Design and Fabrication of High Activity Retention Al-Based Composite Powders for Mild Hydrogen Generation
Abstract
:1. Introduction
2. Materials and Methods
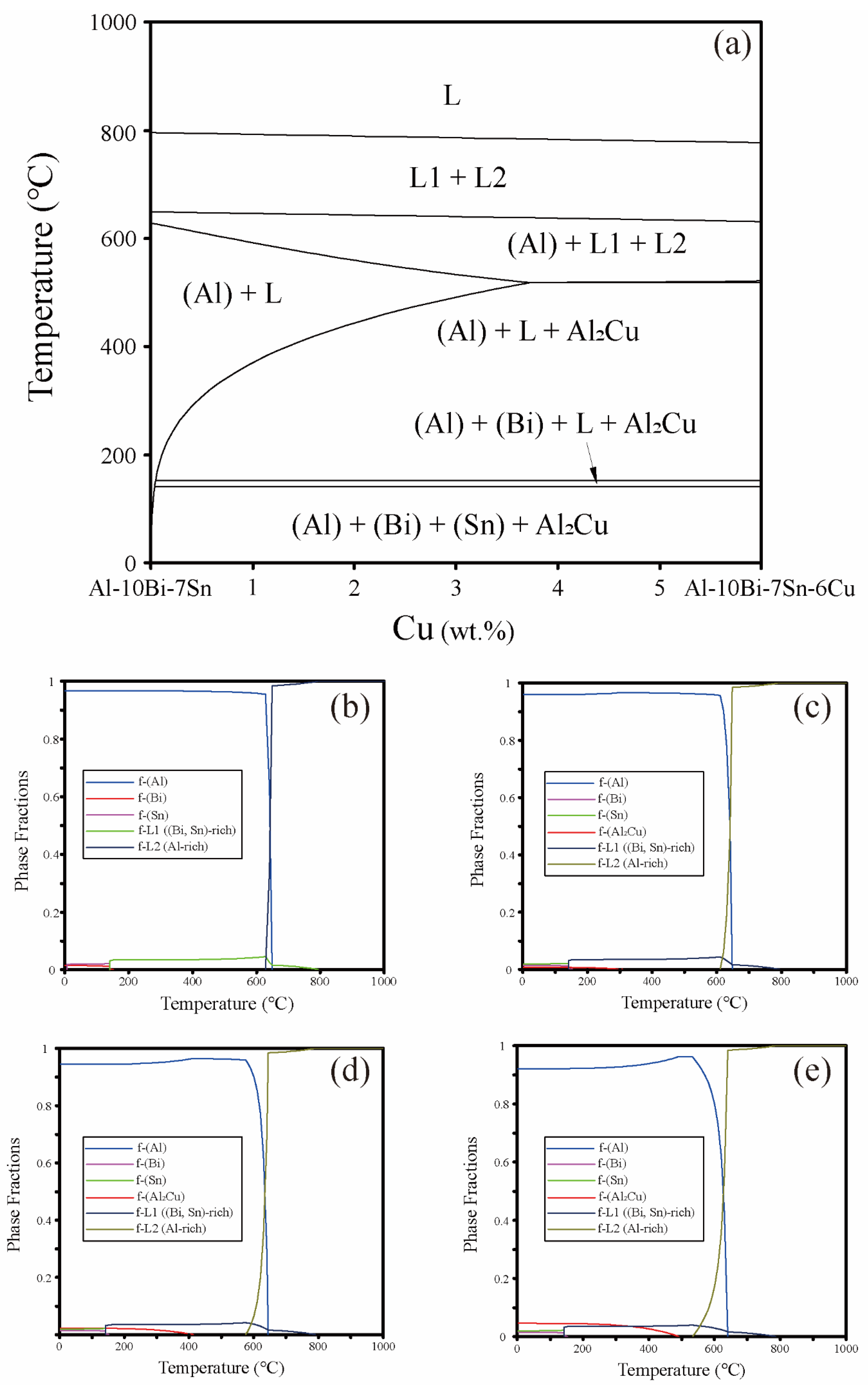
2.1. Design of Powder Composition
2.2. Powder Preparation
2.3. Hydrogen Generation Measurement
2.4. Characterization Methods
3. Results and Discussions
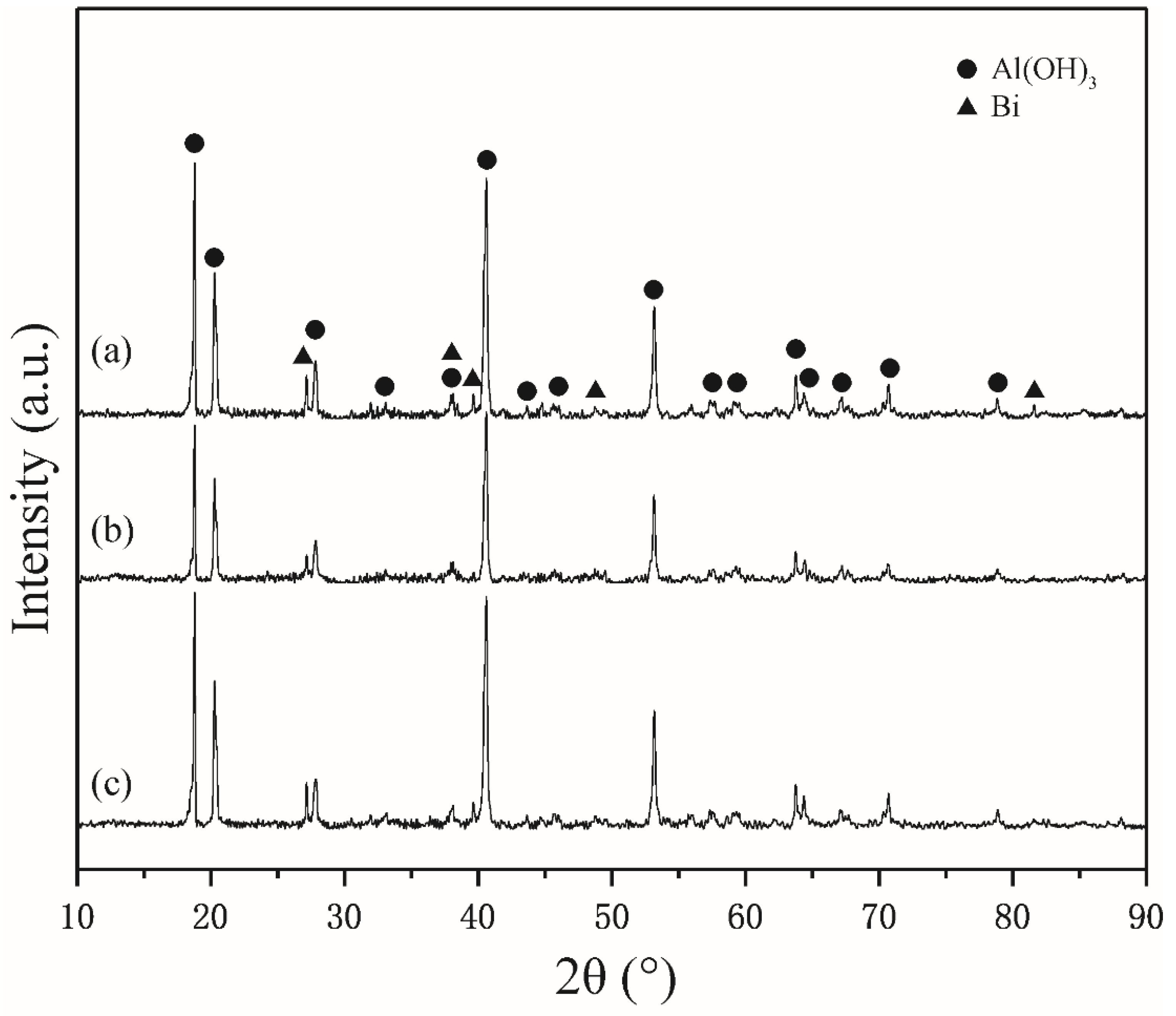
3.1. Morphology Observation and Microstructure Analysis
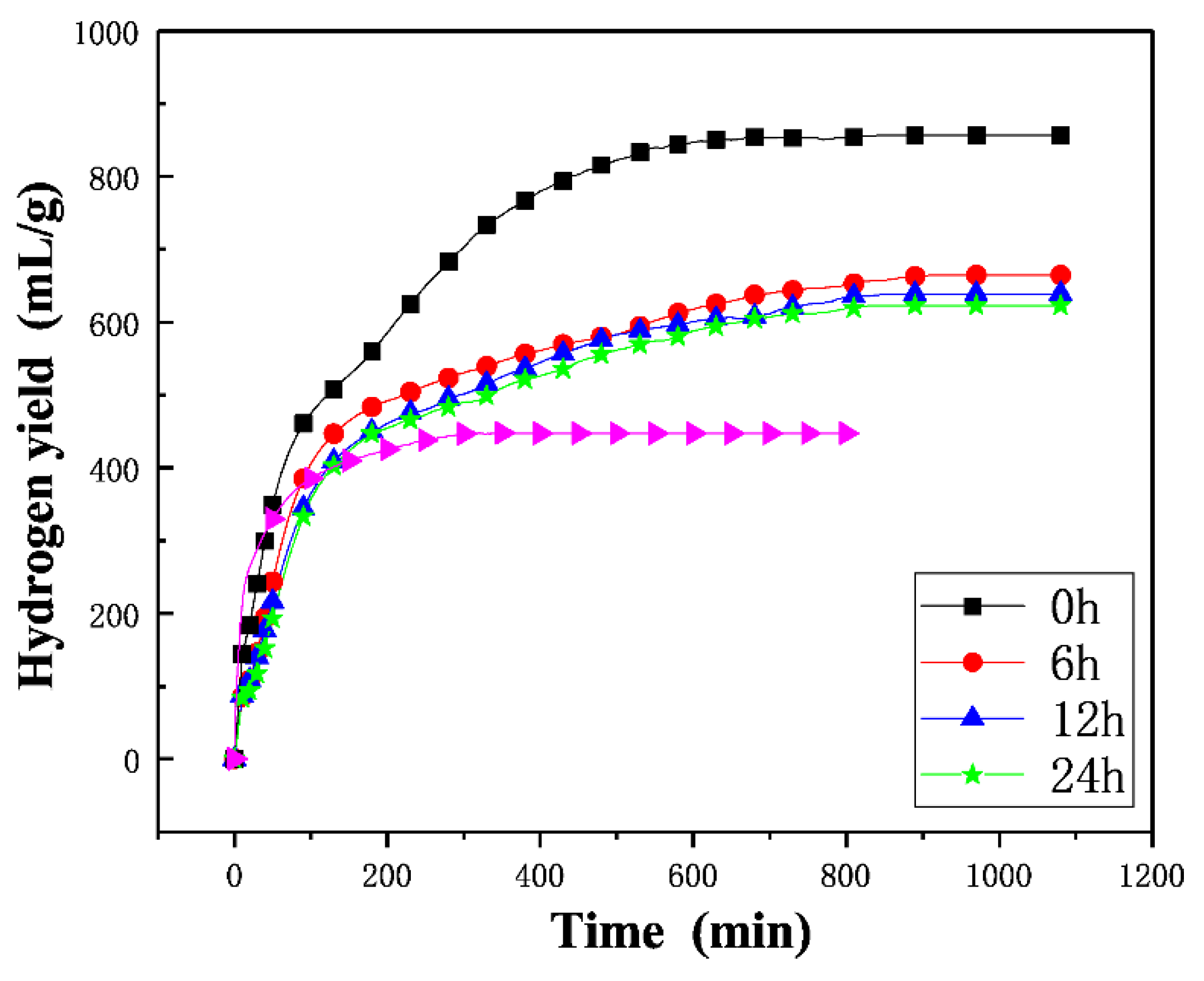
3.2. Hydrogen Generation Performance
3.3. Activity Maintenance Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fan, M.Q.; Xu, F.; Sun, L.X. Studies on hydrogen generation characteristics of hydrolysis of the ball milling Al-based materials in pure water. Int. J. Hydrogen Energy 2007, 32, 2809–2815. [Google Scholar] [CrossRef]
- Soler, L.; Macanas, J.; Munoz, M.; Casado, J. Aluminum and aluminum alloys as sources of hydrogen for fuel cell applications. J. Power Sources 2007, 169, 144–149. [Google Scholar]
- Nizovskii, A.I.; Belkova, S.V.; Novikov, A.A.; Trenikhin, M.V. Hydrogen production for fuel cells in reaction of activated aluminum with water. In Oil and Gas Engineering; Myshlyavtsev, A.V., Likholobov, V.A., Yusha, V.L., Eds.; Procedia Engineering Press: Omsk, Russia, 2015; Volume 113, pp. 8–12. [Google Scholar]
- Budak, Y.; Devrim, Y. Comparative study of PV/PEM fuel cell hybrid energy system based on methanol and water electrolysis. Energy Convers. Manag. 2019, 179, 46–57. [Google Scholar] [CrossRef]
