Effect of Zn/Mg Ratios on Microstructure and Stress Corrosion Cracking of 7005 Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
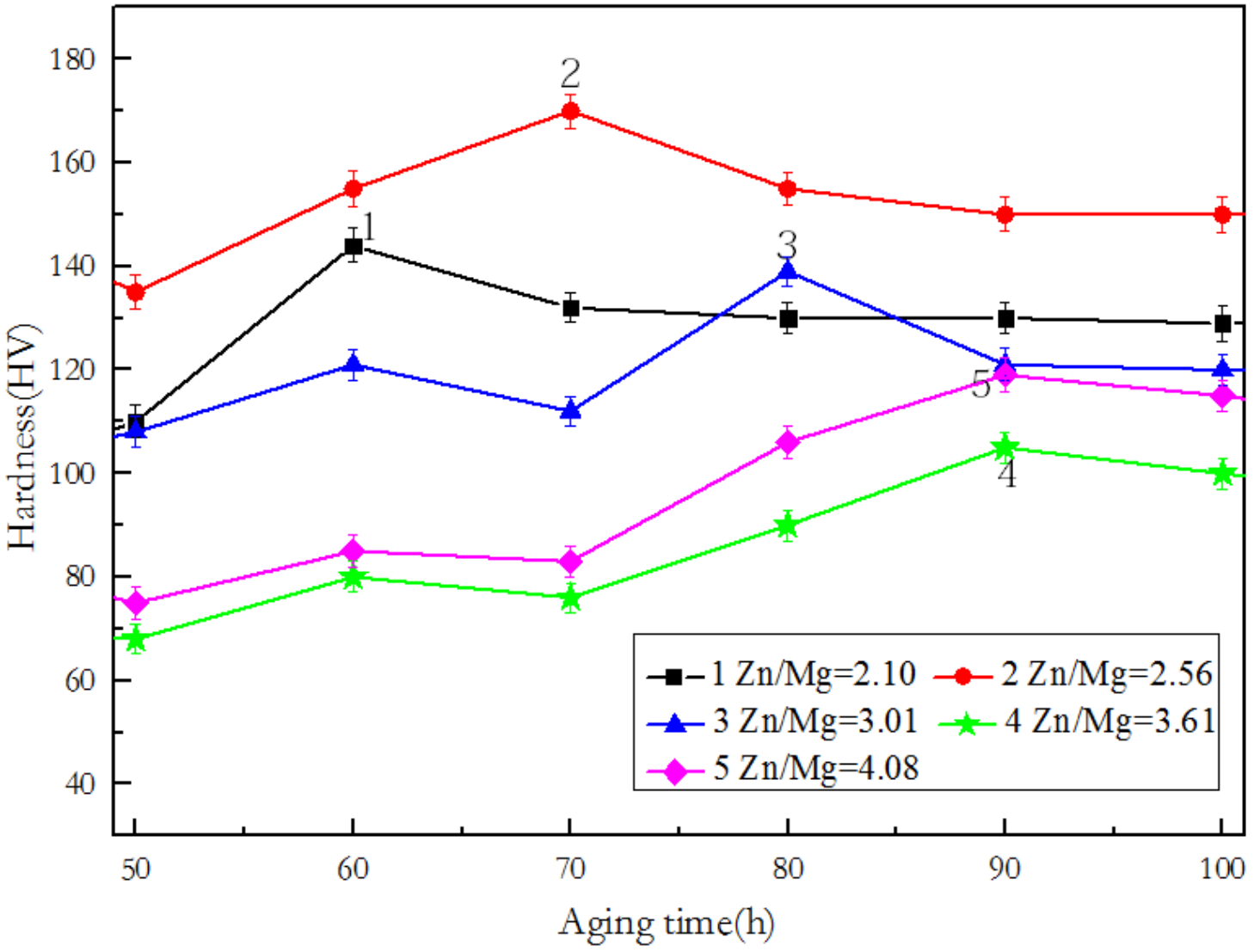
3.1. Hardness and Conductivity in Relation with the Zn/Mg Ratio of the Alloy
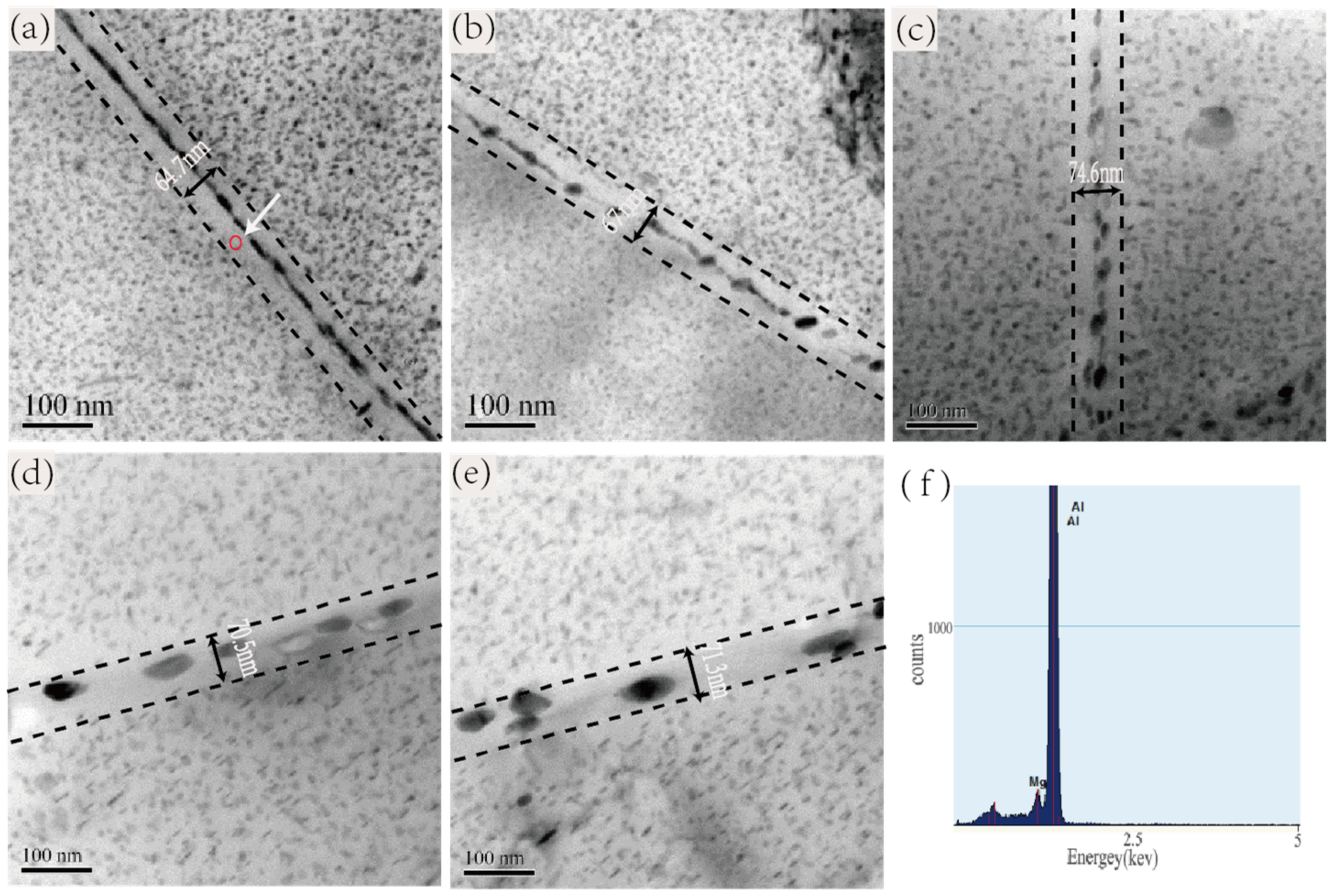
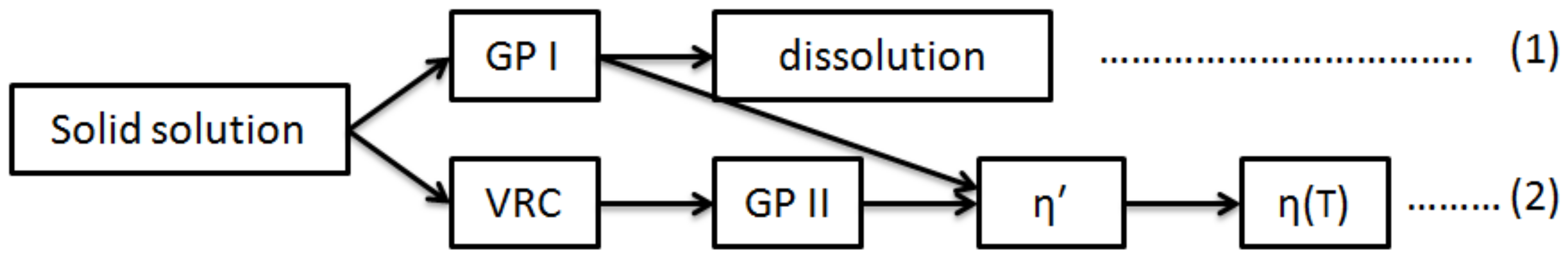
3.2. Precipitation Behaviors in Relation with the Zn/Mg Ratio of the Alloy
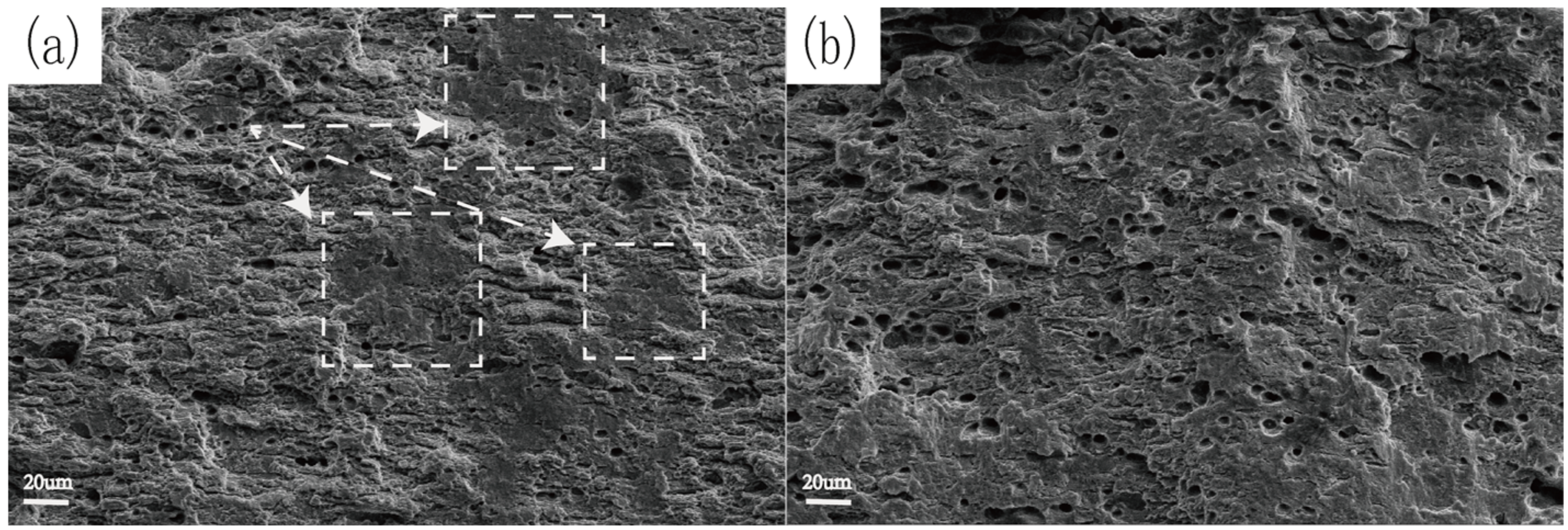
3.3. Mechanical Properties and Resistance of Stress Corrosion Cracking in Relation with the Zn/Mg Ratio of the Alloy
4. Discussion
4.1. Effect of Zn/Mg Ratio on Peak Hardness, Aging Time, and Conductivity
4.2. Influence of Zn/Mg Ratio on SCC Performance
5. Conclusion
- As the Zn/Mg ratio increases, the aging time to reach the peak-aged hardness gradually increases. When the Zn/Mg ≤ 2.6, the intragranular precipitate phases mainly consist of GP I zones and the disc-like η’ phase with small size. The grain boundary precipitates are slender and continuous, and the alloy has poor resistance of stress corrosion cracking in 3.5% NaCl + 0.5% H2O2 aqueous solution.
- When the Zn/Mg ≥ 3.61, the intragranular precipitate phase of alloy are mainly composed of GP II zones and the short-rod η’ phase with larger size. The grain boundary precipitates are larger and intermittently distributed and the PFZ is narrow, which means the alloy has good resistance to stress corrosion cracking in 3.5% NaCl + 0.5% H2O2 aqueous solution.
