Aluminum-Alumina Composites: Part I: Obtaining and Characterization of Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Aluminum Powder
2.2. Aluminum-Alumina Powder Composites
- Oxidation of aluminum powder by water at pressure 0.15–1.80 MPa;
- Powder drying at 120 °C and calcination of the aluminum-alumina composite in a furnace at 600 °C.
2.2.1. Oxidation of Aluminum Powder by Water (Stage 1)
2.2.2. Drying and Calcination (Stage 2)
2.2.3. Characterization
3. Results
3.1. Aluminum Powder Characterization
3.2. Oxidation of Aluminum Powder
3.3. Effect of the Oxidation Mode on the Particles’ Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eyers, D.R.; Potter, A.T. Industrial Additive Manufacturing: A manufacturing systems perspective. Comput. Ind. 2017, 92, 208–218. [Google Scholar] [CrossRef]
- Rauch, E.; Unterhofer, M.; Dallasega, P. Industry sector analysis for the application of additive manufacturing in smart and distributed manufacturing systems. Manuf. Lett. 2017, 120, 305–326. [Google Scholar] [CrossRef]
- Kok, Y.; Tan, X.P.; Wang, P.; Nai, M.L.S.; Loh, N.H.; Liu, E.; Tor, S.B. Anisotropy and heterogeneity of microstructure and mechanical properties in metal additive manufacturing: A critical review. Mater. Des. 2018, 139, 565–586. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Szemkus, S.; Kempf, B.; Jahn, S.; Wiehl, G.; Heringhaus, F.; Rettenmayr, M. Laser additive manufacturing of contact materials. J. Mater. Process. Technol. 2018, 252, 612–617. [Google Scholar] [CrossRef]
- Riberio, F. 3D printing with metals. Comput. Control Eng. J. 1998, 9, 31–38. [Google Scholar] [CrossRef]
- Xu, W.; Brandt, M.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K.; Xia, K.; Qian, M. Additive manufacturing of strong and ductile Ti-6Al-4V by selective laser melting via in situ martensite decomposition. Acta Mater. 2015, 85, 74–84. [Google Scholar] [CrossRef]
