1. Introduction
Functionalization of carbon-based materials, such as graphene and carbon nanotubes for different purposes, is gaining a lot of attention in the field of material science. This interest arises because of their low cost, unique stable physicochemical properties, and broad applications. The former includes energy-harvesting devices [
1], supercapacitors [
2], sensors [
3], field-effect transistors [
4] and medical uses [
5]. Certain extrinsic properties, such as electrical conductivity, high chemical stability, and a zero band-gap, enable some carbon-based materials to perform as semi-metals and semi-conductors [
6]. Graphene, for instance, has a zero band-gap which needs to be manipulated for use in various applications such as solar cells. When graphene is chemically doped, it can change one absorbed photon and cause an increase in power conversion efficiency of solar cells [
7]. However, graphene is a transparent material with a low coefficient of light absorption. Therefore, when graphene is applied in solar cells, it tends to produce a lower power conversion efficiency than solar cells based on heteroatom-doped graphene [
7]. Thus, creating a well-tuned and sizeable band-gap to improve the coefficient of light absorption of graphene is a great challenge but with enormous interest.
The band-gap of graphene can be tuned by altering the surface chemistry through substitutional doping [
8]. This can be achieved using selected heteroatoms to tune and enhance the band structure and conductivity [
9,
10]. Various heteroatoms that have commonly been employed in substitutional doping include boron [
11,
12,
13] and nitrogen [
14,
15,
16]. This is because they possess similar atomic radii and sizes to carbon and impact interesting electron chemistry within the graphene framework [
17]. These heteroatoms have a significant effect on the electrical properties of graphene which is shown by a p-type conductivity for boron whereas nitrogen results in n-type conductivity [
18]. In the case of strong p-type doping, it can be conferred by the interaction with the environment, hence, nitrogen-doping does not always confer n-type conductivity unless graphene is encapsulated [
19]. The nitrogen atom is mostly used in chemical doping of graphene or graphene oxide (GO). This is because nitrogen atom acts as defect site in the crystal structure of graphene and these defective centers can enhance the electrochemical activity of graphene or GO [
20].
Nitrogen-doping suppresses the density of state of graphene near the Fermi level and results to band-gap opening. Furthermore, nitrogen-doping tends to introduce strong electron donor states and leads to n-type or p-type semiconductor behavior depending on the bonding configuration. The conductivity and carrier mobilities of nitrogen-doped graphene are lower than pristine graphene due to the presence of nitrogen atom and defects introduced during the nitrogen-doping process which are capable of functioning as scattering centers that hinder the electron or hole transport [
21]. Boron-doping in graphene, results in a p-type doping and is also highly favorable. This is because B-C bond is about 0.5% longer than the C-C bond while N-C bond is about the same as C-C bond in length, enabling formation of relaxed structure of boron-doped graphene. Boron-doping tends to introduce more holes into the valence band of graphene resulting in a high carrier concentration. Boron-doped graphene is reported to have high conductivity compared to pristine graphene [
22] and nitrogen-doped graphene [
23], due to the large density of state near the Fermi level.
Doping GO with nitrogen, results in nitrogen-doped reduced graphene oxide (N-rGO). The ideal physicochemical properties of N-rGO for optoelectrical applications include a large surface area and high chemical stability. These physicochemical properties of N-rGO can be significantly enhanced by improving the atomic percentage of nitrogen [
24] and the bonding configuration [
25]. Various bonding configurations of nitrogen in N-rGO have been reported, e.g., pyrrolic-N [
25], pyridinic-N [
26], quaternary-N [
27,
28] and oxide-N [
29]. These bonding configurations impart various effects on the carrier concentration which tend to produce well-defined band structures in doped GO [
30].
