Study of the Catalytic Strengthening of a Vacuum Carburized Layer on Alloy Steel by Rare Earth Pre-Implantation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
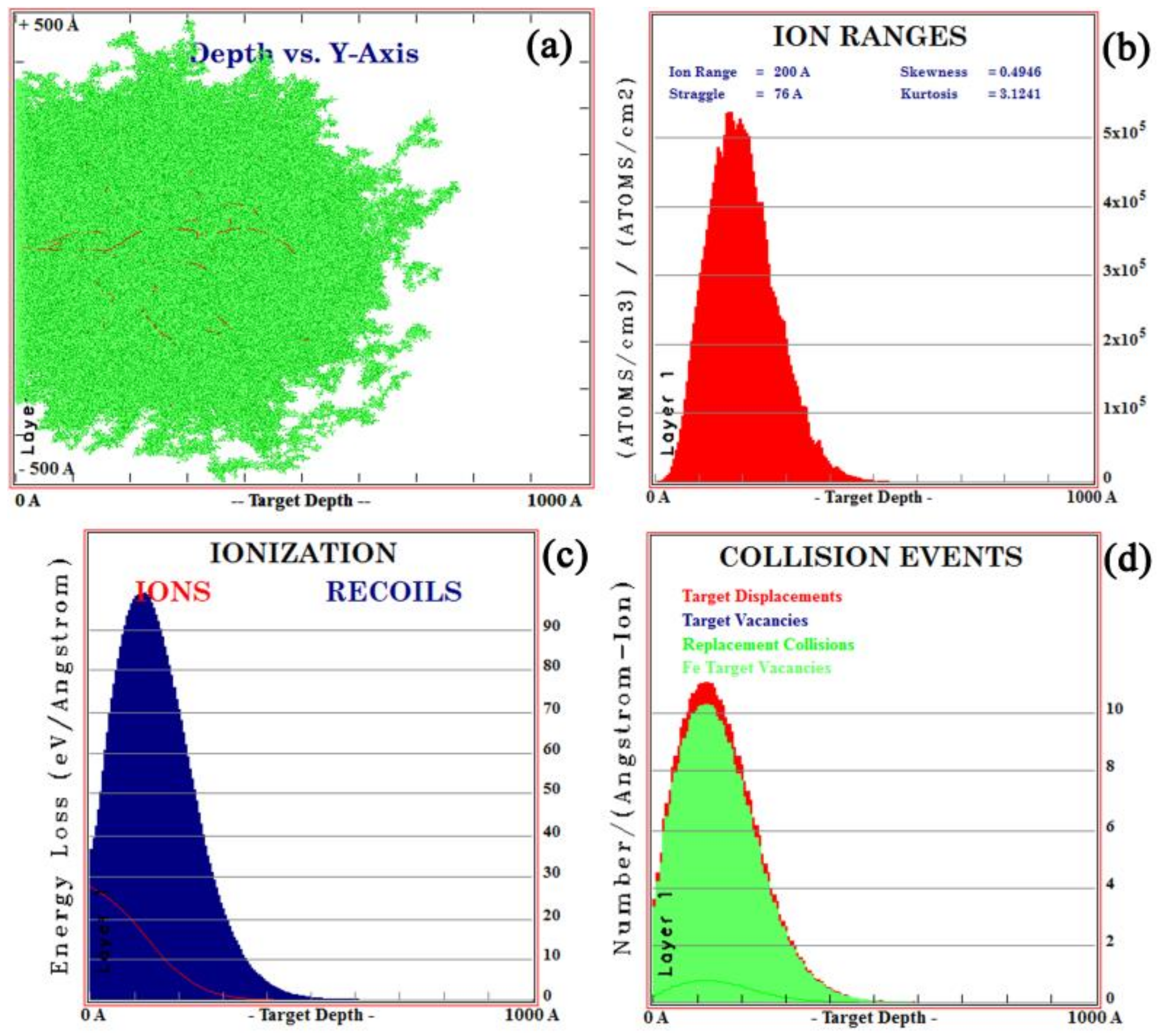
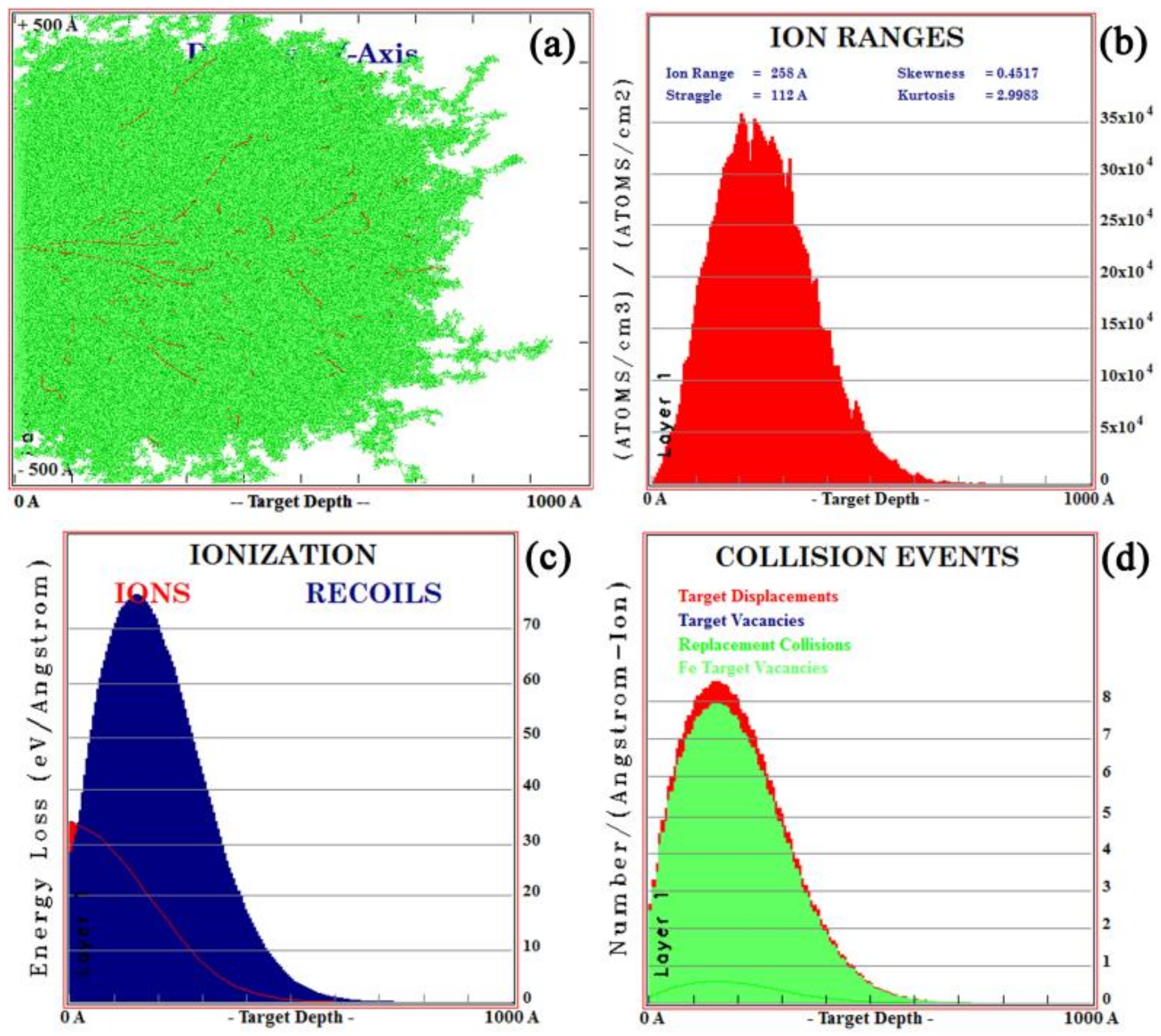
