Influence of Different Pretreatments on the Antibacterial Properties of Chitosan Functionalized Viscose Fabric: TEMPO Oxidation and Coating with TEMPO Oxidized Cellulose Nanofibrils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Chitosan Solution
2.3. Preparation of TEMPO Oxidized Cellulose Nanofibrils
2.4. Pretreatment of Viscose Fabric
- (a)
- TEMPO oxidation carried out under neutral conditions (pH 7) according to a method described previously [25]. Viscose fabric (10 g) was soaked in a 0.05 M sodium phosphate buffer solution at pH 7 (50 mL phosphate buffer solution/g viscose fabric), containing TEMPO (20 mg TEMPO/g viscose fabric). Sodium chlorite (50 mg NaClO2/g viscose fabric) and sodium hypochlorite solution (2.5 mmol NaClO/g viscose fabric) were added to the flask and stirred at 200 rpm and 60 °C for 5 min. After stirring, the oxidation was quenched by adding ethanol (ca 5 mL). The oxidized viscose fabric was thoroughly washed with distilled water by filtration, subsequently with ethanol, and dried at room temperature.
- (b)
- Coating of viscose fabrics with 0.5% (w/v) dispersion of TOCN: The treatment lasted for 30 min at room temperature using a material-liquid bath ratio of 1:50, with a wet pick-up of 100%. Samples were then squeezed onto a laboratory padder (Rapid, Istanbul, Turkey) at a pressure of 2 bars. After the excess liquid was removed, fabric was dried at 40 °C for 30 min in a laboratory oven (Instrumentaria, Zagreb, Croatia).
2.5. Functionalization with Chitosan
2.6. Washing of Functionalized Viscose Fabrics
2.7. The Carboxyl Group Content
2.8. The Aldehyde Group Content
2.9. FTIR Analysis
2.10. Elemental Analyses
2.11. The Streaming Potential Measurement
2.12. SEM Analysis
2.13. Breaking Strength Testing