Author Contributions
Funding
Conflicts of Interest
References
- Qin, C.; Gou, G.Q.; Che, X.L.; Chen, H. Effect of composition on tensile properties and fracture toughness of Al-Zn-Mg alloy (A7N01S-T5) used in high speed trains. Mater. Des. 2016, 91, 278–285. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Chen, H. Influence of heat treatment on the strength and fracture toughness of 7N01 aluminum alloy. J. Alloys Compd. 2016, 678, 160–166. [Google Scholar] [CrossRef]
- Heinz, A.; Haszler, A.; Keidel, C.; Moldenhauer, S.; Benedictus, R.; Miller, W.S. Recent development in aluminium alloys for aerospace applications. Mat. Sci. Eng. A Struct. 2000, 280, 102–107. [Google Scholar] [CrossRef]
- Stiller, K.; Warren, P.J.; Hansen, V.; Angenete, J.; Gjonnes, J. Investigation of precipitation in an Al-Zn-Mg alloy after two-step ageing treatment at 100 °C and 150 °C. Mater. Sci. Eng. A. 1999, 270, 55–63. [Google Scholar] [CrossRef]
- Zhang, X.M.; Deng, Y.L.; Zhang, Y. Development of high strength aluminum alloys and processing techniques for the materials. Acta Metall. Sin. 2015, 51, 257–271. [Google Scholar]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Evolution of precipitate microstructures during the retrogression and re-ageing heat treatment of an Al-Zn-Mg-Cu alloy. Acta Mater. 2010, 58, 4814–4826. [Google Scholar] [CrossRef]
- Peng, X.; Guo, Q.; Liang, X.; Deng, Y.; Gu, Y.; Xu, G. Mechanical properties, corrosion behavior and microstructures of a nonisothermal ageing treated Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A. 2017, 688, 146–154. [Google Scholar] [CrossRef]
- Jiang, J.T.; Xiao, W.Q.; Yang, L.; Shao, W.Z.; Yuan, S.J.; Zhen, L. Ageing behavior and stress corrosion cracking resistance of a non-isothermally aged Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 2014, 605, 167–175. [Google Scholar] [CrossRef]
- Gruhl, W. The stress-corrosion behavior of high-strength Al-Zn-Mg alloys. Aluminium Alloys Aircr. Ind. 1978, 171–174. [Google Scholar]
- Reda, Y.; Abdel-Karim, R.; Elmahallawi, I. Improvements inmechanical and stress corrosion cracking properties in Al-alloy 7075 via retrogression and reaging. Mater. Sci. Eng. A 2008, 485, 468–475. [Google Scholar] [CrossRef]
- Knight, S.; Pohl, K.; Holroyd, N.; Birbilis, N.; Rometsch, P.; Muddle, B.; Goswami, R.; Lynch, S.P. Some effects of alloy composition on stress corrosion cracking in Al-Zn-Mg-Cu alloys. Corros. Sci. 2015, 98, 50–62. [Google Scholar] [CrossRef]
- Scamans, G.M.; Alani, R.; Swann, P.R. Pre-exposure embrittlement and stress corrosion failure in Al Zn Mg alloys. Corros. Sci. 1976, 16, 443–459. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, W.; Berghammer, R.; Gottstein, G. Microstructure evolution and deformation behavior of ultrafine-grained Al-Zn-Mg alloys with fine η′ precipitates. Acta Mater. 2010, 58, 6695–6705. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Early-stage precipitation in Al-Zn-Mg-Cu alloy (7050). Acta Mater. 2004, 52, 4503–4516. [Google Scholar] [CrossRef]
- Sha, G.; Cerezo, A. Kinetic Monte Carlo simulation of clustering in an Al-Zn-Mg-Cu alloy (7050). Acta Mater. 2005, 53, 907–917. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, J.; Li, Z.; Luo, R.; Chen, B. Precipitation in an Al-Zn-Mg-Cu alloy during isothermal aging: Atomicscale HAADF-STEM investigation. Mater. Sci. Eng. A 2017, 691, 60–70. [Google Scholar] [CrossRef]