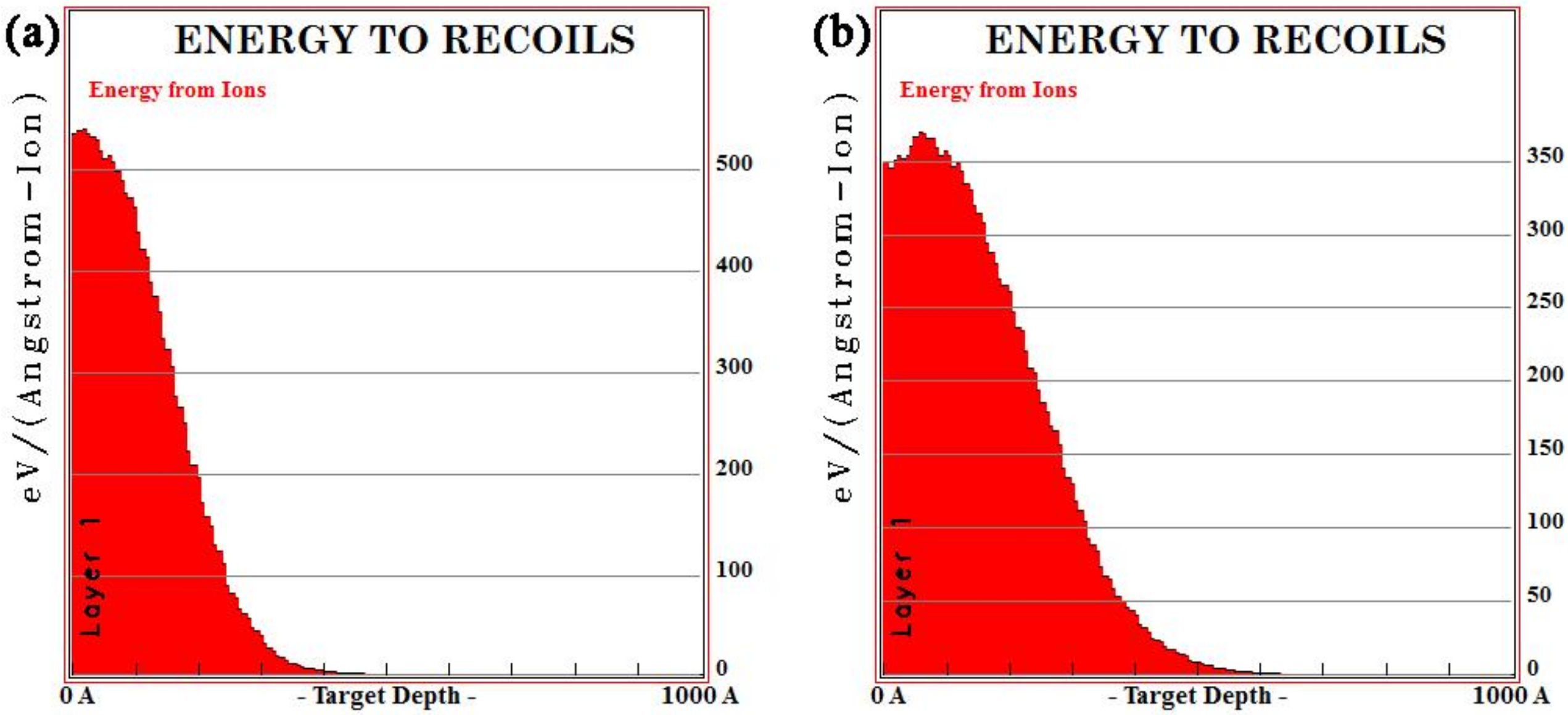
3.1. Calculation of the Implanted Ion Range