2.14. Antibacterial Testing
3. Results and Discussion
3.1. Characterization of Differently Pretreated and Functionalized Viscose Fabrics
3.1.1. Carboxyl and Aldehyde Group Content
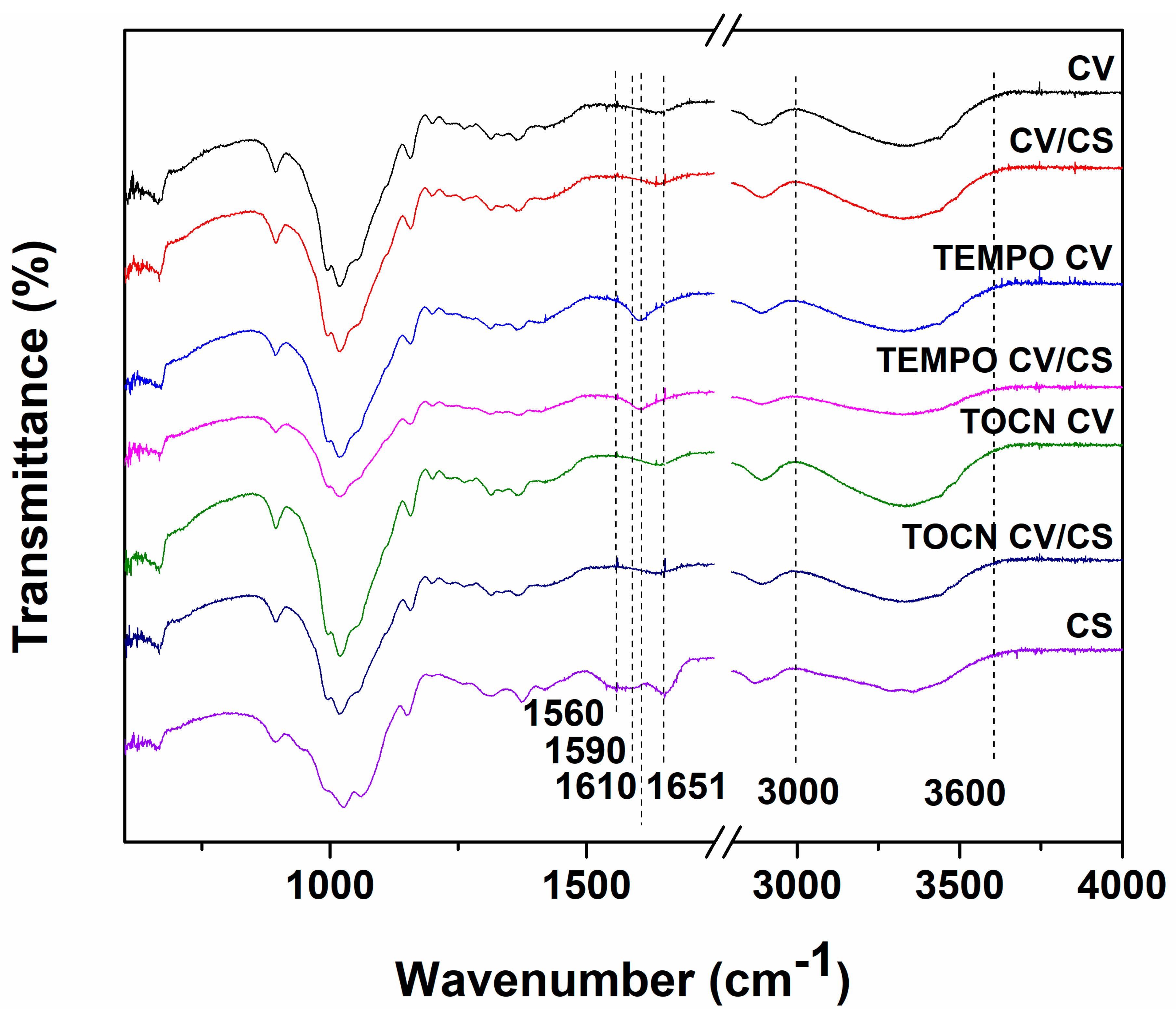
3.1.2. FTIR and Elemental Analyses
3.1.3. Electrokinetic Properties of Viscose Fabrics
3.1.4. Surface Morphology of Viscose Fabrics
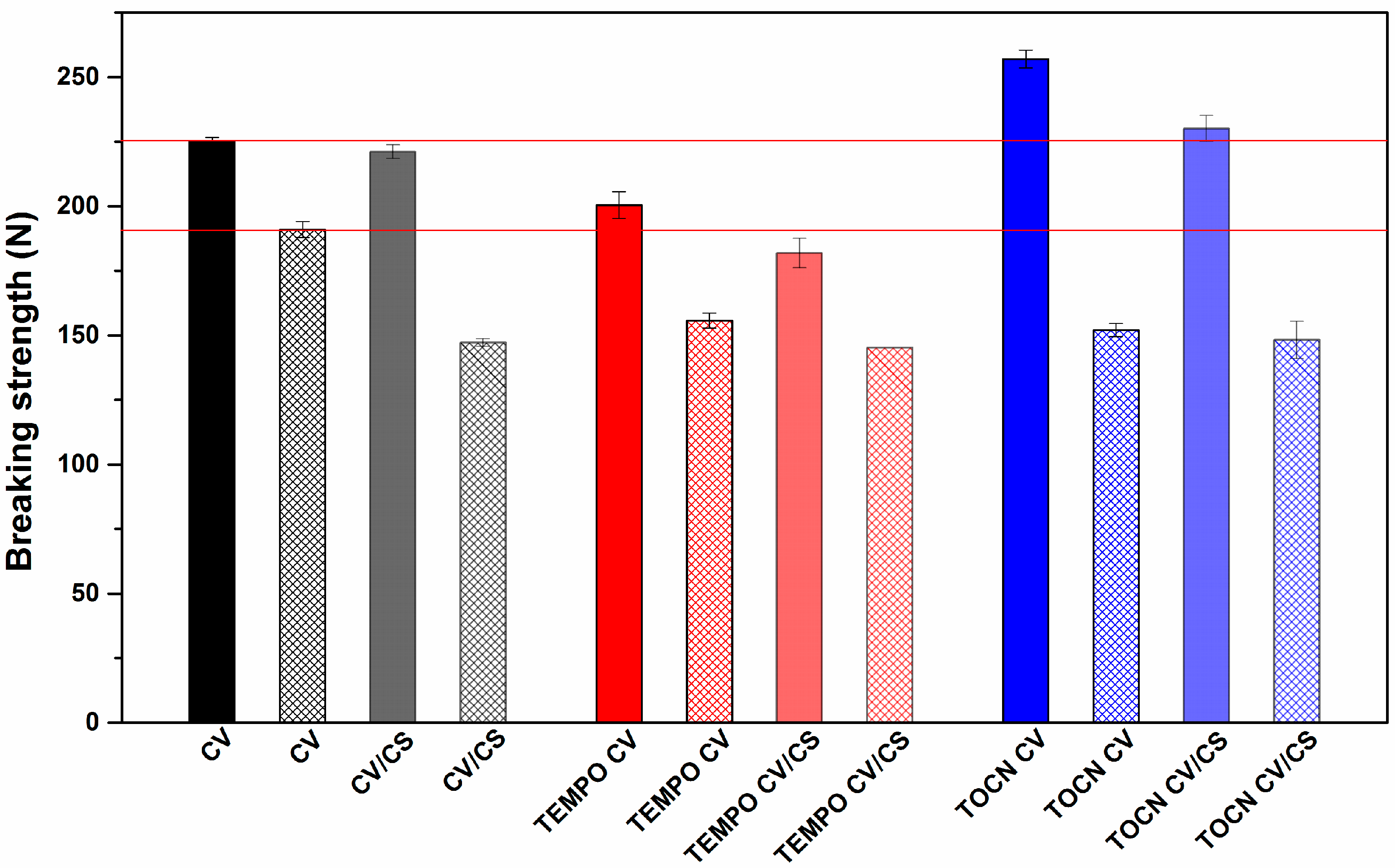
3.1.5. Mechanical Properties of Viscose Fabrics
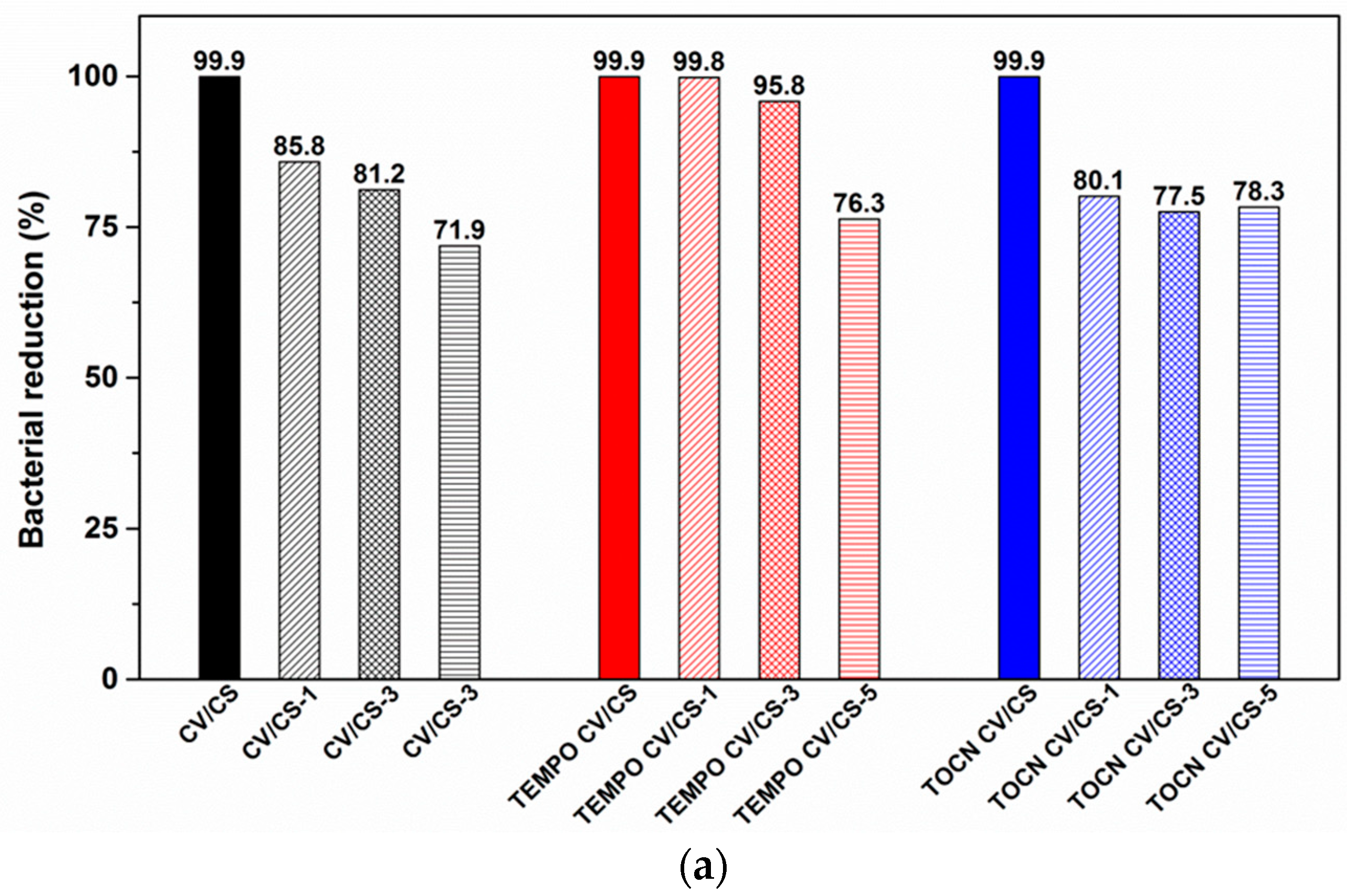
3.1.6. Antibacterial Properties of Viscose Fabrics
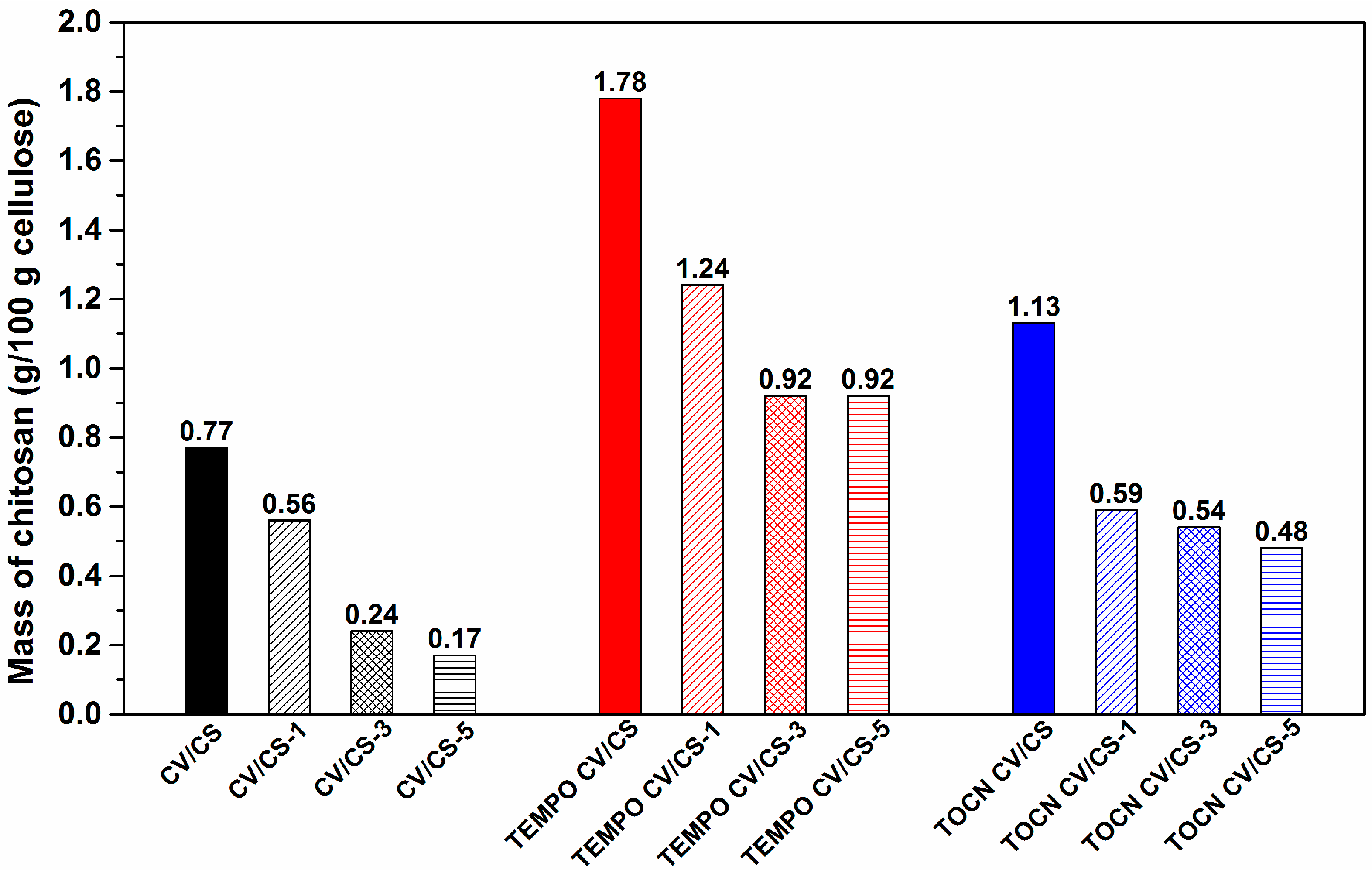
3.2. Washing Durability of Chitosan Functionalized Viscose Fabrics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strnad, S.; Šauperl, O.; Fras-Zemljič, L. Cellulose Fibres Functionalised by Chitosan: Characterization and Application. In Biopolymers; Elnashar, M., Ed.; In Tech: Rijeka, Croatia, 2010; pp. 181–200. [Google Scholar]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Medical Textiles Market-Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013–2019. Available online: https://www.transparencymarketresearch.com/medical-textiles-market.html (accessed on 24 September 2019).