- Li, C.; Pan, Q.L.; Shi, Y.J.; Wang, Y.; Li, B. Influence of aging temperature on corrosion behavior of Al-Zn-Mg-Sc-Zr alloy. Mater. Des. 2014, 55, 551–559. [Google Scholar] [CrossRef]
- Liu, J.Z.; Chen, J.H.; Yang, X.B.; Ren, S.; Wu, C.L.; Xu, H.Y.; Zou, J. Revisiting the precipitation sequence in Al-Zn-Mg-based alloys by high-resolution transimission electron microscopy. Scr. Mater. 2010, 63, 1061–1064. [Google Scholar] [CrossRef]
- Loffler, H.; Kovacs, I.; Lendvai, J. Decomposition processes in Al-Zn-Mg alloys. J. Mater. Sci. 1983, 18, 2215–2240. [Google Scholar] [CrossRef]
- Werenskiold, J.C.; Deschamps, A.; Bre’chet, Y. Characterization and modeling of precipitation kinetics in an Al-Zn-Mg alloy. Mater. Sci. Eng. A 2000, 239, 267–274. [Google Scholar] [CrossRef]
- Berg, L.K.; Gjønnes, J.; Hansen, V.; Li, X.Z.; Knutson-Wedel, M.; Waterloo, G.; Schryvers, D.; Wallenberg, L.R. GP-zones in Al-Zn-Mg alloys and their role in artificial aging. Acta Mater. 2001, 49, 3443–3451. [Google Scholar] [CrossRef]
- Jiang, X.J.; Tafto, J.; Noble, B.; Holme, B.; Waterloo, G. Differential scanning calorimetry and electron diffraction investigation on low-temperature aging in Al-Zn-Mg alloys. Metall. Mater. Trans. A 2000, 31, 339–348. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.K. Guinier-Preston zones in a high-purity Al-Zn-Mg alloy. Philos. Mag. Lett. 1994, 70, 135–140. [Google Scholar] [CrossRef]
- Groma, G.; Kovacs-Csetenyi, E.; Kovács, I.; Lendvai, J.; Ungár, T. The composition of Guinier-Preston zones in Al-Zn-Mg alloys. Philos. Mag. A 1979, 40, 653. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Jayaganthan, R. Effect of ageing on microstructure and mechanical properties of bulk, cryorolled, and room temperature rolled Al 7075 alloy. J. Alloys Compd. 2011, 509, 9609–9616. [Google Scholar] [CrossRef]
- Engdahl, T.; Hansen, V.; Warren, P.J.; Stiller, K. Investigation of fine scale precipitates in Al-Zn-Mg alloys after various heat treatments. Mater. Sci. Eng. A Struct. 2002, 327, 59–64. [Google Scholar] [CrossRef]
- Starink, M.J.; Li, X.M. A model for the electrical conductivity of peak-aged and overaged Al-Zn-Mg-Cu alloys. Metall. Mater. Trans. A 2003, 34, 899–911. [Google Scholar] [CrossRef]
- Winkler, L.; Ryan, M.P.; Flower, H.M. Pitting corrosion in cast 7XXX aluminium alloys and fibre reinforced MMCs. Corros. Sci. 2004, 46, 893–902. [Google Scholar] [CrossRef]
- Starink, M.J.; Wang, S.C. A modle for the yield strength of overaged AL-Zn-Mg-Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Ma, K.; Hu, T.; Yang, H.; Topping, T.; Yousefiani, A. Coupling of dislocations and precipitates: Impact on the mechanical behavior of ultrafine grained Al-Zn-Mg alloys. Acta Mater. 2016, 103, 153–164. [Google Scholar] [CrossRef]
- Gubicza, J.; Schiller, I.; Chinh, N.Q.; Illy, J.; Horita, Z. The effect of severe plastic deformation on precipitation in supersaturated Al-Zn-Mg alloys. Sci. Eng. A Struct. A 2007, 460, 77–85. [Google Scholar] [CrossRef]