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminum alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, N.; Imran, M.; Wischeropp, T.M.; Emmelmann, C.; Siddique, S.; Walther, F. Influence of Process Parameters on the Quality of Aluminum Alloy EN AW 7075 Using Selective Laser Melting (SLM). Phys. Procedia 2016, 83, 918–926. [Google Scholar] [CrossRef]
- Ding, Y.; Muñiz-Lerma, J.A.; Trask, M.; Chou, S.; Walker, A.; Brochu, M. Microstructure and mechanical property considerations in additive manufacturing of aluminum alloys. MRS Bull. 2016, 41, 745–751. [Google Scholar] [CrossRef]
- Gu, H.; Gong, H.; Dilip, J.J.S.; Pal, D.; Hicks, A.; Doak, H.; Stucker, B. Effects of Powder Variation on the Microstructure and Tensile Strength of Ti-6Al-4V Parts Fabricated by Selective Laser Melting. Int. J. Powder Metall. 2015, 51, 470–483. [Google Scholar]
- Yin, S.; Yan, X.; Chen, C.; Jenkins, R.; Liu, M.; Lupoi, R. Hybrid additive manufacturing of Al-Ti6Al4V functionally graded materials with selective laser melting and cold spraying. Mater. Process. Technol. 2018, 255, 650–655. [Google Scholar] [CrossRef]
- Liu, B.; Wildman, R.; Tuck, C.; Ashcroft, I.; Hague, R. Investigaztion the effect of particle size distribution on processing parameters optimisation in selective laser melting process. In Proceedings of the 22nd Annual International Solid Freeform Fabrication Symposium—An Additive Manufacturing Conference, Austin, TX, USA, 8–10 August 2011; pp. 227–238. [Google Scholar]
- Zhang, H.; Zhu, H.; Qi, T.; Hu, Z.; Zeng, X. Selective laser melting of high strength Al–Cu–Mg alloys: Processing, microstructure and mechanical properties. Mater. Sci. Eng. A 2016, 656, 47–54. [Google Scholar] [CrossRef]