The mechanism of formation of N-rGO is still a fascinating phenomenon because it is not well understood and there is more that can be done to manipulate it. Therefore, synthetic procedures for N-rGO need a certain level of control regarding the required extent of doping and the bonding configuration of nitrogen. Different synthetic approaches have been employed in the in-situ synthesis of N-rGO, such as arc discharge [
31], plasma method [
32], thermal annealing [
33] and chemical vapor deposition (CVD) [
34,
35]. The CVD approach is mostly preferred because it is easier to scale-up and produces relatively high-quality N-rGO. Scientific reports on the synthesis of N-rGO via the CVD approach indicate that the mostly used materials are metal catalyst (Cu, Ni, Co or Fe) [
36,
37] and organic molecules [
38].
In the CVD synthesis of N-rGO, several factors including the type of carrier gas, doping temperature and nitrogen precursor (used either as a solid, liquid or in the gaseous phase), influence the nitrogen content and properties of the final product [
39,
40]. Nang et al. [
41] and Panchakarla et al. [
42] reported the use of dimethylformamide and pyridine, respectively, as liquid nitrogen precursors for the synthesis of N-rGO, with the former achieving a very low nitrogen content of 0.64%. The drawback of liquid nitrogen precursors is that they are expensive, dangerous, and highly flammable when used in the CVD method.
The alternative to liquid and gaseous nitrogen precursors is solid nitrogen precursors. The use of solid nitrogen precursors, such as monoethanolamine [
43], urea [
33,
44], 1,3,5-triazine [
24], pentachloropyridine [
36] and the combination of imidazole and melamine [
45] have been reported and observed to result in high doping levels. Lu et al. [
24] reported the CVD synthesis of a few-layered nitrogen-doped graphene oxide containing atomic percentages of between 2.1 and 5.6% nitrogen by making use of the carbon and nitrogen precursor 1,3,5-triazine and Cu foil as catalyst. Doped graphene films with a higher nitrogen content of approximately 5.6% were obtained at a doping temperature of 990 °C, with melamine [
46] as a solid nitrogen precursor. The use of the solid nitrogen precursor, pentachloropyridine, in the synthesis of nitrogen-doped graphene was reported by Wan et al. [
36] to yield a nitrogen content between 4.4 and 7.5%. Solid nitrogen precursors are cost-effective and are easy to handle compared with liquid and gaseous nitrogen precursors.
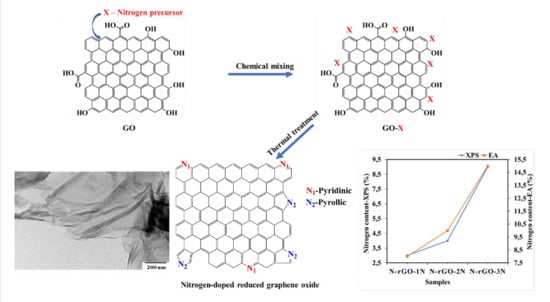
In this work, we report for the first time, the effect of different doping temperatures and solid nitrogen precursors on the physicochemical (nitrogen content, crystallinity, thermal stability and bonding configuration), optical (band-gap energy and charge recombination) and electrical conductivity properties of N-rGO. The synthesis of N-rGO (
Figure 1) was achieved by liquid exfoliation of GO, high temperature vapor reduction of GO and doping it with nitrogen atoms from various solid nitrogen precursors (4-nitroaniline, 4-aminophenol and 4-nitro-
ο-phenylenediamine). These nitrogen precursors were chosen because they possess different number of nitrogen atoms on their structures or frameworks, and therefore the effect of the number of nitrogen atoms contained in the nitrogen precursor was also investigated.
2. Materials and Methods
2.1. Materials and Instrumentation
Graphite powder (99.99% -trace metal basis), sodium nitrate (99%), potassium permanganate (99%), 4-nitroaniline (≥99%), 4-aminophenol (≥99%) and 4-nitro-ο-phenylenediamine (≥99%) were purchased from Sigma-Aldrich, Johannesburg, South Africa. These chemicals were of analytical grade and were used without further purification. Hydrogen peroxide (100%) was purchased from Merck Laboratory Supplies, Johannesburg, South Africa. Sulfuric acid (98%) was purchased from Promark Chemicals, Johannesburg, South Africa. Double distilled water was obtained from a double distiller, Glass Chem water distiller model Ws4lcd was supplied by Shalom Laboratory Supplies, Durban, South Africa. A gas mixture of 10% hydrogen in argon (v/v) was purchased from Afrox Limited Gas Co., Durban, South Africa. Weighing of N-rGO was done on an electronic weighing balance, Mettler AE 200, Mundelein, IL USA. Ultrasonication was performed in a digital ultrasonic water bath (400 W) obtained from Shalom Laboratory Supplies, Durban, South Africa.
