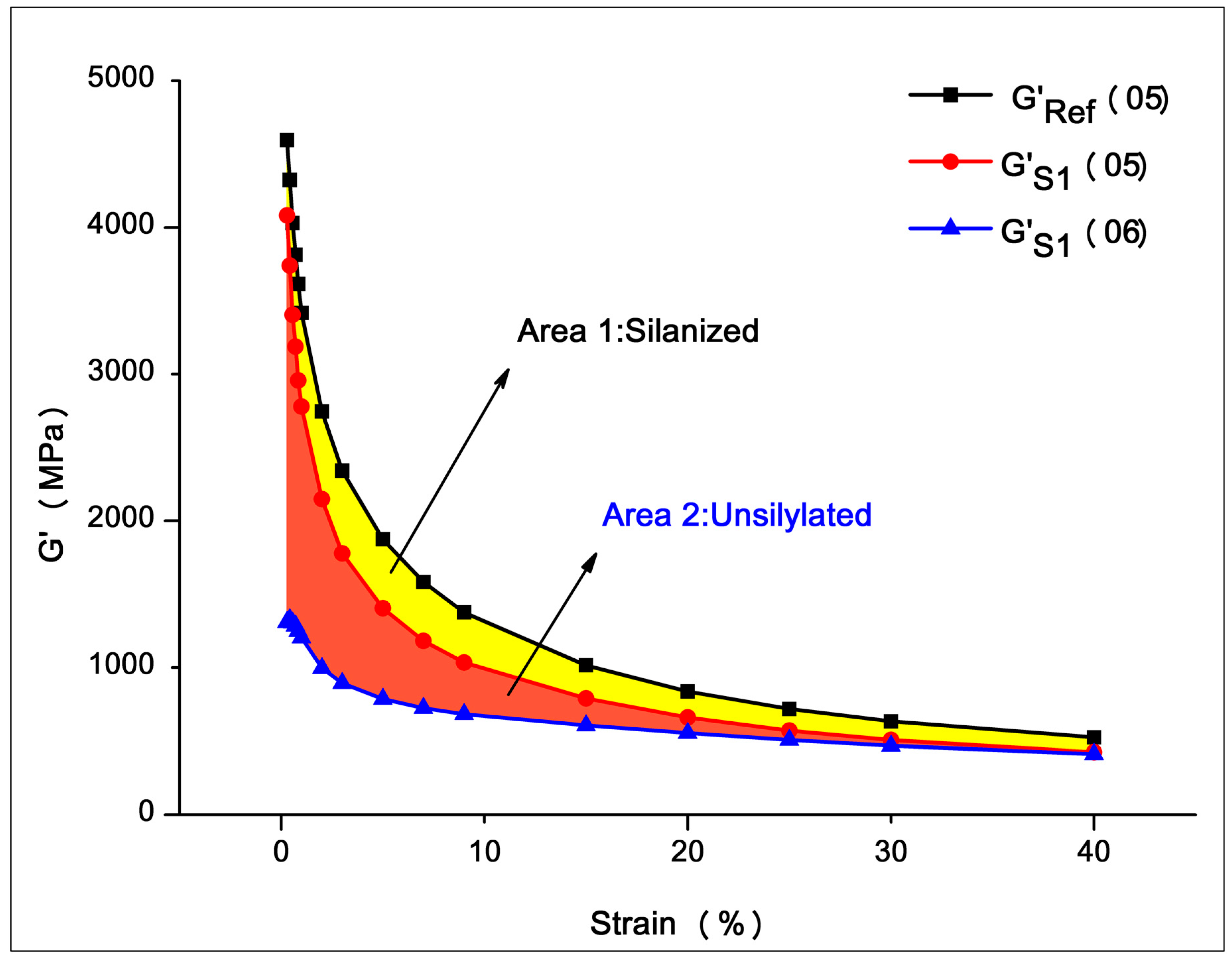
3.2.1. Filler Network (Payne Effect)
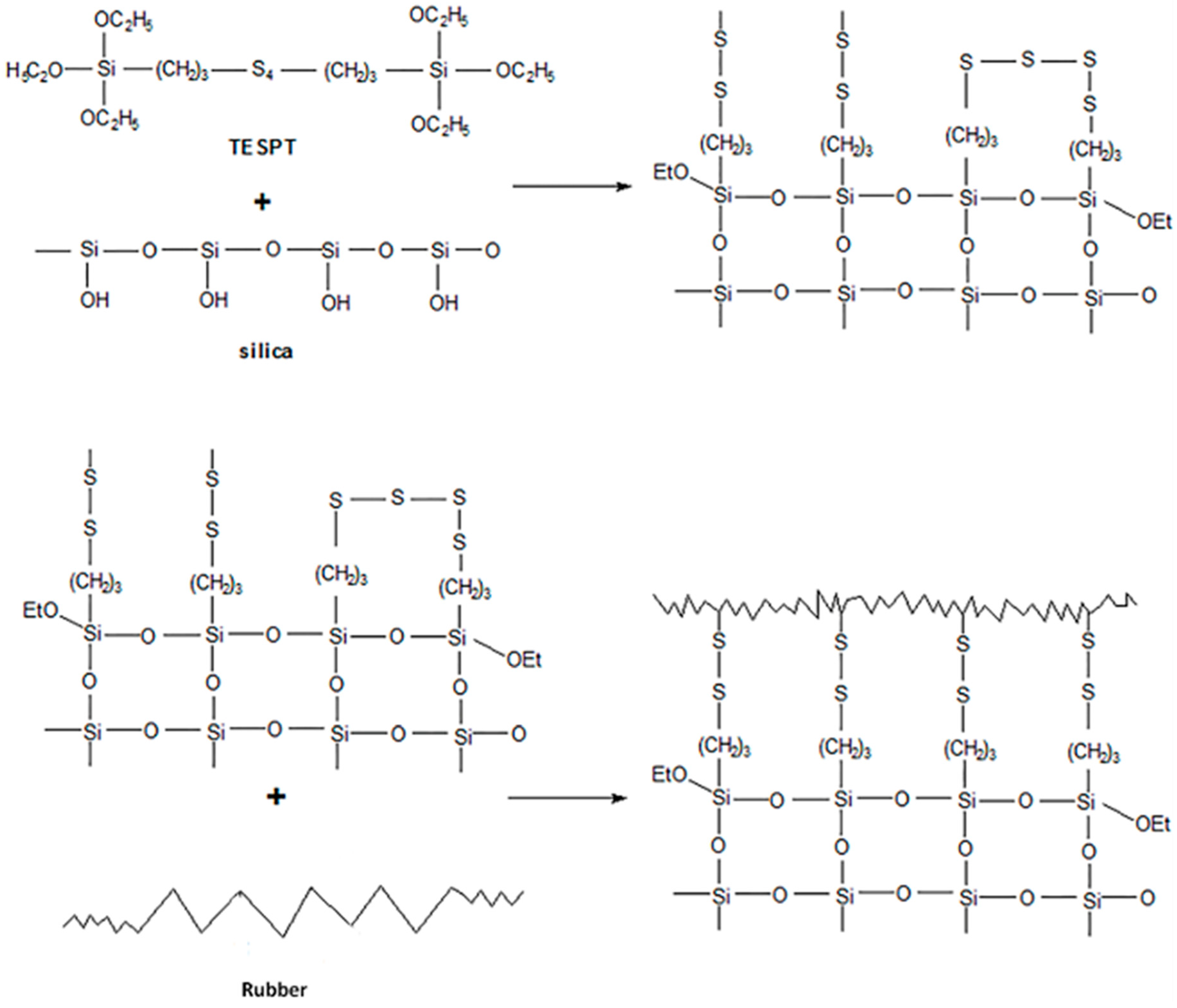
3.2.2. Degree of Silanization Reaction
- (1)
- As the compound advanced through the core reaction mixing zone, the silanization reaction degree increased before remaining steady at a higher level. To some extent, the core reaction mixing zone promoted the silanization reaction.
- (2)
- Silanization did not occur continuously and stably in the core mixing zone, and was affected by the mixing element.