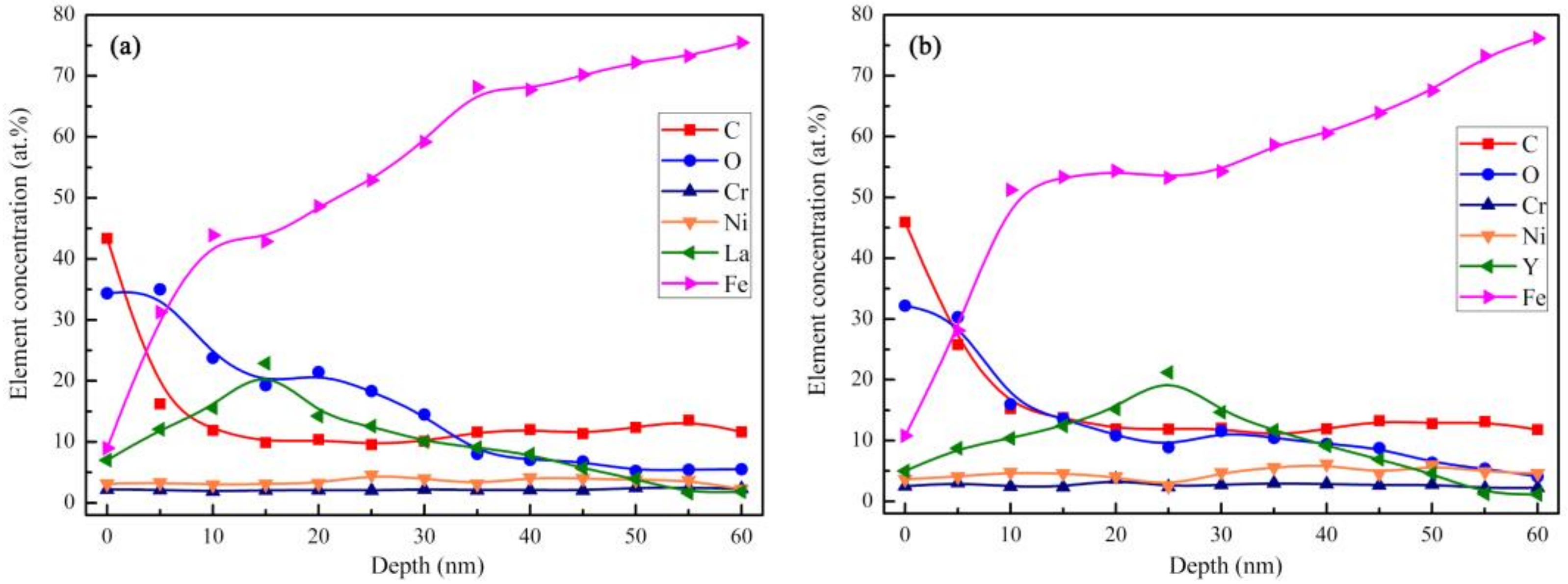
3.2. Chemical Composition and Structure of the Implanted Surface Layer