2.2. Synthesis of N-rGO
A modified Hummer’s method was used to synthesize GO [
47]. In brief, approximately 1 g of graphite powder and 1 g of sodium nitrate were mixed with 50 mL of concentrated sulfuric acid in a 500 mL round-bottom flask placed in an ice-bath and stirred for 30 min. After that, 6 g of potassium permanganate was added slowly to the mixture with the temperature kept at 5 °C to prevent explosion and excessive heating. Thereafter, the mixture was stirred for 3 h at a temperature of 35 °C and then further treated with 200 mL of 3% hydrogen peroxide while stirring for 30 min. The resulting GO was washed with double distilled water until a pH of 6 was achieved. The product was then filtered and dried in the oven for 24 h at a temperature of 80 °C.
The synthesized GO was simultaneously reduced (using 10% hydrogen in argon as a reducing agent) and doped with different nitrogen precursors, namely 4-nitroaniline, 4-aminophenol and 4-nitro-ο-phenylenediamine. This was done by mixing 70 mg of GO and 30 mg of nitrogen precursor in 50 mL double distilled water, followed by sonication (25 °C) for 1 h. The mixture was further stirred and heated for 6 h at a temperature of 100 °C to remove the excess water. After drying, the resulting black solid was heat-treated in a ceramic quartz boat placed in a tube furnace (Elite Thermal Systems Ltd., Model TSH12/50/160) in a mixture of 10% hydrogen in argon (v/v) at a flow rate of 100 mL min−1. The doping temperature of the furnace was set at each of 600, 700 and 800 °C, for each nitrogen precursor. The carrier gas flow rate and the doping temperature were maintained constant throughout the synthesis period of 2 h. After 2 h, the furnace was allowed to cool naturally to room temperature and N-rGO was collected and subsequently characterized.
2.3. Physicochemical Characterization
The surface morphology of N-rGO was investigated by field emission scanning electron microscopy (FE-SEM, Carl Zeiss Ultra Plus, Cambridge, UK). Briefly, the aluminum stub sample holders were coated with piece of a sticky carbon tape; after that the N-rGO was sprinkled on the carbon tape and gold coated thrice before SEM analysis. The microstructural features of the N-rGO were evaluated by means of high-resolution–transmission electron microscopy (HR-TEM, JOEL JEM model 1010, Peabody, MA, USA), and set at an accelerating voltage of 100 kV at different magnifications.
The crystallinity or graphitic nature of the N-rGO was investigated with a Delta Nu Advantage 532TM Raman spectrometer (Laramie, WY, USA) equipped with NuSpecTM software (1.0., Microsoft Publisher, Redmond, WA, USA) and operated at a wavelength (λ) of 514.5 nm. The functional groups present in the N-rGO were investigated with a PerkinElmer Spectrum 100 Fourier transform infrared (FTIR) spectrometer (Akron, OH, USA) equipped with an attenuated total reflectance (ATR) accessory. Approximately 0.22 g of the N-rGO was pressed into a pellet for about 2 min, under a pressure of 10 Tons. The pellets were then placed on the diamond crystal for analysis.
The thermal stability of N-rGO was measured with a TA Instruments Q seriesTM thermal analysis instrument (DSC/thermogravimetric analysis (TGA) (SDT-Q600), New Castle, PA, USA) in air flowing at a rate of 50 mL min−1 and heated from room temperature up to 1000 °C at a ramping rate of 10 °C min−1. N-rGO were further characterized by X-ray photoelectron spectroscopy (XPS, Quantum 2000 with an X-ray source of monochromatic Al Kα (1486.7 eV), Chanhassen, MN, USA) to investigate the surface chemical composition of carbon and nitrogen.