3.3. Comparison of Vulcanization Properties: Serial Mixing and Two-Stage Mixing
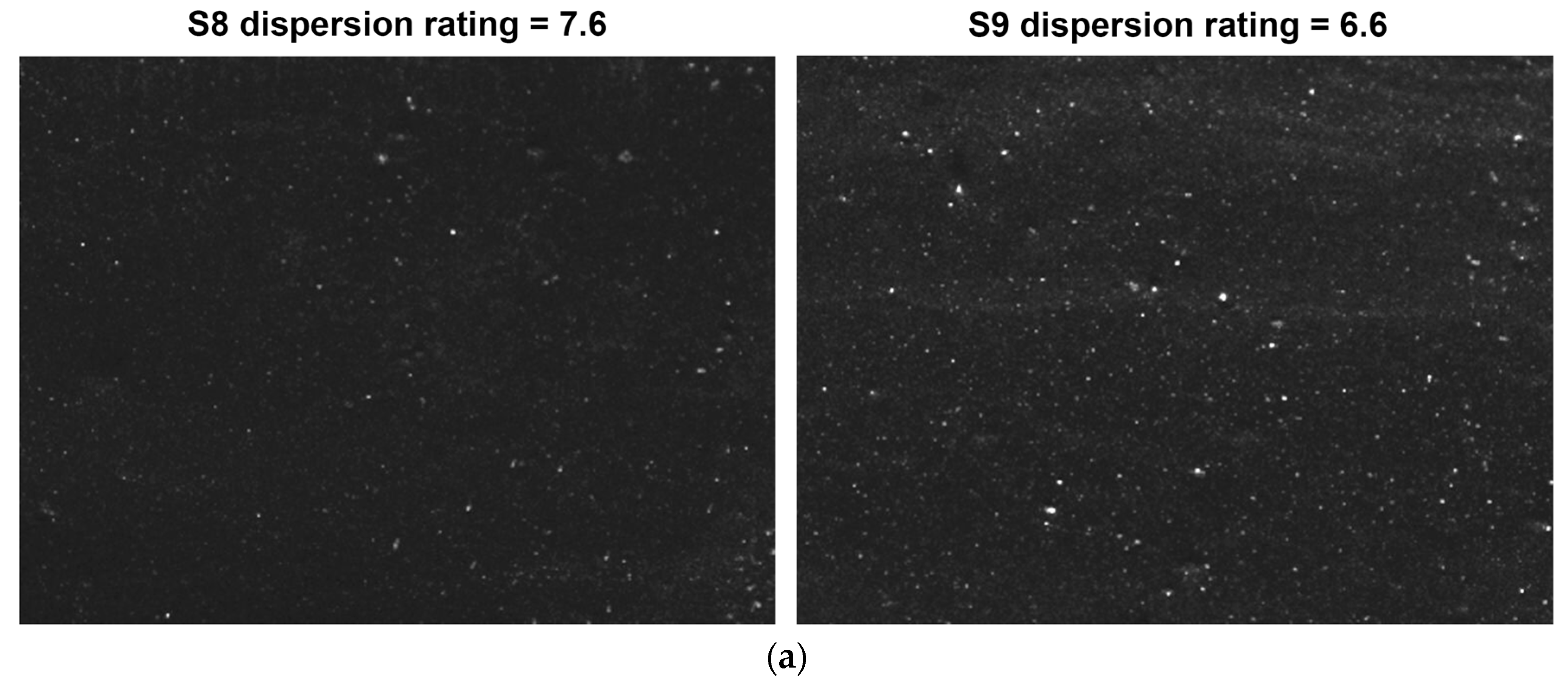
3.3.1. Filler Dispersion
3.3.2. Mechanical and Machining Properties
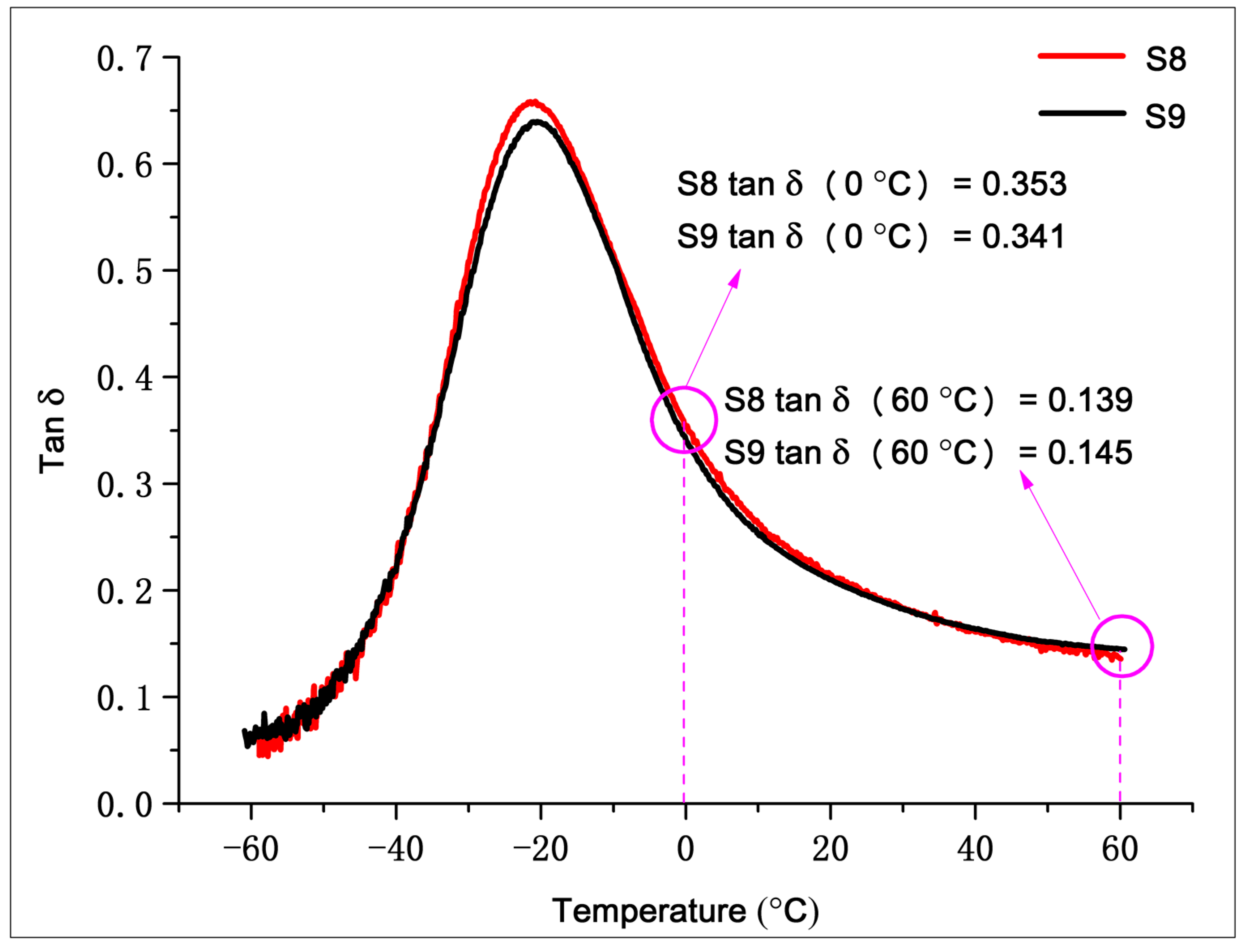
3.3.3. Dynamic Mechanical Properties and Abrasion
4. Conclusions
- (1)
- The core reaction mixing zone effectively improved the filler dispersion and promoted the continuous silanization reaction.
- (2)
- Both shear mixing and tensile mixing elements played important roles in improving the dispersion, and the tensile mixing element further improved dispersion during the later mixing period. The synchronous four-wing serrated rotor, combined kneading block, and eccentric roller all had good dispersing abilities. Among them, the eccentric roller element was more suitable during later mixing.
- (3)
- In general, the serial continuous mixing process suitably controlled the silanization temperature, and the reaction degree gradually increased. However, the grooving section had little effect on promoting the dispersion and reaction. Thus, distribution effect should be further verified.
- (4)
- The performance of the mixture slightly decreased after passing through the head, which was related to secondary filler agglomerates and the breakage of the weak interactions between the rubber and filler.
- (5)
- Compared with a traditional two-stage mixing process, the serial continuous mixing improved the filler dispersion, physical, and mechanical properties and dynamic mechanical properties to different degrees, and was more suitable for the mixing of filler. This was related to the filler network, silanization reaction degree, and bonded rubber content.
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Han, B.; Liu, L.; Zhang, F.; Zhang, L.; Wen, S.; Lu, Y.; Yang, H.; Shen, J. Surface modification of silica by two-step method and properties of solution styrene butadiene rubber (SSBR) nanocomposites filled with modified silica. Compos. Sci. Technol. 2013, 88, 69–75. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Luo, Y.; Jia, D.; Peng, Z. Interfacial interaction between the epoxidized natural rubber and silica in natural rubber/silica composites. Appl. Surf. Sci. 2015, 328, 306–313. [Google Scholar] [CrossRef]
- Van Hoek, J.W.; Heideman, G.; Noordermeer, J.W.M.; Dierkes, W.K.; Blume, A. Implications of the Use of Silica as Active Filler in Passenger Car Tire Compounds on Their Recycling Options. Materials 2019, 12, 725. [Google Scholar] [CrossRef] [PubMed]