- Emam, H.E. Antimicrobial cellulosic textiles based on organic compounds. 3 Biotech. 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Fras Zemljic, L.; Volmajer, J.; Ristić, T.; Bracic, M.; Sauperl, O.; Kreže, T. Antimicrobial and antioxidant functionalization of viscose fabric using chitosan–curcumin formulations. Tex. Res. J. 2014, 84, 819–830. [Google Scholar] [CrossRef]
- Windler, L.; Height, M.; Nowack, B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013, 53, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; He, J.; Yan, T.; Liu, C.; Wei, X.; Li, J.; Huang, Y. Antibacterial and hemostatic composite gauze of N,O-carboxymethyl chitosan/oxidized regenerated cellulose. RSC Adv. 2016, 6, 94429–94436. [Google Scholar] [CrossRef]
- Fras Zemljič, L.; Peršin, Z.; Stenius, P. Improvement of Chitosan Adsorption onto Cellulosic Fabrics by Plasma Treatment. Biomacromolecules 2009, 10, 1181–1187. [Google Scholar] [CrossRef]
- Fras Zemljič, L.; Peršin, Z.; Šauperl, O.; Rudolf, A.; Kostic, M. Medical textiles based on viscose rayon fabrics coated with chitosan-encapsulated iodine: Antibacterial and antioxidant properties. Tex. Res. J. 2018, 88, 2519–2531. [Google Scholar] [CrossRef]
- Raza, Z.A.; Bilal, U.; Noreen, U.; Munim, S.A.; Riaz, S.; Abdullah, M.U.; Abid, S. Chitosan Mediated Formation and Impregnation of Silver Nanoparticles on Viscose Fabric in Single Bath for Antibacterial Performance. Fibers Polym. 2019, 20, 1360–1367. [Google Scholar] [CrossRef]
- Fras, L.; Ristić, T.; Tkavc, T. Adsorption and Antibacterial Activity of Soluble and Precipitated Chitosan on Cellulose Viscose Fibers. J. Eng. Fibers Fabr. 2012, 7, 50–57. [Google Scholar] [CrossRef]
- Brodnjak, U.V.; Gregor-Svetec, D.; Klančnik, M. Influence of enzymatic treatment on the structural, sorption and dyeing properties of viscose and chitosan/cellulose fibers. Text. Res. J. 2015, 86, 990–1005. [Google Scholar] [CrossRef]
- He, J.; Wang, F.; Wu, Y.; Huang, Y.; Zhang, H. Preparation of the water-soluble chitosan-coated oxidized regenerated cellulose gauze. Cellulose 2011, 18, 1651–1659. [Google Scholar] [CrossRef]
- Korica, M.; Fras Zemljič, L.; Bračič, M.; Kargl, R.; Spirk, S.; Reishofer, D.; Mihajlovski, K.; Kostić, M. Novel protein-repellent and antimicrobial polysaccharide multilayer thin films. Holzforschung 2019, 73, 93–103. [Google Scholar] [CrossRef]
- Bethke, K.; Palantöken, S.; Andrei, V.; Roß, M.; Raghuwanshi, V.S.; Kettemann, F.; Greis, K.; Ingber, T.T.K.; Stückrath, J.B.; Valiyaveettil, S.; et al. Functionalized cellulose for water purification, antimicrobial applications, and sensors. Adv. Funct. Mater. 2018, 28, 1800409. [Google Scholar] [CrossRef]
- Zeronian, S.H.; Inglesby, M.K. Bleaching of cellulose by hydrogen peroxide. Cellulose 1995, 2, 265–272. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, P.; Wu, R.; Peng, H.; Wu, R. Preparation and properties of oxidized regenerated cellulose by hydrogen peroxide. CIESC J. 2016, 67, 2401–2409. [Google Scholar] [CrossRef]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Bajerová, M.; Krejčová, K.; Rabišková, M.; Gajdziok, J.; Masteiková, R. Oxycellulose: Significant characteristics in relation to its pharmaceutical and medical applications. Adv. Polym. Technol. 2009, 28, 199–208. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose – Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Gray, D.; Dorris, A.; Lindstrom, T.; Aknerfors, M. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2012, 50, 5438–5466. [Google Scholar] [CrossRef]
- Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Marković, D.; Korica, M.; Kostić, M.; Radovanović, Ž.; Šaponjić, Z.; Mitrić, M.; Radetić, M. In situ synthesis of Cu/Cu2O nanoparticles on the TEMPO oxidized cotton fabrics. Cellulose 2018, 25, 829–841. [Google Scholar] [CrossRef]
- Kumar, V.; Yang, T. HNO3/H3PO4–NaNO2 mediated oxidation of cellulose preparation and characterization of bioabsorbable oxidized celluloses in high yields and with different levels of oxidation. Carbohydr. Polym. 2002, 48, 403–412. [Google Scholar] [CrossRef]
- Parks, E.J.; Hebert, R.L. Thermal analysis of ion-exchange reaction products of wood pulps with calcium and aluminum cations. Tappi J. 1972, 55, 1510–1514. [Google Scholar]
- Luxbacher, T. The Zeta Potential for Solid Surface Analysis, 1st ed.; Anton Paar GmbH: Graz, Austria, 2014; pp. 42–72. [Google Scholar]
- Široký, J.; Blackburn, R.; Bechtol, T.; Taylor, J.; White, P. Attenuated total reflectance Fourier-transform Infrared spectroscopy analysis of crystallinity changes in lyocell following continuous treatment with sodium hydroxide. Cellulose 2010, 17, 103–115. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Ifuku, S.; Tsuji, M.; Morimoto, M.; Saimoto, H.; Yano, H. Synthesis of Silver Nanoparticles Templated by TEMPO-Mediated Oxidized Bacterial Cellulose Nanofibers. Biomacromolecules 2009, 10, 2714–2717. [Google Scholar] [CrossRef] [PubMed]
- Coseri, S.; Biliuta, G.; Fras Zemljič, L.; Stevanic Srndovic, J.; Larsson, P.T.; Strnad, S.; Kreže, T.; Naderi, A.; Lindström, T. One-shot carboxylation of microcrystalline cellulose in the presence of nitroxyl radicals and sodium periodate. RSC Adv. 2015, 5, 85889–85897. [Google Scholar] [CrossRef]
- Khan, A.; Othman, M.B.H.; Razak, K.A.; Akil, H.M. Synthesis and physicochemical investigation of chitosan-PMAA-based dual-responsive hydrogels. J. Polym. Res. 2013, 20, 273–282. [Google Scholar] [CrossRef]
- Trivedi, P.; Saloranta-Simell, T.; Maver, U.; Gradisnik, L.; Prabhakar, N.; Smått, J.-H.; Mohan, T.; Gericke, M.; Heinze, T.; Fardim, P. Chitosan–Cellulose Multifunctional Hydrogel Beads: Design, Characterization and Evaluation of Cytocompatibility with Breast Adenocarcinoma and Osteoblast Cells. Bioengineering 2018, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kwon, G.-J.; Hwang, K.; Kim, D.-Y. Cellulose–Chitosan Antibacterial Composite Films Prepared from LiBr Solution. Polymers 2018, 10, 1058. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.; Trovatti, E.; Carvalho, A.J.F.; Gandini, A. Continuous microfiber drawing by interfacial charge complexation between anionic cellulose nanofibers and cationic chitosan. J. Mater. Chem. A 2017, 5, 13098–13103. [Google Scholar] [CrossRef]
- Köstler, S.; Ribitsch, V.; Stana-Kleinschek, K.; Jakopic, G.; Strnad, S. Electrokinetic investigation of polyelectrolyte adsorption and multilayer formation on a polymer surface. Colloids Surf. A Physicochem. Eng. Asp. 2005, 270, 107–114. [Google Scholar] [CrossRef]
- Praskalo, J.; Kostic, M.; Potthast, A.; Popov, G.; Pejic, B.; Skundric, P. Sorption properties of TEMPO-oxidized natural and man-made cellulose fibers. Carbohydr. Polym. 2009, 77, 791–798. [Google Scholar] [CrossRef]
- Nikolic, T.; Korica, M.; Milanovic, J.; Kramar, A.; Petronijevic, Z.; Kostic, M. TEMPO-oxidized cotton as a substrate for trypsin immobilization: Impact of functional groups on proteolytic activity and stability. Cellulose 2017, 24, 1863–1875. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R.; Muzzarelli, R.A.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A.J.; Muzzarelli, R. Chitosan Chemistry and Pharmaceutical Perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef]