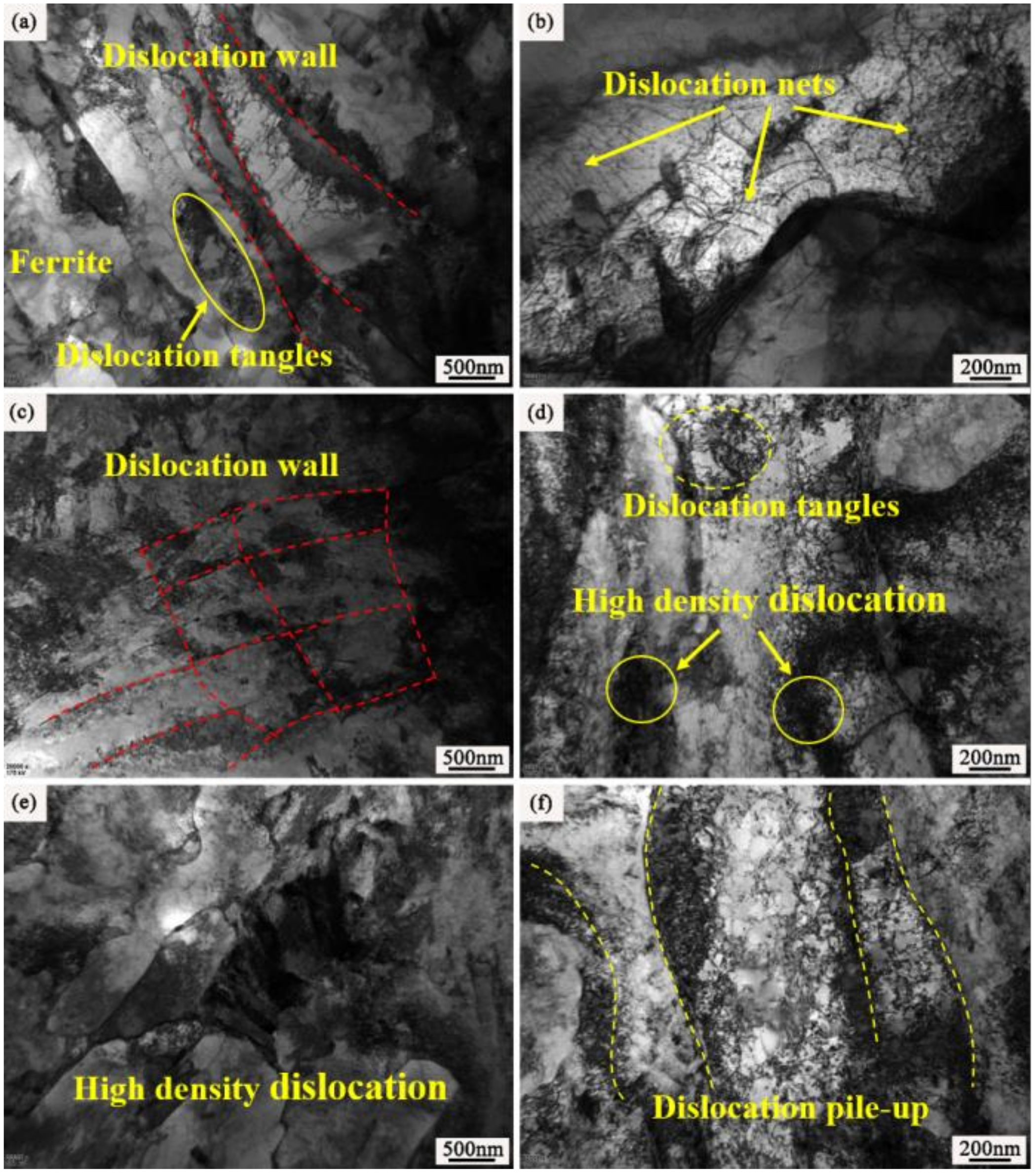
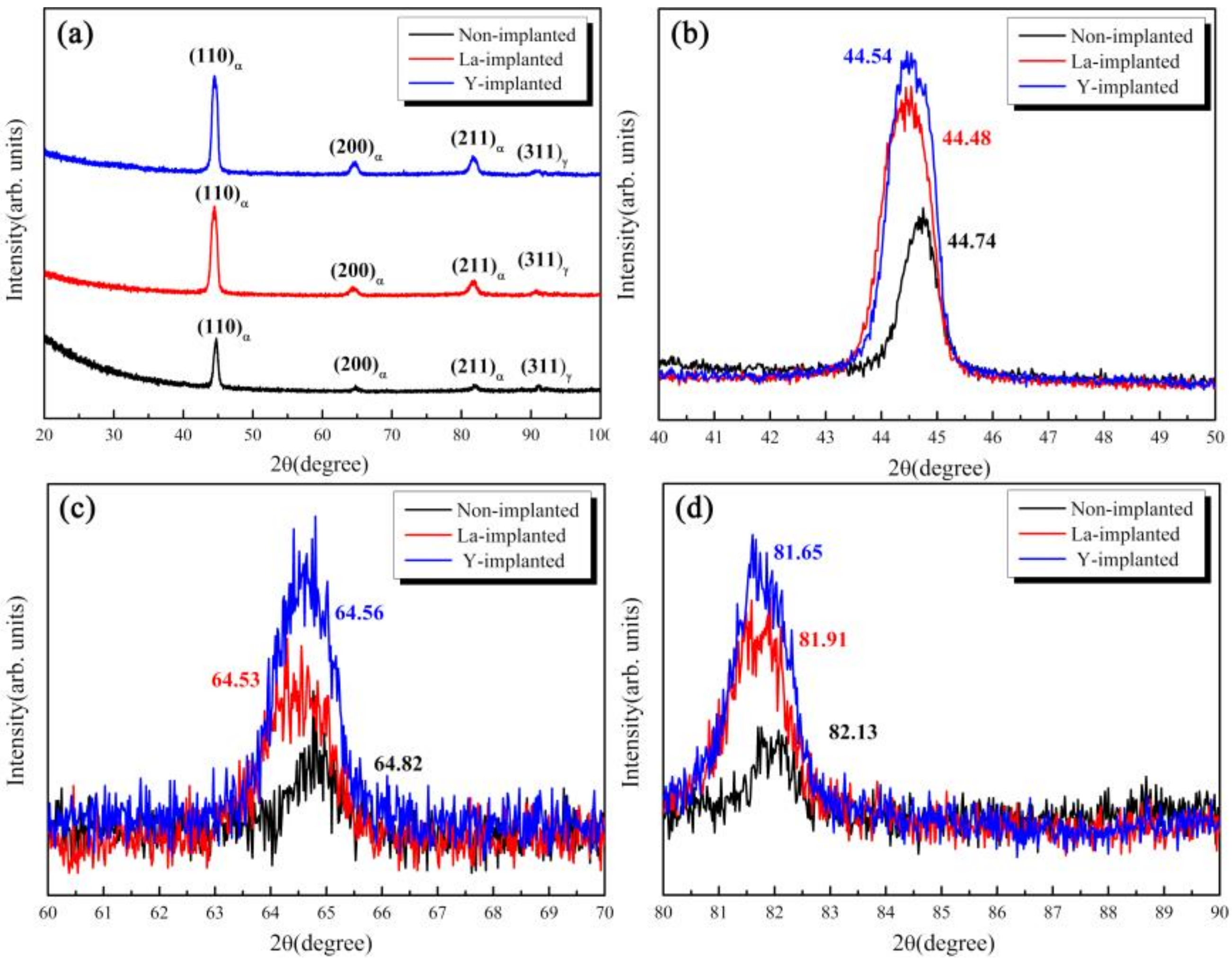
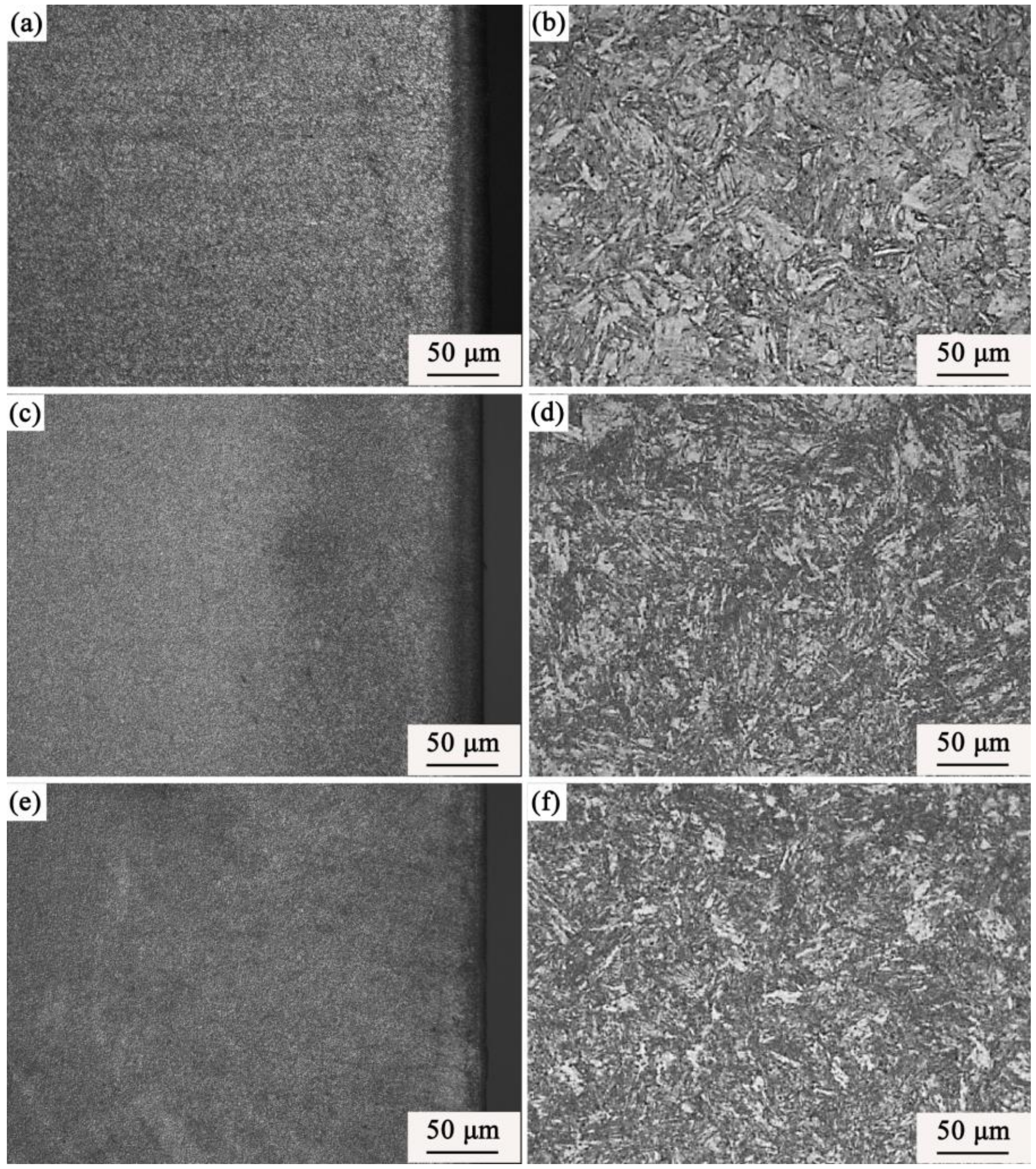
