Combustion Mechanism of Alloying Elements Cr in Ti-Cr-V Alloys
Abstract
:1. Introduction
2. Experimental
2.1. Material Preparation
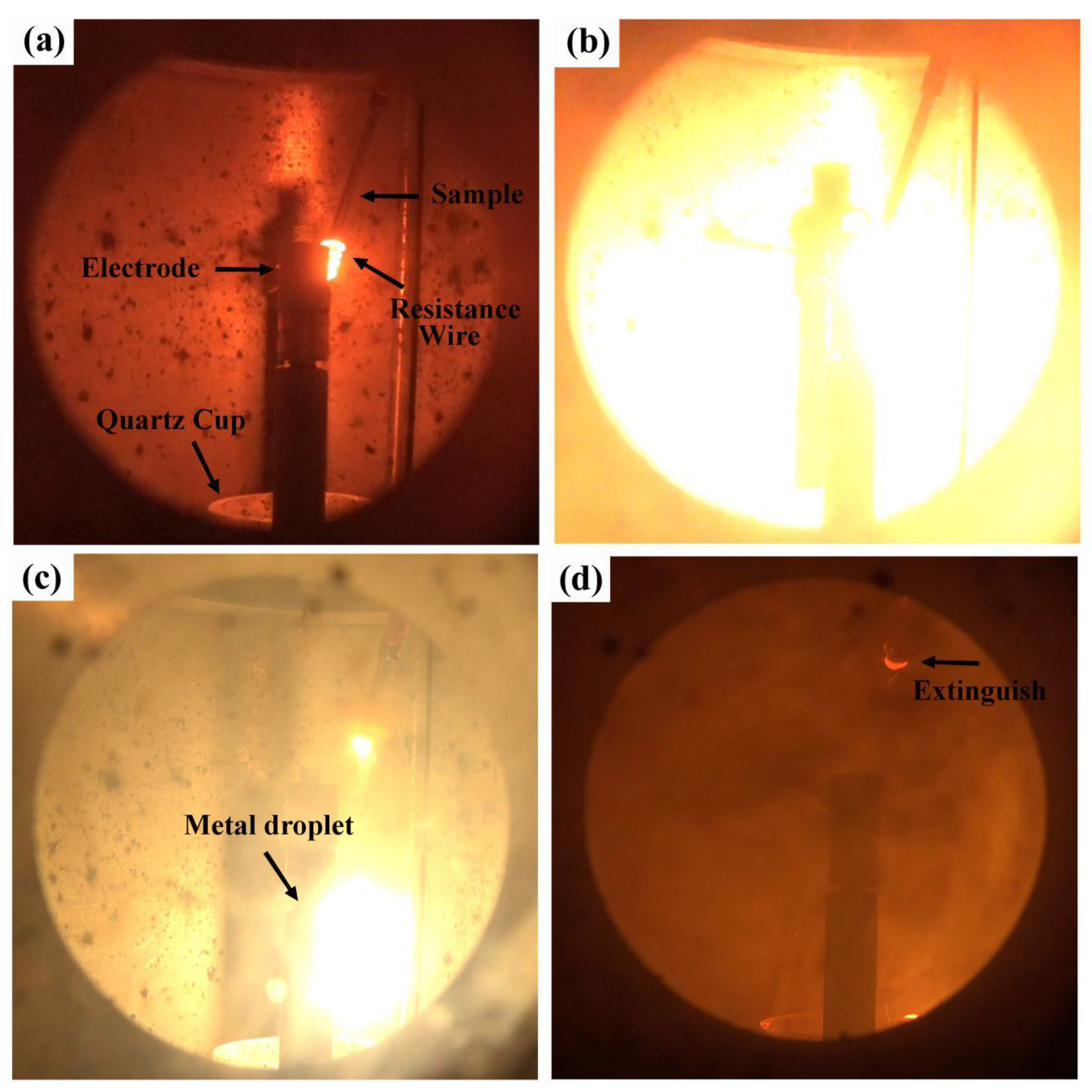
2.2. Experimental Process
2.3. Characterization Methods
3. Results and Discussion
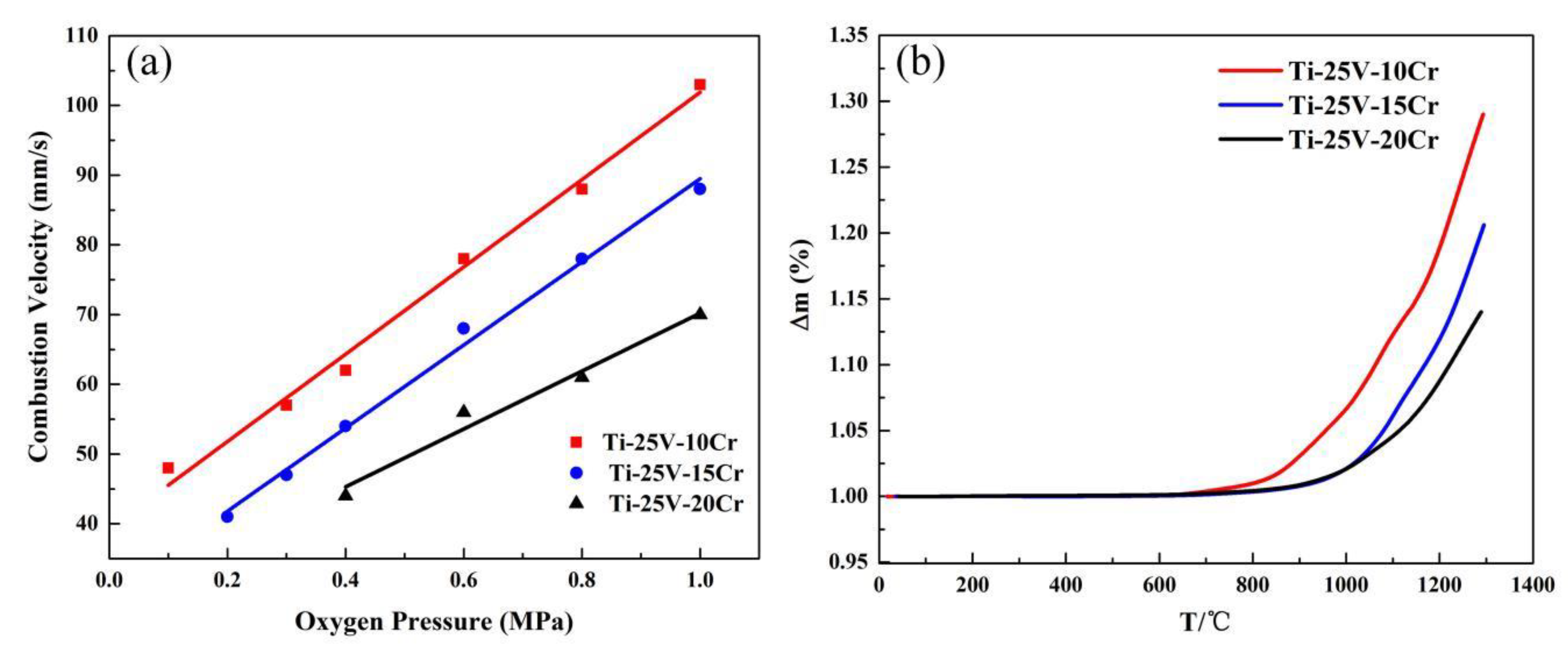
3.1. Combustion Characteristics
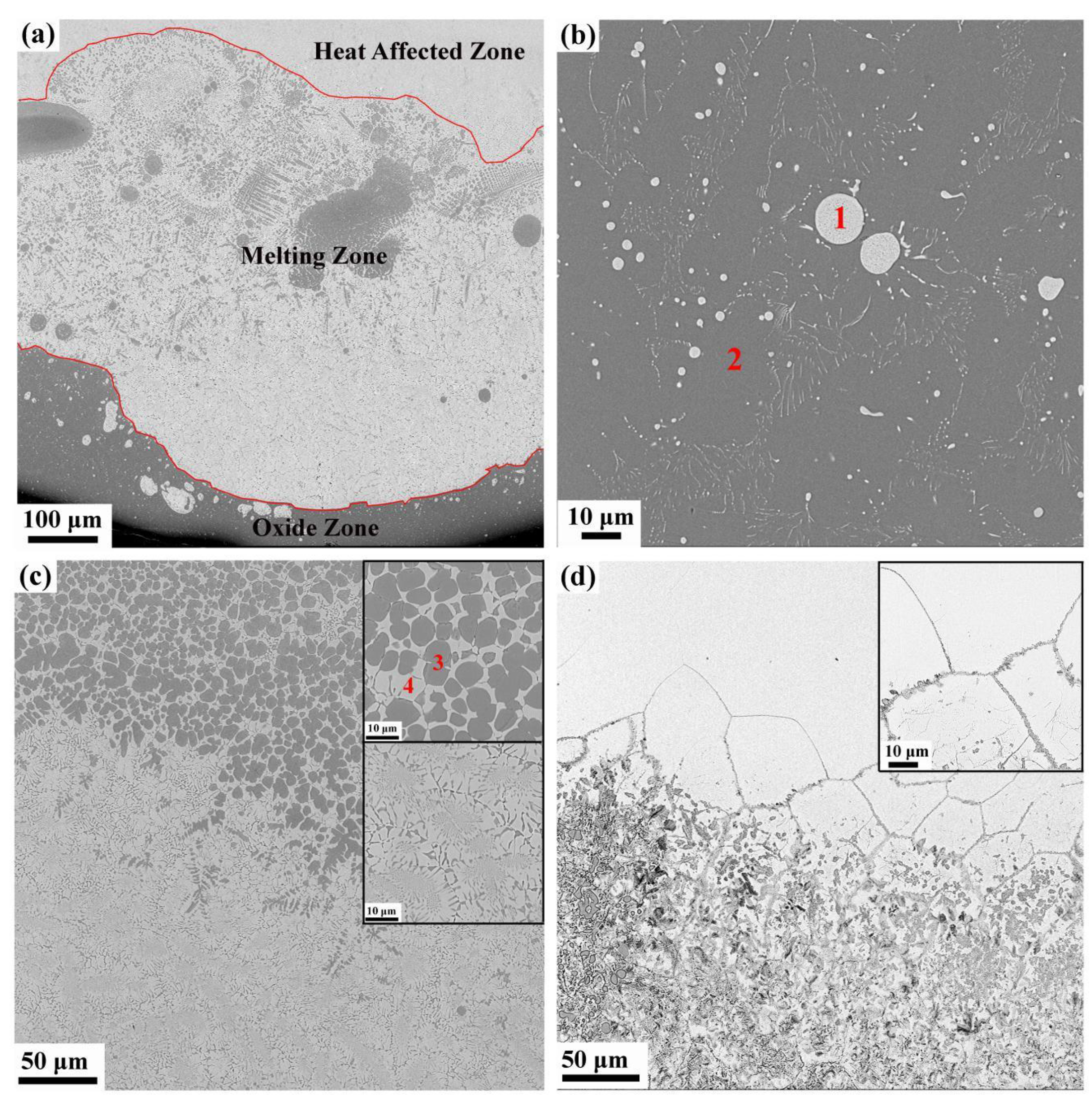
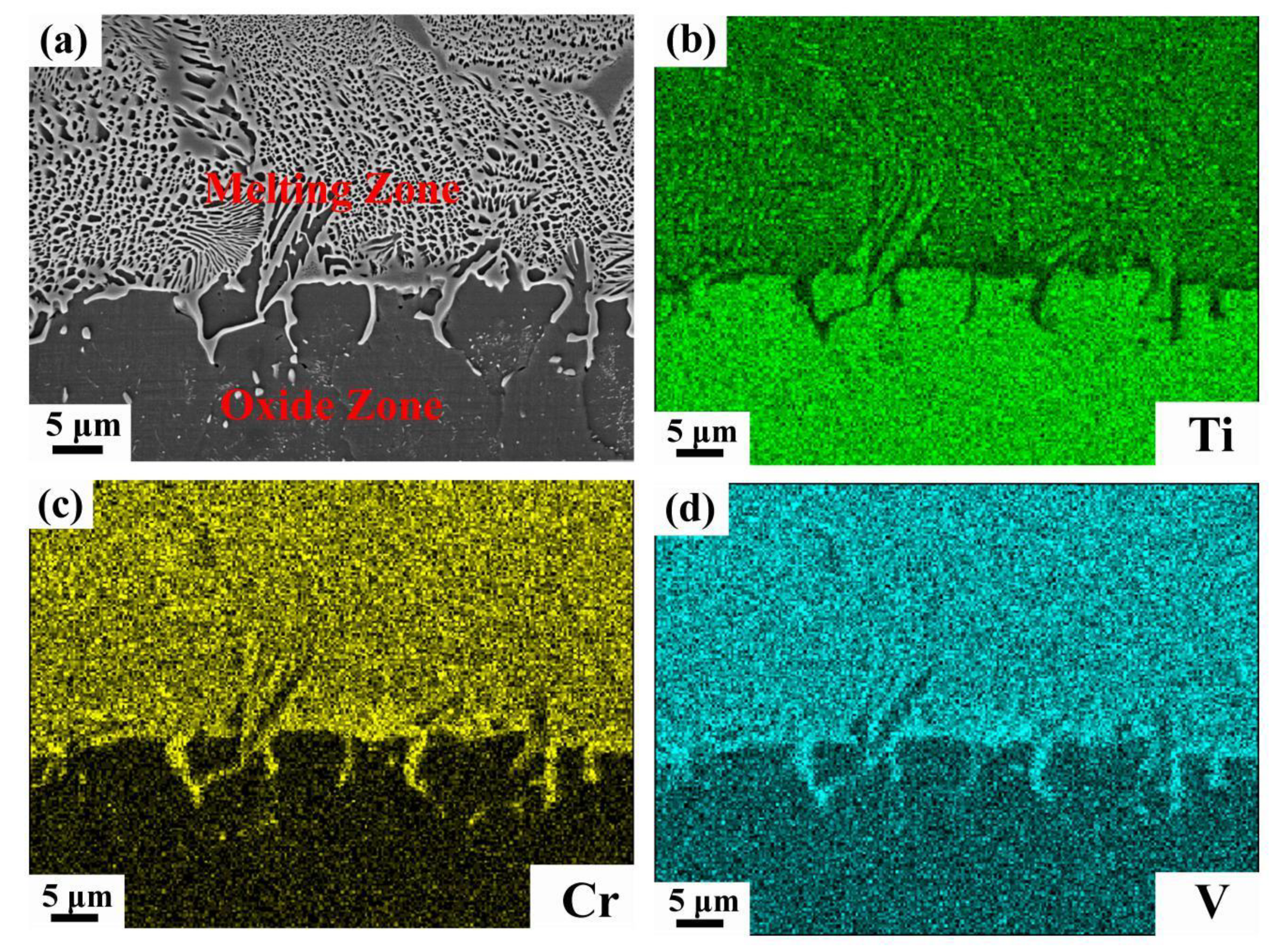
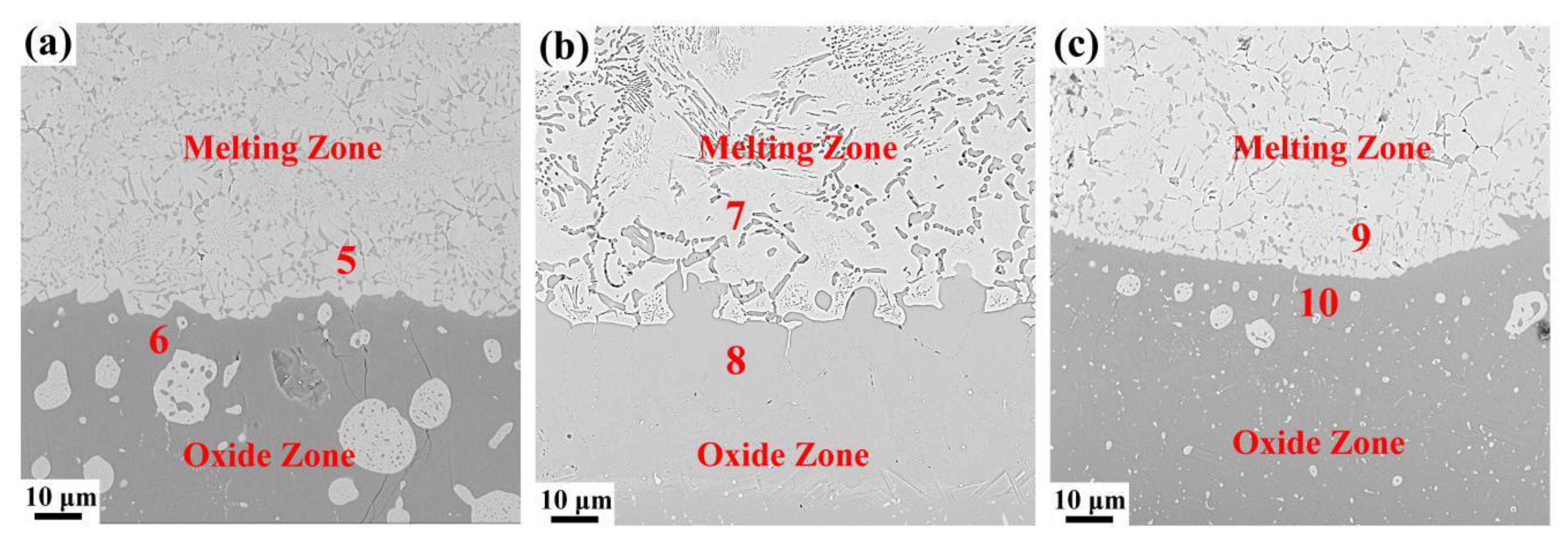
3.2. Microstructure and Composition
3.3. The Role of Alloying Elements
4. Conclusions
- The post-combustion Ti-Cr-V alloys consist of three different zones, namely the heat-affected zone, melting zone and oxide zone.
- The oxide zone is composed of titanium oxides, Cr2O3 and V2O5, moreover, the Cr2O3/V2O5 composites are spherical and they randomly disperse in the oxide zone.
- The combustion velocity decreases with increasing Cr content, and the variation in the combustion velocity is related to the enrichment of Cr and V in the melting zone. This enrichment phenomenon in the melting zone reduces the heat created by combustion and further slows the combustion velocity.