- Reischl, M.; Ribitsch, V.; Stana-Kleinschek, K. Electrokinetic Investigations of Oriented Cellulose Polymers. Macromol. Symp. 2006, 244, 31–47. [Google Scholar] [CrossRef]
- Dhiman, G.; Chakraborty, J.N. Antimicrobial performance of cotton finished with triclosan, silver and chitosan. Fash. Text. 2015, 2, 181. [Google Scholar] [CrossRef]
- Rahman Bhuiyan, M.A.; Hossain, M.A.; Zakaria, M.; Islam, M.N.; Zulhash Uddin, M. Chitosan Coated Cotton Fiber: Physical and Antimicrobial Properties for Apparel Use. J. Polym. Environ. 2017, 25, 334–342. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. Engl. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Goy, C.R.; Britto, D.; Assis, B.G.O. A Review of the Antimicrobial Activity of Chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Ristić, T.; Hribernik, S.; Fras-Zemljič, L. Electrokinetic properties of fibres functionalised by chitosan and chitosan nanoparticles. Cellulose 2015, 22, 3811–3823. [Google Scholar] [CrossRef]




| Description of Samples | Washing Cycles | |||
|---|---|---|---|---|
| 0 | 1 | 3 | 5 | |
| Pristine viscose | CV | – | – | – |
| TEMPO oxidized viscose | TEMPO CV | – | – | – |
| Viscose coated with TOCN | TOCN CV | – | – | – |
| CV + chitosan solution | CV/CS | CV/CS–1 | CV/CS–3 | CV/CS–5 |
| TEMPO CV + chitosan solution | TEMPO CV/CS | TEMPO CV/CS–1 | TEMPO CV/CS–3 | TEMPO CV/CS–5 |
| TOCN CV + chitosan solution | TOCN CV/CS | TOCN CV/CS–1 | TOCN CV/CS–3 | TOCN CV/CS–5 |
| Sample | COOH, mmol/kg | CHO, mmol/kg | COOH + CHO, mmol/kg |
|---|---|---|---|
| CV | 64 | 18 | 82 |
| TEMPO CV | 438 | 440 | 878 |
| TOCN | 830 | 90 | 920 |
| TOCN CV | 86 | 27 | 113 |
| Sample | Bacterial Reduction, % | |
|---|---|---|
| Staphylococcus aureus | Escherichia coli | |
| CV/CS | 99.9 | 97.9 |
| TEMPO CV/CS | 99.9 | 99.9 |
| TOCN CV/CS | 99.9 | 99.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korica, M.; Peršin, Z.; Trifunović, S.; Mihajlovski, K.; Nikolić, T.; Maletić, S.; Fras Zemljič, L.; Kostić, M.M. Influence of Different Pretreatments on the Antibacterial Properties of Chitosan Functionalized Viscose Fabric: TEMPO Oxidation and Coating with TEMPO Oxidized Cellulose Nanofibrils. Materials 2019, 12, 3144. https://doi.org/10.3390/ma12193144
Korica M, Peršin Z, Trifunović S, Mihajlovski K, Nikolić T, Maletić S, Fras Zemljič L, Kostić MM. Influence of Different Pretreatments on the Antibacterial Properties of Chitosan Functionalized Viscose Fabric: TEMPO Oxidation and Coating with TEMPO Oxidized Cellulose Nanofibrils. Materials. 2019; 12(19):3144. https://doi.org/10.3390/ma12193144
Chicago/Turabian StyleKorica, Matea, Zdenka Peršin, Snežana Trifunović, Katarina Mihajlovski, Tanja Nikolić, Slavica Maletić, Lidija Fras Zemljič, and Mirjana M. Kostić. 2019. "Influence of Different Pretreatments on the Antibacterial Properties of Chitosan Functionalized Viscose Fabric: TEMPO Oxidation and Coating with TEMPO Oxidized Cellulose Nanofibrils" Materials 12, no. 19: 3144. https://doi.org/10.3390/ma12193144
APA StyleKorica, M., Peršin, Z., Trifunović, S., Mihajlovski, K., Nikolić, T., Maletić, S., Fras Zemljič, L., & Kostić, M. M. (2019). Influence of Different Pretreatments on the Antibacterial Properties of Chitosan Functionalized Viscose Fabric: TEMPO Oxidation and Coating with TEMPO Oxidized Cellulose Nanofibrils. Materials, 12(19), 3144. https://doi.org/10.3390/ma12193144