- Thongsang, S.; Sombatsompop, N.; Ansarifar, A. Effect of fly ash silica and precipitated silica fillers on the viscosity, cure, and viscoelastic properties of natural rubber. Polym. Adv. Technol. 2008, 19, 1296–1304. [Google Scholar] [CrossRef]
- Martin, P.J.; Brown, P.; Chapman, A.V.; Cook, S. Silica-reinforced epoxidized natural rubber tire treads—Performance and durability. Rubber Chem. Technol. 2015, 88, 390–411. [Google Scholar] [CrossRef]
- Brinke, J.W.T.; Van Swaaij, P.J.; Reuvekamp, L.A.E.M.; Noordermeer, J.W.M. The Influence of Silane Sulfur and Carbon Rank on Processing of a Silica Reinforced Tire Tread Compound. Rubber Chem. Technol. 2003, 76, 12–35. [Google Scholar] [CrossRef]
- Yan, H.; Tian, G.; Sun, K.; Zhang, Y.; Zhang, Y. Effect of silane coupling agent on the polymer-filler interaction and mechanical properties of silica-filled NR. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 573–584. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W. Mechanistic aspects of silane coupling agents with different functionalities on reinforcement of silica-filled natural rubber compounds. Polym. Eng. Sci. 2015, 55, 836–842. [Google Scholar] [CrossRef]
- Majesté, J.-C.; Vincent, F. A kinetic model for silica-filled rubber reinforcement. J. Rheol. 2015, 59, 405–427. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Hu, D.; Luo, Y.; Jia, D. Reinforcement and reinforcing mechanism of styrene–butadiene rubber by antioxidant-modified silica. Compos. Part A Appl. Sci. Manuf. 2015, 78, 303–310. [Google Scholar] [CrossRef]
- Bandzierz, K.; Reuvekamp, L.; Dryzek, J.; Dierkes, W.; Blume, A.; Bielinski, D. Influence of Network Structure on Glass Transition Temperature of Elastomers. Materials 2016, 9, 607. [Google Scholar] [CrossRef] [PubMed]
- Brinke, J.; Debnath, S.; Reuvekamp, L.; Noordermeer, J. Mechanistic aspects of the role of coupling agents in silica–rubber composites. Compos. Sci. Technol. 2003, 63, 1165–1174. [Google Scholar] [CrossRef]
- Choi, S.-S. Difference in bound rubber formation of silica and carbon black with styrene-butadiene rubber. Polym. Adv. Technol. 2002, 13, 466–474. [Google Scholar] [CrossRef]
- Guyard, A.; Persello, J.; Boisvert, J.-P.; Cabane, B.; Boisvert, J. Relationship between the polymer/silica interaction and properties of silica composite materials. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1134–1146. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Optimization of mixing conditions for silica-reinforced natural rubber tire tread compounds. Rubber Chem. Technol. 2012, 85, 277–294. [Google Scholar] [CrossRef]