Author Contributions
Funding
Conflicts of Interest
References
- Qian, J.H. Application and developement of new titanium alloys for aerospace. Chin. J. Rare Met. 2000, 24, 218–223. [Google Scholar]
- Zhao, Y.Q.; Xi, Z.P.; Qu, H. Current situation of titanium alloy materials used for national aviation. J. Aeronaut. Mater. 2003, 23, 215–219. [Google Scholar]
- Lutering, G.; Willams, J.C. Titanium; Springer: Berlin, Germany, 2003. [Google Scholar]
- Zhang, G.H.; Zhang, P.Z.; Huang, G.Q.; Peng, X.; Zheng, S.Q. Study on the burn-resistant properties of titanium alloy Ti-6Al-4V surface by diffusing copper. Rare Metal Mater. Eng. 2011, 40, 286–289. [Google Scholar]
- Zhao, Y.Q.; Zhu, K.Y.; Qu, H.L.; Wu, H. Microstructures of a burn resistant highly stabilized titanium alloy. Mater. Sci. Eng. A 2000, 282, 153–157. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhao, X.M.; Zhu, K.Y. Effect of major alloying elements on microstructure and mechanical properties of a highly β stabilized titanium alloy. Alloys Compd. 2009, 481, 190–194. [Google Scholar] [CrossRef]