- Pozdniakov, A.V.; Churyumov, A.Y.; Loginova, I.S.; Daubarayte, D.K.; Ryabov, D.K.; Korolev, V.A. Microstructure and properties of novel AlSi11CuMn alloy manufactured by selective laser melting. Mater. Lett. 2018, 225, 33–36. [Google Scholar] [CrossRef]
- Manca, D.R.; Churyumov, A.Y.; Pozdniakov, A.V.; Ryabov, D.K.; Korolev, V.A.; Daubarayte, D.K. Novel heat-resistant Al-Si-Ni-Fe alloy manufactured by selective laser melting. Mater. Lett. 2019, 236, 676–679. [Google Scholar] [CrossRef]
- Sercombe, T.B.; Li, X. Selective laser melting of aluminium and aluminium metal matrix composites: Review. Mater. Technol. 2016, 31, 77–85. [Google Scholar] [CrossRef]
- Yu, W.H.; Sling, S.L.; Chua, C.K.; Kuo, C.N.; Tian, X.L. Particle-reinforced metal matrix nanocomposites fabricated by selective laser melting: A state of the art review. Prog. Mater. Sci. 2019, 104, 330–379. [Google Scholar] [CrossRef]
- Durai, T.G.; Das, K.; Das, S. Synthesis and characterization of Al matrix composites reinforced by in situ alumina particulates. Mater. Sci. Eng. A 2007, 445–446, 100–105. [Google Scholar] [CrossRef]
- Surappa, M.K. Aluminium matrix composites: Challenges and opportunities. Sadhana 2003, 28, 319–334. [Google Scholar] [CrossRef]
- Pramod, S.L.; Bakshi, S.R.; Murty, B.S. Aluminum-Based Cast In Situ Composites: A Review. J. Mater. Eng. Perform. 2015, 24, 2185–2207. [Google Scholar] [CrossRef]
- Tikhov, S.F.; Fenelonov, V.B.; Sadykov, V.A.; Potapova, Y.V.; Salanov, A.N. Porous Al2O3/Al Metal Ceramics Prepared by the Oxidation of Aluminum Powder under Hydrothermal Conditions Followed by Thermal Dehydration: I. Composition and Macrocharacteristics of Composites. Kinet. Catal. 2000, 41, 826–834. [Google Scholar] [CrossRef]