- Thaptong, P.; Sae-Oui, P.; Sirisinha, C. Effects of silanization temperature and silica type on properties of silica-filled solution styrene butadiene rubber (SSBR) for passenger car tire tread compounds. J. Appl. Polym. Sci. 2016, 133, 43–50. [Google Scholar] [CrossRef]
- Li, Y.; Han, B.; Wen, S.; Lu, Y.; Yang, H.; Zhang, L.; Liu, L. Effect of the temperature on surface modification of silica and properties of modified silica filled rubber composites. Compos. Part A Appl. Sci. Manuf. 2014, 62, 52–59. [Google Scholar] [CrossRef]
- Wolff, S. Optimization of Silane-Silica OTR Compounds. Part 1: Variations of Mixing Temperature and Time during the Modification of Silica with Bis-(3-Triethoxisilylpropyl)-Tetrasulfide. Rubber Chem. Technol. 1982, 55, 967–989. [Google Scholar] [CrossRef]
- Reuvekamp, L.A.E.M.; Brinke, J.W.T.; Van Swaaij, P.J.; Noordermeer, J.W.M. Effects of Time and Temperature on the Reaction of Tespt Silane Coupling Agent During Mixing with Silica Filler and Tire Rubber. Rubber Chem. Technol. 2002, 75, 187–198. [Google Scholar] [CrossRef]
- Jang, S.H.; Kim, W.S.; Kang, Y.G.; Han, M.-H.; Chang, S.M. Study on mixing condition of the rubber composite containing functionalized S-SBR, silica and silane: I. Effect of mixing temperature. Elastom. Compos. 2013, 48, 94–102. [Google Scholar] [CrossRef]
- Kim, S.; Cho, H.; Kim, J.; Kim, K. Effects of processing geometry on the mechanical properties and silica dispersion of silica-filled isobutylene-isoprene rubber (iir) compounds. Elastom. Compos. 2010, 45, 223–229. [Google Scholar]
- Jennissen, J. Highly Efficient Mixing with Tangential Internal Mixers. Int. Polym. Sci. Technol. 2017, 44, 1–6. [Google Scholar] [CrossRef]
- Liu, J.; Li, F.; Zhang, L.; Yang, H. Numerical simulation of flow of rubber compounds in partially filled internal mixer. J. Appl. Polym. Sci. 2015, 32, 132–139. [Google Scholar] [CrossRef]
- Freakley, P.K.; Patel, S.R. Internal Mixing: A Practical Investigation of the Flow and Temperature Profiles during a Mixing Cycle. Rubber Chem. Technol. 1985, 58, 751–773. [Google Scholar] [CrossRef]
- White, J.L. Development of Internal-Mixer Technology for the Rubber Industry. Rubber Chem. Technol. 1992, 65, 527–579. [Google Scholar] [CrossRef]