- Xin, S.W.; Zhao, Y.Q.; Zeng, W.D.; Wu, H. Research on thermal stability of Ti40 alloy at 550 °C. Mater. Sci. Eng. A 2008, 477, 372–378. [Google Scholar] [CrossRef]
- Mi, G.B.; Huang, X.; Cao, J.X.; Cao, C.X.; Huang, X.S. Frictional ignition of Ti40 fireproof titanium alloys for aero-engine in oxygen-containing media. Trans. Nonferrous Metals Soc. China 2013, 23, 2270–2275. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhou, L.; Zhou, Y.G.; Qu, H.L.; Wu, H.; Yang, H.Y. Research on basic theories of Ti40 burn resistant titanium alloy. J. Aeronaut. Mater. 2006, 26, 233–237. [Google Scholar]
- Mi, G.B.; Huang, X.; Cao, J.X.; Cao, C.X. Ignition resistance performance and its theoretical analysis of Ti-V-Cr type fireproof titanium alloy. Acta Metall. Sin. 2014, 50, 575–586. [Google Scholar]
- Mi, G.B.; Cao, C.X.; Huang, X.; Cao, J.X.; Wang, B. Ignition resistance performance and its mechanism of TC11 titanium alloy for aero-engine. J. Aeronaut. Mater. 2014, 34, 83–91. [Google Scholar]
- Tian, W.; Wang, B. Combustion Characteristics of Titanium Alloy TC11. Gas Turb. Exp. Res. 2012, 25, 40–43. [Google Scholar]