- Neef, H.J. International overview of hydrogen and fuel cell research. Energy 2009, 34, 327–333. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, C.Y.; Lee, S.C. Technology forecasting and patent strategy of hydrogen energy and fuel cell technologies. Int. J. Hydrogen Energy 2011, 36, 6957–6969. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Kumar, A.; Prasad, R.; Upadhyay, S.N. Ethanol steam reforming for hydrogen production: Latest and effective catalyst modification strategies to minimize carbonaceous deactivation. Renew. Sustain. Energy Rev. 2017, 74, 89–103. [Google Scholar] [CrossRef]
- Li, A.; Sun, Y.; Yao, T.; Han, H. Earth-Abundant Transition-Metal-Based Electrocatalysts for Water Electrolysis to Produce Renewable Hydrogen. Chem. Eur. J. 2018, 24, 18334–18355. [Google Scholar] [CrossRef]
- Wang, H.Z.; Leung, D.Y.C.; Leung, M.K.H.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Tan, Z.; Ouyang, L.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Hydrogen generation by hydrolysis of Mg-Mg2Si composite and enhanced kinetics performance from introducing of MgCl2 and Si. Int. J. Hydrogen Energy 2018, 43, 2903–2912. [Google Scholar] [CrossRef]
- Herzog, F.; Glaubitz, D. Production of Hydrogen from the Na/Nah-Process. Int. J. Hydrogen Energy 1990, 15, 13–19. [Google Scholar] [CrossRef]
- Wegner, K.; Ly, H.C.; Weiss, R.J.; Pratsinis, S.E.; Steinfeld, A. In situ formation and hydrolysis of Zn nanoparticles for H2 production by the 2-step ZnO/Zn water-splitting thermochemical cycle. Int. J. Hydrogen Energy 2006, 31, 55–61. [Google Scholar] [CrossRef]