- Hu, G.-H.; Kadri, I.; Hu, G. Modeling reactive blending: An experimental approach. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 2153–2163. [Google Scholar] [CrossRef]
- Kim, K.J.; White, J.L. Breakdown of silica agglomerates and other particles during mixing in an internal mixer and their processing character. J. Ind. Eng. Chem. 2000, 6, 262–269. [Google Scholar]
- Kim, K.-J.; Vanderkooi, J. Reactive Batch Mixing for Improved Silica-Silane Coupling. Int. Polym. Process. 2004, 19, 364–373. [Google Scholar] [CrossRef]
- Salahudeen, S.A.; Elleithy, R.H.; Alothman, O.Y.; Alzahrani, S. Comparative study of internal batch mixer such as cam, banbury and roller: Numerical simulation and experimental verification. Chem. Eng. Sci. 2011, 66, 2502–2511. [Google Scholar] [CrossRef]
- Koolhiran, C.; White, J.L. Comparison of intermeshing rotor and traditional rotors of internal mixers in dispersing silica and other fillers. J. Appl. Polym. Sci. 2000, 78, 1551–1554. [Google Scholar] [CrossRef]
- Dierkes, W.; Noordermeer, J.W.M. Modeling and Practice of Ethanol-devolatilization of Silica-silane Rubber Compoundsin an Internal Mixer. Int. Polym. Process. 2007, 22, 259–265. [Google Scholar] [CrossRef]
- Kim, K.-J.; Vanderkooi, J. Moisture Effects on TESPD-Silica/CB/SBR Compounds. Rubber Chem. Technol. 2005, 78, 84–104. [Google Scholar] [CrossRef]
- Charman, M.; Léonardi, F.; Dominguez, S.; Bissuel, C.; Derail, C. Dispersion of multiwalled carbon nanotubes in a rubber matrix using an internal mixer: Effects on rheological and electrical properties. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1597–1604. [Google Scholar] [CrossRef]
- Peter, J.; Weckerle, G. The tandem-mixing process. Kautsch. Gummi Kunstst. 1993, 46, 545–549. [Google Scholar]
- Limper, A.; Keuter, H. Tandem Mixing: A New Concept Revolutionizes A Traditional Process. ThyssenKrupp Techforum 2004, 7, 68–74. [Google Scholar]
- Hwang, S.Y. Tandem Mixer for Rubber Mixing. Korea Patent 20140170243A, 2 December 2014. [Google Scholar]
- Markhart, G.T. Continuous Mixer. U.S. Patent 4,542,992, 24 September 1985. [Google Scholar]
- Shon, K.; Bumm, S.H.; White, J.L. A comparative study of dispersing a polyamide 6 into a polypropylene melt in a Buss Kneader, continuous mixer, and modular intermeshing corotating and counter-rotating twin screw extruders. Polym. Eng. Sci. 2008, 48, 756–766. [Google Scholar] [CrossRef]