- Huang, H.F.; Liu, S.L. Crack Growth Behavior in Titanium Alloy TC11 at Room and High Temperature. Acta Metall. Sin. 1999, 12, 77–81. [Google Scholar]
- Chen, Y.N.; Yang, W.Q.; Bo, A.X.; Zhan, H.F.; Zhao, Y.Q.; Zhao, Q.Y.; Wan, M.P.; Gu, Y.T. Underlying burning resistant mechanisms for titanium alloy. Mater. Des. 2018, 156, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.N.; Yang, W.Q.; Bo, A.X.; Zhan, H.F.; Huo, Y.Z. Tailorable Burning Behavior of Ti14 Alloy by Controlling Semi-Solid Forging Temperature. Materials 2016, 9, 697. [Google Scholar] [CrossRef]
- Chen, Y.N.; Huo, Y.Z.; Song, X.D.; Bi, Z.Z.; Cao, Y.; Zhao, Y.Q. Burn-resistant behavior and mechanism of Ti14 alloy. Int. J. Min. Metall. Materials 2016, 23, 215–221. [Google Scholar]
- Zhu, K.Y.; Zhao, Y.Q.; Qu, H.L.; Wu, Z.L.; Zhao, X.M. Microstructure and properties of burn-resistant Ti-Al-Cu alloys. J. Mater. Sci. 2000, 35, 5609–5612. [Google Scholar] [CrossRef]
- Kikuchi, M.; Takada, Y.; Kiyosue, S.; Yoda, M.; Woldu, M.; Cai, Z.; Okuno, O. Mechanical properties and microstructures of cast Ti-Cu alloys. Dent. Mater. 2003, 19, 174–181. [Google Scholar] [CrossRef]