- Yang, W.; Ji, S.; Wang, M.; Li, Z. Precipitation behaviour of Al-Zn-Mg-Cu alloy and diffraction analysis. J. Alloys Compd. 2014, 610, 623–629. [Google Scholar] [CrossRef]
- Umamaheshwer Rao, A.C.; Vasu, V.; Govindaraju, M.; Saisrinadh, K.V. Stress corrosion cracking behaviour of 7xxx aluminum alloys: A literature review. Trans. Nonferrous Met. Soc. China 2016, 26, 1447–1471. [Google Scholar]
- Oliveira Jr, A.F.; De Barros, M.C.; Cardoso, K.R.; Travessa, D.N. The effect of RRA on the strength and SCC resistance on AA7050 and AA7150 aluminium alloys. Mater. Sci. Eng. A 2004, 379, 321–326. [Google Scholar] [CrossRef]
- Buha, J.; Lumley, R.N.; Crosky, A.G. Secondary ageing in an aluminium alloy 7050. Mater. Sci. Eng. A 2008, 492, 1–10. [Google Scholar] [CrossRef]
- Kotsikosa, G.; Sutcliffe, J.M.; Holroyd, N.J.H. Hydrogen effcts in the corrosion fatigue behaviour of the white zones of 7xxx series aluminium alloy welds. Corros. Sci. 2000, 42, 17–33. [Google Scholar] [CrossRef]



| No. | Zn | Mg | Mn | Cu | Cr | Zr | Al | Zn/Mg |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.201 | 1.850 | 0.256 | 0.099 | 0.148 | 0.145 | 93.301 | 2.27 |
| 2 | 4.953 | 1.890 | 0.254 | 0.097 | 0.149 | 0.145 | 92.512 | 2.62 |
| 3 | 4.556 | 1.512 | 0.254 | 0.099 | 0.146 | 0.146 | 93.287 | 3.01 |
| 4 | 4.149 | 1.149 | 0.256 | 0.098 | 0.148 | 0.145 | 94.055 | 3.61 |
| 5 | 4.905 | 1.202 | 0.251 | 0.097 | 0.145 | 0.145 | 93.255 | 4.08 |
| No. | Rod Length of MPs (nm) | Disk Diameter of MPs (nm) | Average Area Fraction of MPs (%) | Average Length of GBPs (nm) | Average Width of GBPs (nm) | Average Width of PFZ (nm) |
|---|---|---|---|---|---|---|
| 1 | 5.7–17.5 | 3.5–18.5 | 41.2 ± 2.4 | 80.3 ± 9.5 | 15.36 ± 5.5 | 62.22 |
| 2 | 5.5–25.2 | 5.3–30.9 | 45.8 ± 2.2 | 30.3 ± 13.2 | 25.55 ± 7.1 | 65.08 |
| 3 | 11.4–30.2 | 10.6–45.5 | 33.6 ± 2.2 | 31.4 ± 10.8 | 35.8 ± 10.1 | 72.26 |
| 4 | 18.9–45.1 | 17.3–55.1 | 25.1 ± 2.2 | 59.8 ± 16.9 | 62.2 ± 20.5 | 69.33 |
| 5 | 32.8–65.8 | 22.2–65.7 | 27.5 ± 2.3 | 64.4 ± 21.4 | 70.5 ± 16.5 | 70.57 |
| No. | σy(air) (MPa) | σy(NaCl + H2O2) (MPa) | ∆(air) (%) | ∆(NaCl + H2O2) (%) | Rscc |
|---|---|---|---|---|---|
| 1 | 451 | 377 | 13.33 | 3.1 | 0.835 |
| 2 | 503 | 426 | 13.74 | 3.24 | 0.846 |
| 3 | 422 | 381 | 15.66 | 3.86 | 0.906 |
| 4 | 311 | 288 | 17.09 | 4.25 | 0.925 |
| 5 | 336 | 314 | 17.11 | 4.47 | 0.936 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Luo, B.; Bai, Z.; He, C.; Tan, S.; Jiang, G. Effect of Zn/Mg Ratios on Microstructure and Stress Corrosion Cracking of 7005 Alloy. Materials 2019, 12, 285. https://doi.org/10.3390/ma12020285
Wang S, Luo B, Bai Z, He C, Tan S, Jiang G. Effect of Zn/Mg Ratios on Microstructure and Stress Corrosion Cracking of 7005 Alloy. Materials. 2019; 12(2):285. https://doi.org/10.3390/ma12020285
Chicago/Turabian StyleWang, Shuai, Binghui Luo, Zhenhai Bai, Chuan He, Sizhi Tan, and Gen Jiang. 2019. "Effect of Zn/Mg Ratios on Microstructure and Stress Corrosion Cracking of 7005 Alloy" Materials 12, no. 2: 285. https://doi.org/10.3390/ma12020285
APA StyleWang, S., Luo, B., Bai, Z., He, C., Tan, S., & Jiang, G. (2019). Effect of Zn/Mg Ratios on Microstructure and Stress Corrosion Cracking of 7005 Alloy. Materials, 12(2), 285. https://doi.org/10.3390/ma12020285