- Yavor, Y.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L. Comparative reactivity of industrial metal powders with water for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 1026–1036. [Google Scholar] [CrossRef]
- John, P.; George, T. Reaction of Aluminum with Water to Produce Hydrogen. US Dep. Energy 2008, 1, 1–26. [Google Scholar]
- Parmuzina, A.V.; Kravchenko, O.V. Activation of aluminium metal to evolve hydrogen from water. Int. J. Hydrogen Energy 2008, 33, 3073–3076. [Google Scholar] [CrossRef]
- Czech, E.; Troczynski, T. Hydrogen generation through massive corrosion of deformed aluminum in water. Int. J. Hydrogen Energy 2010, 35, 1029–1037. [Google Scholar] [CrossRef]
- Ho, C.Y.; Huang, C.H. Enhancement of hydrogen generation using waste aluminum cans hydrolysis in low alkaline de-ionized water. Int. J. Hydrogen Energy 2016, 41, 3741–3747. [Google Scholar] [CrossRef]
- Fang, C.S.; Gai, W.Z.; Deng, Z.Y. Al Surface Modification by a Facile Route. J. Am. Ceram. Soc. 2014, 97, 44–47. [Google Scholar] [CrossRef]
- Du Preez, S.P.; Bessarabov, D.G. Hydrogen generation by the hydrolysis of mechanochemically activated aluminum-tin-indium composites in pure water. Int. J. Hydrogen Energy 2018, 43, 21398–21413. [Google Scholar] [CrossRef]
- Yavor, Y.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L.; Stowe, R.; Ringuette, S. Enhanced hydrogen generation from aluminum-water reactions. Int. J. Hydrogen Energy 2013, 38, 14992–15002. [Google Scholar] [CrossRef]