- ASTM. ASTM G124 Standard Test Method for Determining the Burning Behavior of Metallic Materials in Oxygen-Enriched Atmospheres, Annual Book of ASTM Standards; ASTM Int.: West Conshohocken, PA, USA, 2010; Volume 14.04, pp. 1–10. [Google Scholar]
- Muller, M.; ElRabii, H.; Fabbro, R. Liquid phase combustion of iron in an oxygen atmosphere. J. Mater. Sci. 2015, 50, 3337–3350. [Google Scholar] [CrossRef]
- ElRabii, H.; Kazakov, K.A.; Muller, M. Experimental and theoretical study of iron and mild steel combustion in oxygen flows. Phys. Fluids 2017, 29, 1–17. [Google Scholar]
- Zhao, Y.Q.; Zhou, L.; Deng, J. Study on the burning behavior of Ti-Cr binary. alloys and their burning products. J. Aeronaut. Mater. 2001, 1, 6–9. [Google Scholar]
- Zhao, Y.Q.; Zhou, L.; Deng, J. Effect of Alloying Element,Cr,on the Burning Behavior of Titanium Alloys. Rare Met. Mater. Eng. 1999, 28, 132–135. [Google Scholar]
- Zhao, Y.Q.; Zhou, L.; Zhu, K.Y.; Qu, H.L.; Wu, H. Mechanism of burn resistance of alloy Ti40. J. Mater. Sci. Technol. 2001, 17, 677–678. [Google Scholar]