- Rai, A.; Park, K.; Zhou, L.; Zachariah, M.R. Understanding the mechanism of aluminum nanoparticle oxidation. Combust. Theory Modell. 2006, 10, 843–859. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; Lerner, M.; Rodkevich, N.; Nie, H.; Abraham, A.; Schoenitz, M.; Dreizin, E.L. Oxidation of nano-sized aluminum powders. Thermochimica Acta 2016, 636, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Antipina, S.A.; Zmanovskii, S.V.; Gromov, A.A.; Teipel, U. Air and water oxidation of aluminum flake particles. Powder Technol. 2017, 307, 184–189. [Google Scholar] [CrossRef]
- Streletskii, A.N.; Kolbanev, I.V.; Borunova, A.B.; Butyagin, P.Y. Mechanochemical Activation of Aluminum: 3. Kinetics of Interaction between Aluminum and Water. Colloid J. 2005, 67, 631–637. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Shkolnikov, E.I.; Lisicyn, A.V.; Bersh, A.V.; Zhuk, A.Z. Computational and experimental investigation on thermodynamics of the reactor of aluminum oxidation in saturated wet steam. Int. J. Hydrog. Energy 2010, 35, 1888–1894. [Google Scholar] [CrossRef]
- Wang, H.Z.; Leung, D.Y.C.; Leung, M.K.H.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Kravchenko, O.V.; Semenenko, K.N.; Bulychev, B.M.; Kalmykov, K.B. Activation of aluminum metal and its reaction with water. J. Alloy. Compd. 2005, 397, 58–62. [Google Scholar] [CrossRef]
- Shmelev, V.; Nikolaev, V.; Lee, J.H.; Yim, C. Hydrogen production by reaction of aluminum with water. Int. J. Hydrog. Energy 2016, 41, 16664–16673. [Google Scholar] [CrossRef]
- Shkolnikov, E.I.; Shaitura, N.S.; Vlaskin, M.S. Structural properties of boehmite produced by hydrothermal oxidation of aluminum. J. Supercrit. Fluids 2013, 73, 10–17. [Google Scholar] [CrossRef]
- Lysenko, A.P.; Nalivayko, A.Y. Optimization of electrolysis during the high-pure aluminium oxide obtaining, using electrochemical method of aluminium oxidation. Tsvetnye Met. 2017, 1, 28–32. [Google Scholar] [CrossRef]