- Saiwari, S.; Van Hoek, J.W.; Dierkes, W.K.; Reuvekamp, L.E.; Heideman, G.; Blume, A.; Noordermeer, J.W. Upscaling of a Batch De-Vulcanization Process for Ground Car Tire Rubber to a Continuous Process in a Twin Screw Extruder. Materials 2016, 9, 724. [Google Scholar] [CrossRef]
- White, J.L.; Liu, D.; Bumm, S.H. Development of dispersion in rubber-particle compounds in internal and continuous mixers. J. Appl. Polym. Sci. 2006, 102, 3940–3943. [Google Scholar] [CrossRef]
- Kim, K.J.; White, J.L. Silica agglomerate breakdown in three-stage mix including a continuous ultrasonic extruder. J. Ind. Eng. Chem. 2000, 6, 372–379. [Google Scholar]
- Grace, H.P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar] [CrossRef]
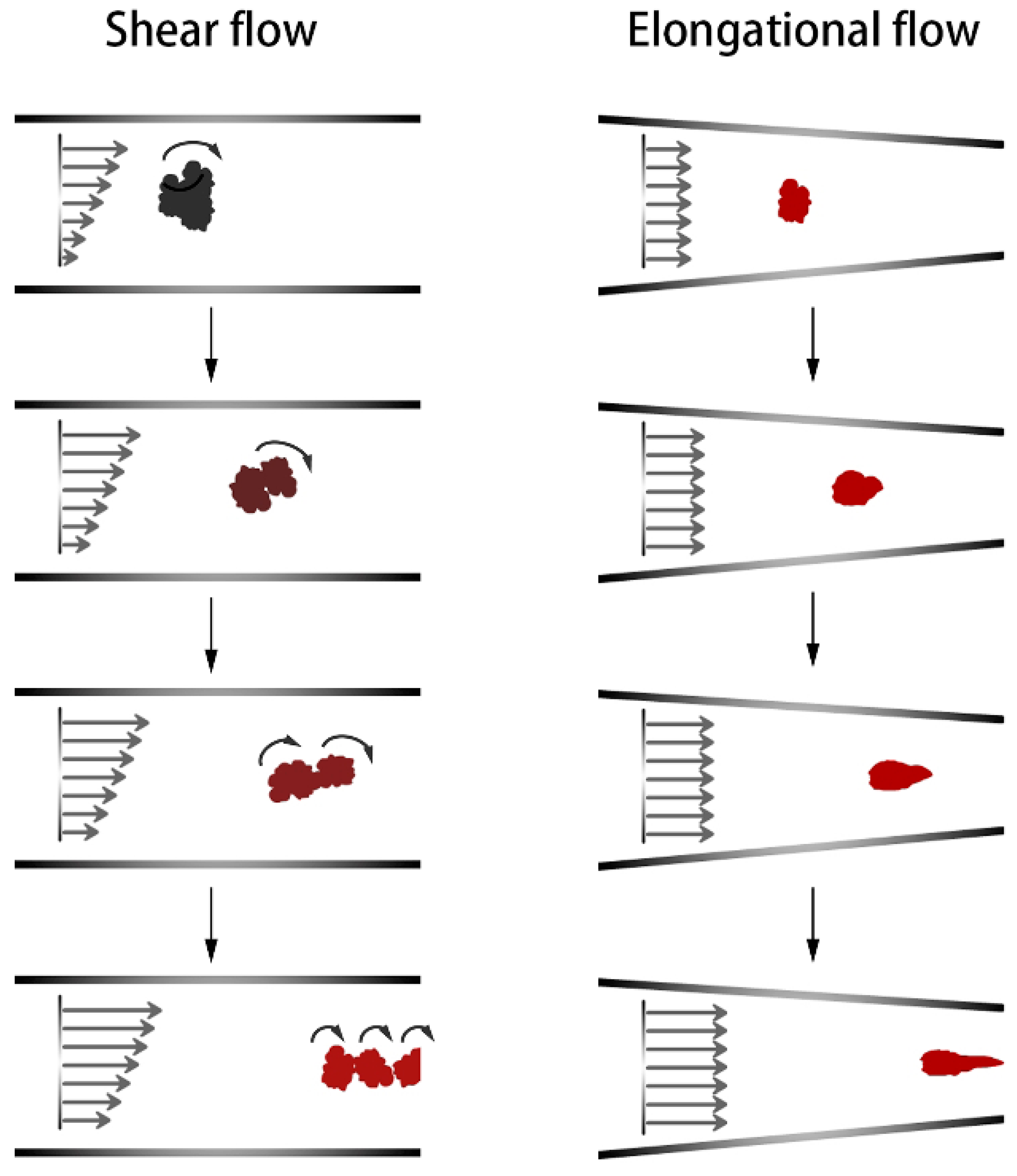
- Bourry, D.; Khayat, R.E.; Utracki, L.A.; Godbille, F.; Picot, J.; Luciani, A. Extensional flow of polymeric dispersions. Polym. Eng. Sci. 1999, 39, 1072–1086. [Google Scholar] [CrossRef]
- Wang, C. The Theoretical and Experimental Research about Mixing Rubber in Synohronous Rotor Internal Mixer. Ph.D. Thesis, Beijing University of Chemical Technology, Beijing, China, 2000. [Google Scholar]
- Payne, A.R. Effect of dispersion on the dynamic properties of filler-loaded rubbers. J. Appl. Polym. Sci. 1965, 9, 2273–2284. [Google Scholar] [CrossRef]
- Lin, C.J.; York, W.M.; Cody, R.J. Silanization characterization and compound properties of silica-filled rubber containing a blocked mercapto silane. Rubber Chem. Technol. 2017, 90, 126–145. [Google Scholar] [CrossRef]
- Chen, S.; Li, H.; Liu, H.; Zheng, K.; Yuan, J.; Xu, Y. Application of end-modified polystyrene butadiene rubber in all-weather tire tread. Tire Ind. 2019, 39, 344–348. [Google Scholar]



| Supplier | Category | Trade Name | Content (phr) |
|---|---|---|---|
| SINOPEC (Beijing, China) | Rubber | SSBR | 96.25 |
| Rubber | BR | 30 | |
| Activator | Zinc Oxide | 2 | |
| Processing aids | Stearic Acid | 2 | |
| SOLVAY (Brussels, Belgium) | Filler | Silica | 45 |
| CABOT (Boston, MA, USA) | Filler | CB N234 | 25 |
| Nanjing Shuguang (Nanjing, China) | Coupling agent | TESPT(Rsi-B) | 5.4 |
| RheinChemie (Mannheim, Germany) | Antioxidant | Microcrystalline Wax | 1.5 |
| Hansen & Rosenthal (Hamburg, Germany) | Plasticizer | Aromatic Oil | 3 |
| Shandong Shangshun (Heze, China) | Antioxidant | DMPPD | 2 |
| Accelerator | DPG | 0.8 | |
| Accelerator | CZ | 1.8 | |
| Vulcanizer | Sulfur | 1.3 |
| Equipment | Model | Manufacturer |
|---|---|---|
| Mill | XK-160E | Dalian Rubber and Plastics Machinery Co., Ltd. (Dalian, China) |