3.3. Phase and Microstructure of the Carburized Layer After Ion Implantation
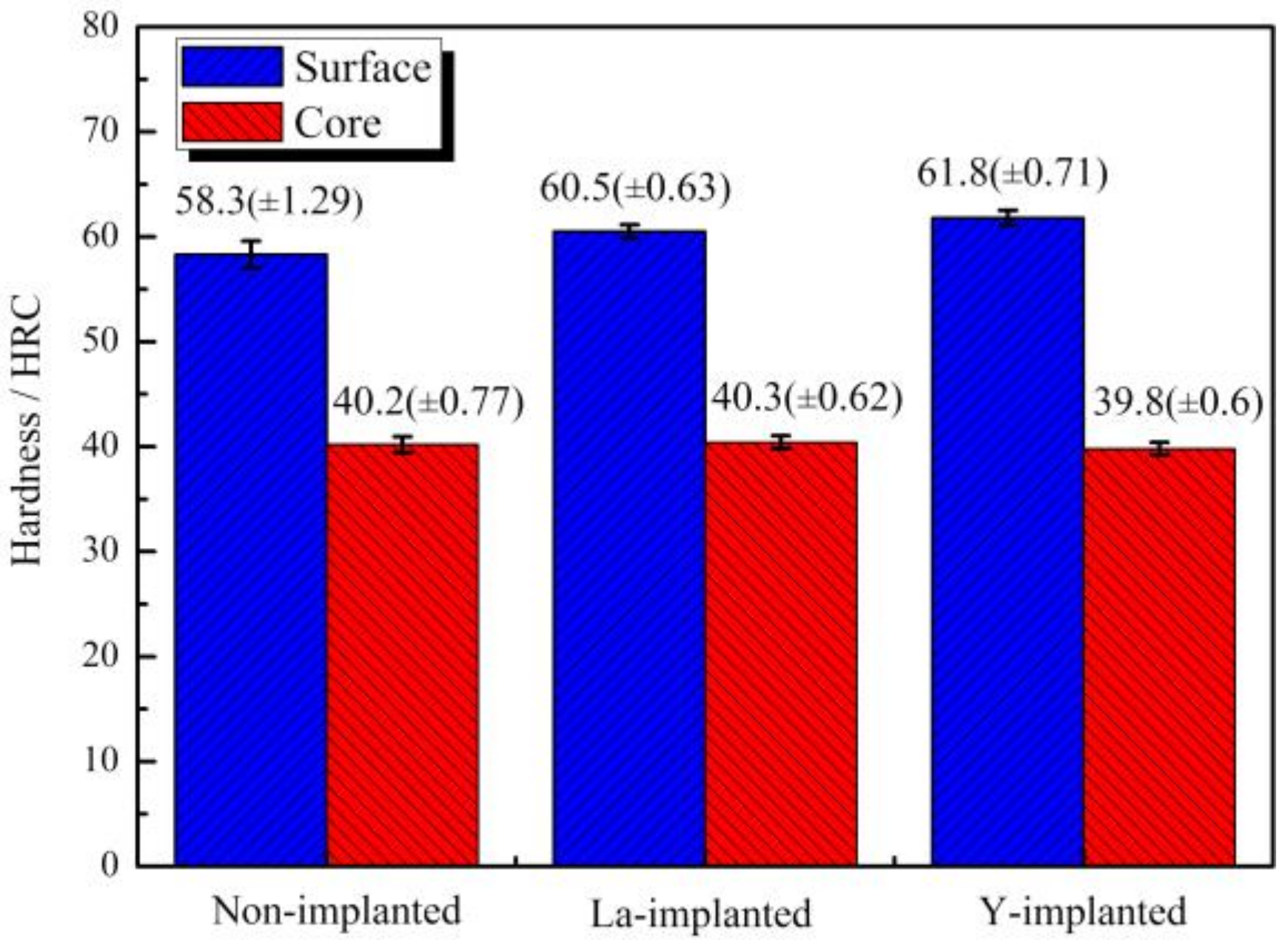