- Liu, Y.Q.; Bai, K.W.; Shen, J.Y.; Zhang, Z. Thermodynamic Analysis of Burn-resistance Mechanism for Ti-Cr-V and Ti-Cr-Mo Alloys. Chin. J. Rare Met. 1998, 22, 204–207. [Google Scholar]
- Ouyang, P.; Mi, G.; Cao, J.; Huang, X.; He, L.; Li, P. Microstructure Characteristics after combustion and fireproof mechanism of TiAl-based alloys. Mater. Today Commun. 2018, 16, 364–373. [Google Scholar] [CrossRef]
- Newton, B.; Hull, W.C.; Stradling, J. A Database for Metallic and Nonmetallic Materials Commonly Utilized in Oxygen Service. ASTM Spec. Tech. Publ. 1997, 19, 458–467. [Google Scholar]


| Region | Composition | |||
|---|---|---|---|---|
| Ti | Cr | V | O | |
| 1 (at%) | 7.47 | 30.01 | 39.06 | 23.45 |
| 1 (wt%) | 8.35 | 36.43 | 46.45 | 8.76 |
| 2 (at%) | 61.42 | 0.10 | 2.21 | 36.27 |
| 2 (wt%) | 80.82 | 0.14 | 3.09 | 15.95 |
| 3 (at%) | 65.94 | 4.20 | 9.06 | 20.80 |
| 3 (wt%) | 75.72 | 5.23 | 11.07 | 8.76 |
| 4 (at%) | 7.47 | 30.01 | 39.06 | 15.84 |
| 4 (wt%) | 11.22 | 37.49 | 45.71 | 5.58 |
| Region | Composition | |||
|---|---|---|---|---|
| Ti | Cr | V | O | |
| 5 (at%) | 27.71 | 16.61 | 23.13 | 32.55 |
| 5 (wt%) | 34.11 | 22.21 | 30.29 | 13.39 |