| Flat-Panel Vulcanizer | QLB-400X400X2 | Qingdao Yadong Machinery Group Co., Ltd. (Qingdao, China) |
| Rubber Process Analyzer | RPA2000 | ALPHA (Akron, OH, USA) |
| Dispersion Tester | Disper GRADER | |
| Universal Tester | TS2005b | U-CAN (Taiwan, China) |
| Mooney Viscometer | UM-2050 | |
| Dynamic Mechanical Thermal Analyzer | DMA/SDTA861e | METTLER TOLEDO (Zurich, Switzerland) |
| Din Abrasion Tester | GT-2012-D | GOTECH (Dongguan, China) |
| Zone | Parameter | Value | Process |
|---|---|---|---|
| Initial Mixing | Temperature Controller | 55 °C | 1. Add rubber, and mix for 15 s 2. Add carbon black, 1/2 silica, and the rest additives, then mix for 15 s 3. Add the rest of the silica, and mix to 110 °C 4. Sweep and add oil, then mix to 140 °C and drop 5. Sequentially enter the interconnection zone and the core reaction mixing zone |
| rotor speed | 50 rpm | ||
| Filling Factor | 0.75 | ||
| Interconnection | Temperature Controller | 140 °C | |
| Double-cone screw speed | 20 rpm | ||
| Core Reaction Mixing | Temperature Controller 1 | 145 °C | |
| Temperature Controller 2 | 140 °C | ||
| Temperature Controller 3 | 130 °C | ||
| Twin rotor speed | 40 rpm |
| Stage | Frequency (Hz) | Temperature (°C) | Time (min) | Strain | Test Parameters |
|---|---|---|---|---|---|
| 1 | 0.1 | 60 | 5 | 0.28% | - |
| 2 | 1 | 60 | - | 0.28%–40% | G′02 |
| 3 | 1 | 60 | - | 0.28%–40% | G′03 |
| 4 | 0.1 | 60/160/160 | 0/2.5/5 | 0.28% | - |
| 5 | 1 | 60 | - | 0.28%–40% | G′05 |
| 6 | 1 | 60 | - | 0.28%–40% | G′06 |
| Sample | Hardness (Shore A) | M100 (MPa) | M300 (MPa) | M300/M100 | Tensile Strength (MPa) | Tear Strength (kN/m) | Mooney Viscosity |
|---|---|---|---|---|---|---|---|
| S8 | 60 | 1.9 | 8.7 | 4.60 | 18.2 | 54.9 | 66.5 |
| S9 | 61 | 1.8 | 7.9 | 4.39 | 16.3 | 50.3 | 75.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Pan, Y.; Tian, X.; Liu, H.; Bian, H.; Wang, C. Continuous Preparation and Properties of Silica/Rubber Composite Using Serial Modular Mixing. Materials 2019, 12, 3118. https://doi.org/10.3390/ma12193118
Zhu L, Pan Y, Tian X, Liu H, Bian H, Wang C. Continuous Preparation and Properties of Silica/Rubber Composite Using Serial Modular Mixing. Materials. 2019; 12(19):3118. https://doi.org/10.3390/ma12193118
Chicago/Turabian StyleZhu, Lin, Yiren Pan, Xiaolong Tian, Huaqiao Liu, Huiguang Bian, and Chuansheng Wang. 2019. "Continuous Preparation and Properties of Silica/Rubber Composite Using Serial Modular Mixing" Materials 12, no. 19: 3118. https://doi.org/10.3390/ma12193118