A Micromeritics Tristar II 3020 surface area and porosity analyzer (Norcross, GA, USA) was used to determine the textural properties of N-rGO. Typically, a mass of approximately 0.1 g of N-rGO was degassed at 90 °C for 1 h, the temperature was then raised to 160 °C and the sample further degassed for 12 h using Micromeritics Vacprep 061 (sample degas system), before fitting N-rGO in the Micromeritics Tristar II instrument for analysis. The textural properties of the N-rGO were investigated at a temperature of −196 °C with N2 as the adsorbate. The specific surface areas were calculated with the Brunauer, Emmett and Teller (BET) model and the pore volumes were obtained by applying the Barrett-Joyner-Halenda (BJH) model.
The phase characteristics of the synthesized N-rGO were determined by X-ray powder diffraction (XRD, Rigaku/Dmax RB, The Woodlands, TX, USA) and the measurements were performed with graphite monochromated high-density with a 𝜃-𝜃 scan in locked coupled mode, using a Cu kα radiation source (λ = 0.15406 nm). The absorbance of N-rGO was recorded with an ultraviolet-visible spectrophotometer (UV-Vis, Shimadzu, UV-1800, Roodepoort, South Africa). The GO and N-rGO were first dispersed in absolute ethanol and then sonicated for 30 min before UV-Visible spectrophotometric analysis. Charge recombination analysis of N-rGO was investigated with a PerkinElmer LS 55 spectrofluorometer (Akron, OH, USA) fitted with solid sample accessory. Excitation was performed at 310 nm, and the emission spectrum recorded from 450 to 550 nm with an excitation slit and emission slit at 5 nm and 2 nm, respectively (slid position). Electrical conductivity of N-rGO was determined by four-point probe (Keithley 2400 source-meter, Beaverton, OR, USA) measurements which were carried out on pellets with a thickness of 0.2 mm formed from N-rGO (0.03 g).
4. Conclusions
In conclusion, N-rGO has been successfully synthesized from solid nitrogen precursors (4-aminophenol, 4-nitroaniline and 4-nitro-ο-phenylenediamine). The incorporation of N atom into the GO lattice at various doping temperatures caused a significant effect on the physicochemical properties such as surface morphology, surface chemistry, surface area, and porosity. Microscopic studies showed a more wrinkled-like structure on N-rGO than for GO due to the presence of nitrogen atoms in the GO framework. By lowering the doping temperature, a higher nitrogen content was incorporated into the GO lattice. The nitrogen content of N-rGO varied for different nitrogen precursors. N-rGO exhibited lower thermal stability as the level of nitrogen-doping increased, due to more defects and distortions experienced in the N-rGO structure. The enhancement of surface area and high degree of disorder on N-rGO were attributed to the removal of oxygen-containing functional groups.
N-rGO-1N-600 °C, N-rGO-2N-600 °C and N-rGO-3N-600 °C had a nitrogen content of 3.0, 3.7 and 8.5%, respectively. The nitrogen-rich precursor, 4-nitro-ο-phenylenediamine, lead to higher doping of N-rGO. N-rGO-3N-600 °C was found to have the highest nitrogen content of 8.5% and a high surface area of 154.02 m2 g−1 though it was less crystalline and manifested low thermal stability. The peak fitting of N 1s in all N-rGO samples produced two major components of pyridinic-N and pyrrolic-N with different nitrogen content. N-rGO showed good absorption and luminescence in the near UV region. The photoluminescence peak intensity and band-gap values were highly dependent on nitrogen content. A higher nitrogen content in N-rGO exhibited a smaller optical band-gap of 4.5 eV with lower photoluminescence peak intensity. N-rGO-3N-600 °C exhibited higher electrical conductivity of 0.133 S cm−1.