- Fan, M.Q.; Sun, L.X.; Xu, F. Hydrogen production for micro-fuel-cell from activated Al-Sn-Zn-X (X: hydride or halide) mixture in water. Renew. Energy 2011, 36, 519–524. [Google Scholar] [CrossRef]
- Huang, T.; Gao, Q.; Liu, D.; Xu, S.; Guo, C.; Zou, J.; Wei, C. Preparation of Al-Ga-In-Sn-Bi quinary alloy and its hydrogen production via water splitting. Int. J. Hydrogen Energy 2015, 40, 2354–2362. [Google Scholar] [CrossRef]
- Wei, C.; Liu, D.; Xu, S.; Cui, T.; An, Q.; Liu, Z.; Gao, Q. Effects of Cu additives on the hydrogen generation performance of Al-rich alloys. J. Alloys Compd. 2018, 738, 105–110. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Liu, H.; Yang, T.; Chen, X.; Yang, S.; Liu, X. A Novel Self-Assembling Al-based Composite Powder with High Hydrogen Generation Efficiency. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, X.; Chen, X.; Yang, S.; Wang, C. Hydrogen generation from hydrolysis of activated Al-Bi, Al-Sn powders prepared by gas atomization method. Int. J. Hydrogen Energy 2017, 42, 10943–10951. [Google Scholar] [CrossRef]
- Pukrushpan, J.T.; Stefanopoulou, A.G.; Peng, H. Control of fuel cell breathing. IEEE Control Syst. Mag. 2004, 24, 30–46. [Google Scholar]
- Edwards, P.P.; Kuznetsov, V.L.; David, W.I.F.; Brandon, N.P. Hydrogen and fuel cells: Towards a sustainable energy future. Energy Policy 2008, 36, 4356–4362. [Google Scholar] [CrossRef]
- Kaban, I.; Hoyer, W. Effect of Cu and Sn on liquid-liquid interfacial energy in ternary and quaternary Al-Bi-based monotectic alloys. Mater. Sci. Eng. A 2008, 495, 3–7. [Google Scholar] [CrossRef]