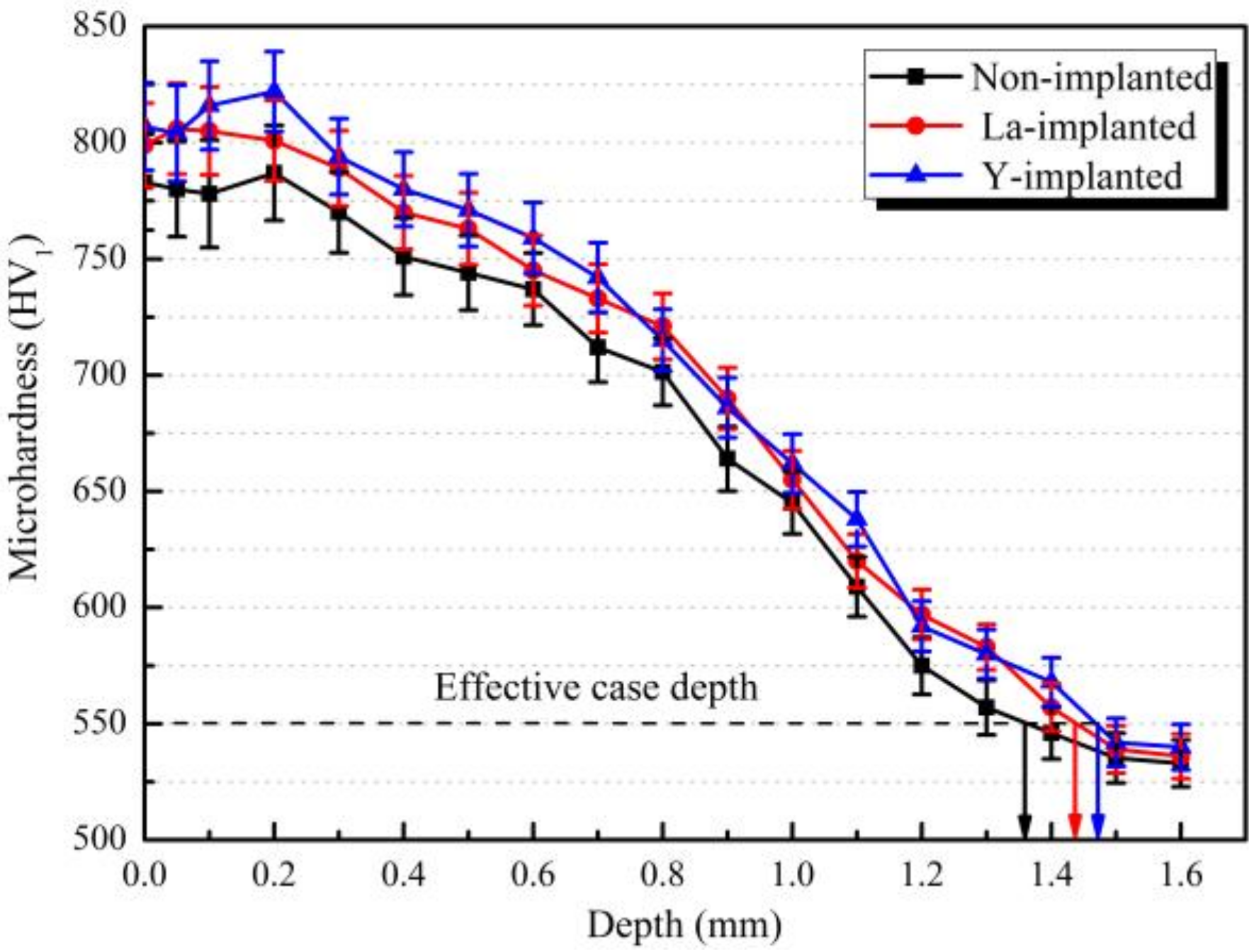
3.4. Hardness Distribution and Effective Hardening Depth of the Carburized Layer