- Nalivaiko, A.Y.; Lysenko, A.P.; Pak, V.I.; Ivanov, M.A. Feasibility Assessment for Leucosapphire Production from Aluminum Oxide Prepared Electrochemically. Refract. Ind. Ceram. 2018, 55, 80–84. [Google Scholar] [CrossRef]
- Ilyukhina, A.V.; Kravchenko, O.V.; Bulychev, B.M. Studies on microstructure of activated aluminum and its hydrogen generation properties in aluminum/water reaction. J. Alloy. Compd. 2017, 690, 321–329. [Google Scholar] [CrossRef]
- Ambaryan, G.; Valyano, G.; Zhuk, A.; Shkolnikov, E.; Gromov, A.; Zmanovsky, S.; Vlaskin, M. Partial oxidation of aluminum powder for obtaining a controlled amount of aluminum oxide on the surface of aluminum. IOP Conf. Ser. Earth Env. Sci. 2018, 168, 012021. [Google Scholar] [CrossRef] [Green Version]
- Lisitsyn, A.V.; Dombrovsky, L.A.; Mendeleyev, V.Y.; Grigorenko, A.V.; Vlaskin, M.S.; Zhuk, A.Z. Near-infrared optical properties of a porous alumina ceramics produced by hydrothermal oxidation of aluminum. Infrared Phys. Technol. 2016, 77, 162–170. [Google Scholar] [CrossRef]
- Grigorenko, A.V.; Ambaryan, G.N.; Valyano, G.E.; Vlaskin, M.S.; Gromov, A.A.; Zmanovsky, S.V. Kinetics of Aluminum Micron Powder Oxidation in Hot Distilled Water and Product Microstructure Investigation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 381, 012028. [Google Scholar] [CrossRef]
- Nie, H.; Schoenitz, M.; Dreizin, E.L. Oxidation of differently prepared Al-Mg alloy powders in oxygen. J. Alloy. Compd. 2016, 685, 402–410. [Google Scholar] [CrossRef] [Green Version]


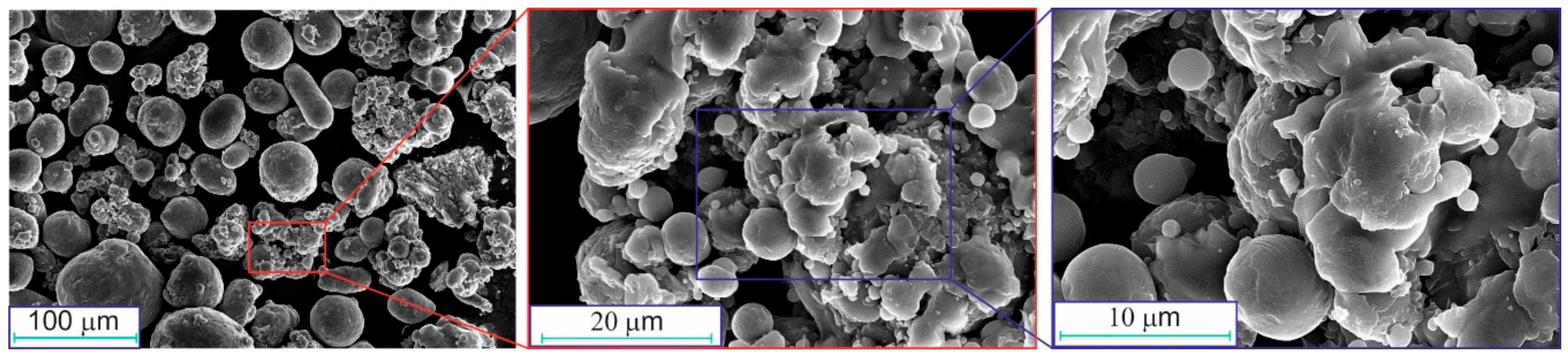
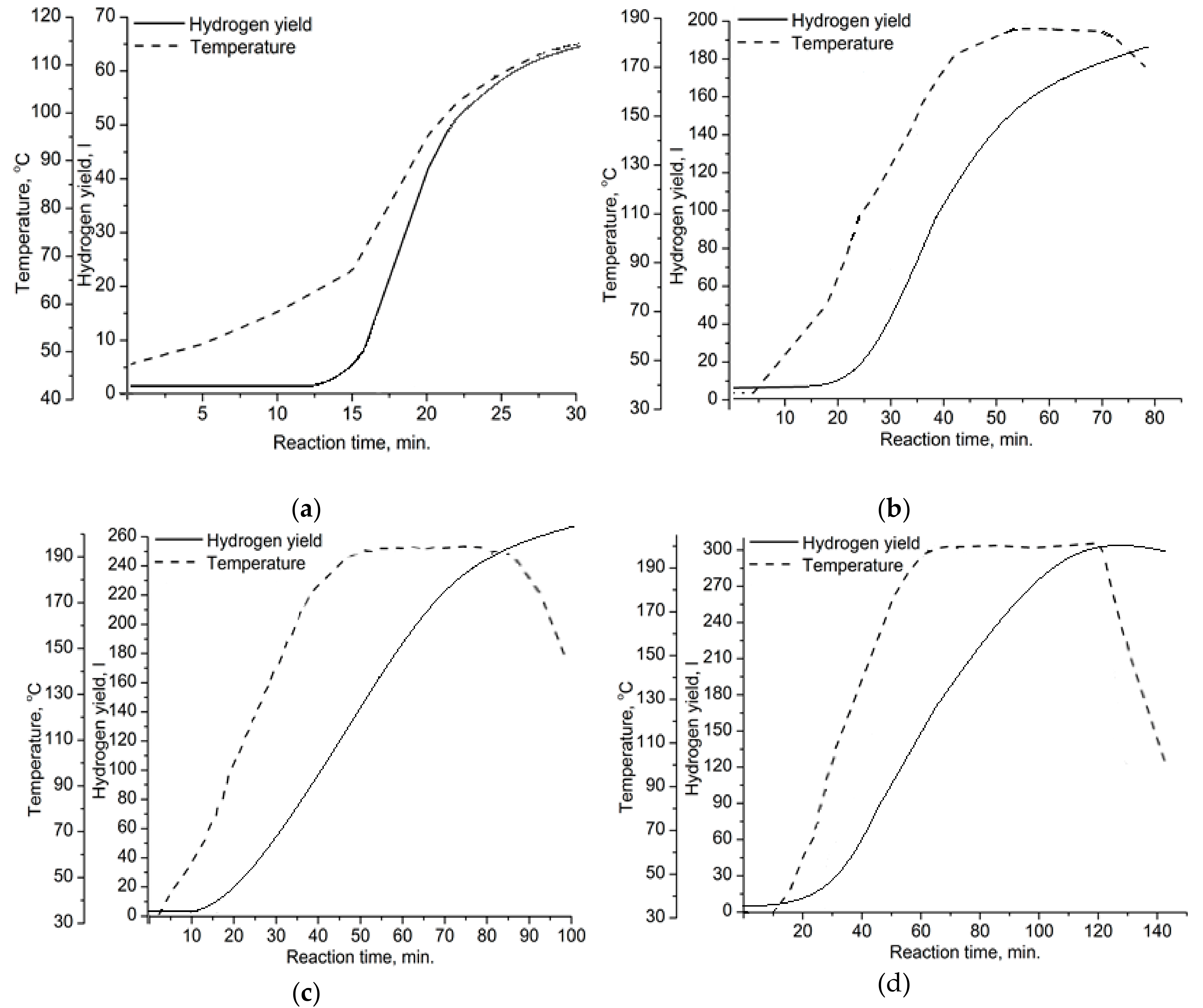

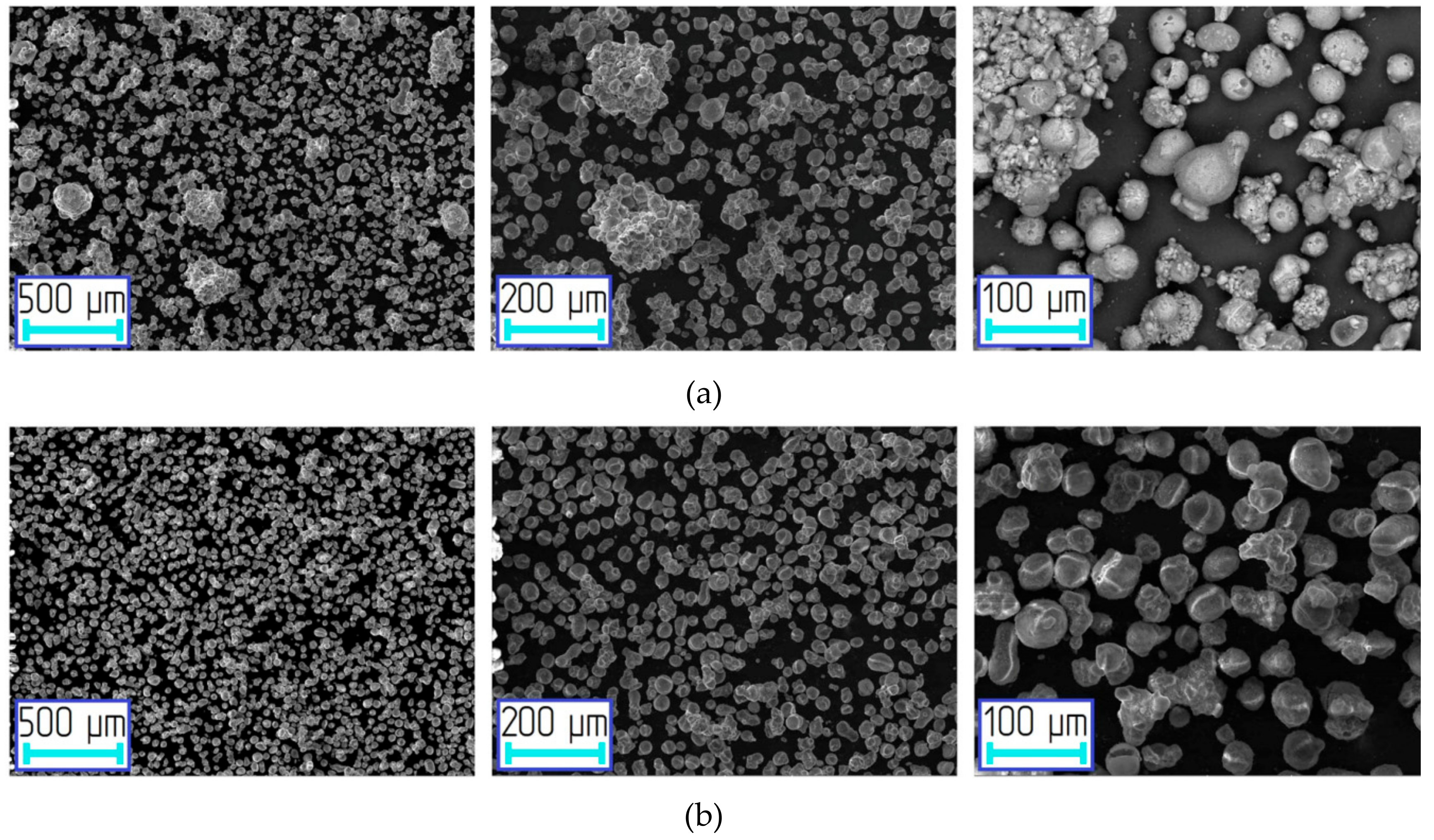


| Mode | Parameters of Stage 1 | ||||
|---|---|---|---|---|---|
| Maximal Temperature, °C | Pressure, MPa | Volume of the Released H2, Liters | Alumina Content, wt. % | Mean Alumina Layer Thickness *, µm | |