| 6 (at%) | 54.11 | -- | 1.02 | 44.87 |
| 6 (wt%) | 77.09 | -- | 1.55 | 21.37 |
| 7 (at%) | 23.85 | 22.86 | 25.29 | 28 |
| 7 (wt%) | 28.08 | 31.68 | 31.68 | 11.02 |
| 8 (at%) | 63.33 | 0.53 | 3.02 | 33.12 |
| 8 (wt%) | 80.99 | 0.74 | 4.11 | 14.16 |
| 9 (at%) | 7.65 | 25.20 | 32.78 | 34.37 |
| 9 (wt%) | 9.40 | 33.63 | 42.86 | 14.11 |
| 10 (at%) | 52.24 | 0.11 | 1.14 | 46.52 |
| 10 (wt%) | 75.58 | 0.17 | 1.76 | 22.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, L.; Wang, Y.; Xie, G.; Li, H.; Xiong, J.; Yu, J.; He, G.; Huang, J. Combustion Mechanism of Alloying Elements Cr in Ti-Cr-V Alloys. Materials 2019, 12, 3206. https://doi.org/10.3390/ma12193206
Shao L, Wang Y, Xie G, Li H, Xiong J, Yu J, He G, Huang J. Combustion Mechanism of Alloying Elements Cr in Ti-Cr-V Alloys. Materials. 2019; 12(19):3206. https://doi.org/10.3390/ma12193206
Chicago/Turabian StyleShao, Lei, Yayu Wang, Guoliang Xie, Hongying Li, Jiashuai Xiong, Jiabin Yu, Guangyu He, and Jinfeng Huang. 2019. "Combustion Mechanism of Alloying Elements Cr in Ti-Cr-V Alloys" Materials 12, no. 19: 3206. https://doi.org/10.3390/ma12193206