- Al-Bi-Sn Liquidus Projection of Ternary Phase Diagram. Available online: https://materials.springer.com/isp/phase-diagram/docs/c_0950975 (accessed on 14 June 2019).
- Liu, T.; Leazer, J.D.; Menon, S.K.; Brewer, L.N. Microstructural analysis of gas atomized Al-Cu alloy feedstock powders for cold spray deposition. Surf. Coat. Technol. 2018, 350, 621–632. [Google Scholar] [CrossRef]
- Kim, M.; Eom, K.; Kwon, J.; Cho, E.; Kwon, H. On-board hydrogen production by hydrolysis from designed Al-Cu alloys and the application of this technology to polymer electrolyte membrane fuel cells. J. Power Sources 2012, 217, 345–350. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Lin, K.; Liu, Y.; Chen, X.; Zou, H.; Qiu, C.; Yang, S.; Liu, X. Design and Fabrication of High Activity Retention Al-Based Composite Powders for Mild Hydrogen Generation. Materials 2019, 12, 3328. https://doi.org/10.3390/ma12203328
Wang C, Lin K, Liu Y, Chen X, Zou H, Qiu C, Yang S, Liu X. Design and Fabrication of High Activity Retention Al-Based Composite Powders for Mild Hydrogen Generation. Materials. 2019; 12(20):3328. https://doi.org/10.3390/ma12203328
Chicago/Turabian StyleWang, Cuiping, Kairui Lin, Yuheng Liu, Xinren Chen, Hongwei Zou, Changrui Qiu, Shuiyuan Yang, and Xingjun Liu. 2019. "Design and Fabrication of High Activity Retention Al-Based Composite Powders for Mild Hydrogen Generation" Materials 12, no. 20: 3328. https://doi.org/10.3390/ma12203328