| A | 120 | 0.15 | 65 | 10.0 | 1.45 |
| B | 180 | 1.35 | 190 | 14.5 | 2.14 |
| C | 190 | 1.50 | 260 | 17.4 | 2.60 |
| D | 200 | 1.80 | 300 | 20.0 | 3.00 |
| Element, wt. % | |
|---|---|
| Al | 99.20 |
| Ga | 0.09 |
| Zn | 0.08 |
| Ce | 0.08 |
| La | 0.07 |
| Fe | 0.07 |
| V | 0.03 |
| Mg | 0.02 |
| B | 0.01 |
| Cr, Ti, Co, Y, Cu, and volatiles | 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gromov, A.A.; Nalivaiko, A.Y.; Ambaryan, G.N.; Vlaskin, M.S.; Buryakovskaya, O.A.; Kislenko, S.A.; Zhuk, A.Z.; Shkolnikov, E.I.; Slyusarskiy, K.V.; Osipenkova, A.A.; et al. Aluminum-Alumina Composites: Part I: Obtaining and Characterization of Powders. Materials 2019, 12, 3180. https://doi.org/10.3390/ma12193180
Gromov AA, Nalivaiko AY, Ambaryan GN, Vlaskin MS, Buryakovskaya OA, Kislenko SA, Zhuk AZ, Shkolnikov EI, Slyusarskiy KV, Osipenkova AA, et al. Aluminum-Alumina Composites: Part I: Obtaining and Characterization of Powders. Materials. 2019; 12(19):3180. https://doi.org/10.3390/ma12193180
Chicago/Turabian StyleGromov, Alexander A., Anton Yu. Nalivaiko, Grayr N. Ambaryan, Mikhail S. Vlaskin, Olesya A. Buryakovskaya, Sergey A. Kislenko, Andrey Z. Zhuk, Evgeniy I. Shkolnikov, Konstantin V. Slyusarskiy, Alexandra A. Osipenkova, and et al. 2019. "Aluminum-Alumina Composites: Part I: Obtaining and Characterization of Powders" Materials 12, no. 19: 3180. https://doi.org/10.3390/ma12193180
APA StyleGromov, A. A., Nalivaiko, A. Y., Ambaryan, G. N., Vlaskin, M. S., Buryakovskaya, O. A., Kislenko, S. A., Zhuk, A. Z., Shkolnikov, E. I., Slyusarskiy, K. V., Osipenkova, A. A., & Arnautov, A. N. (2019). Aluminum-Alumina Composites: Part I: Obtaining and Characterization of Powders. Materials, 12(19), 3180. https://doi.org/10.3390/ma12193180