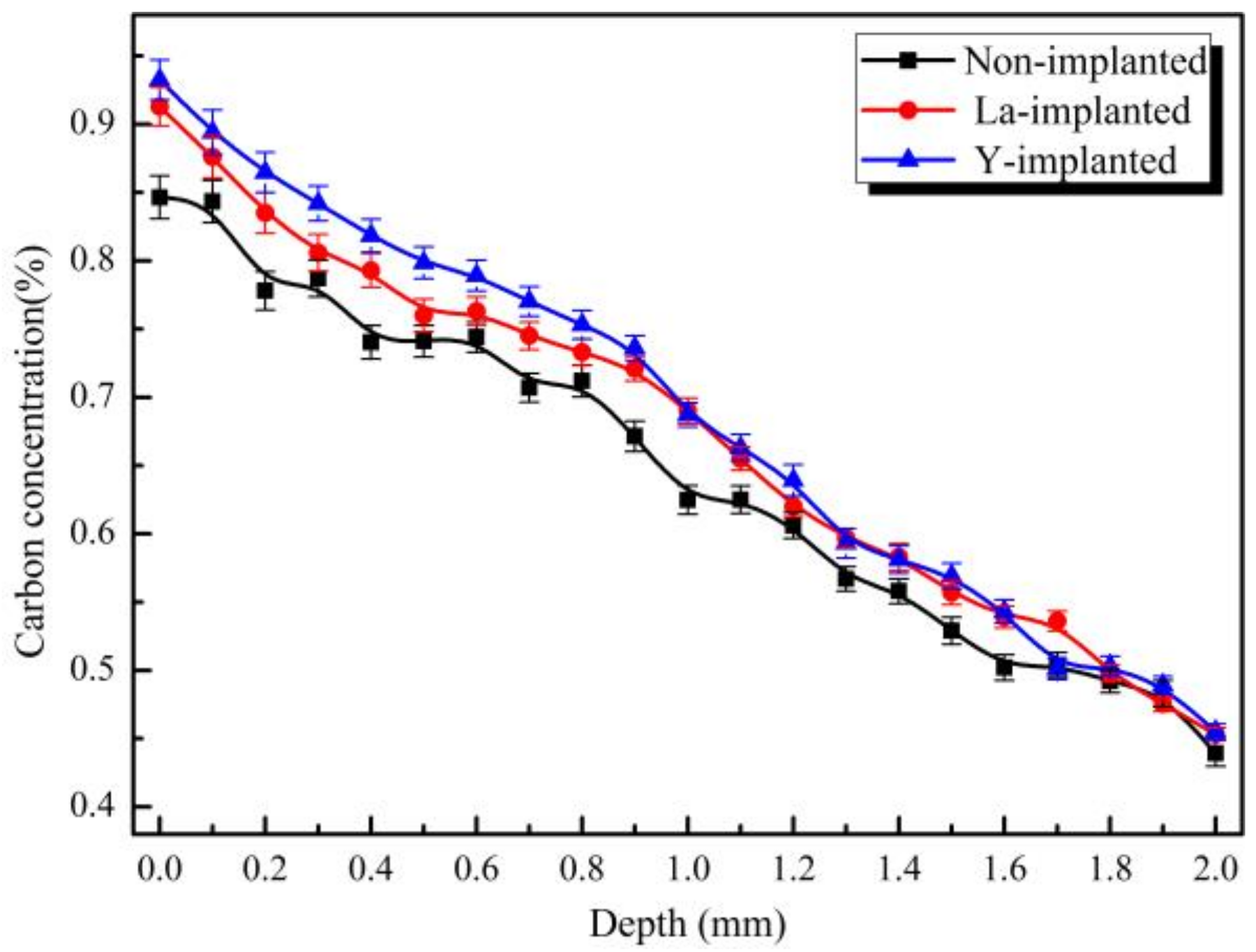
3.5. Calculation of the Carbon Diffusion Coefficient
4. Discussion
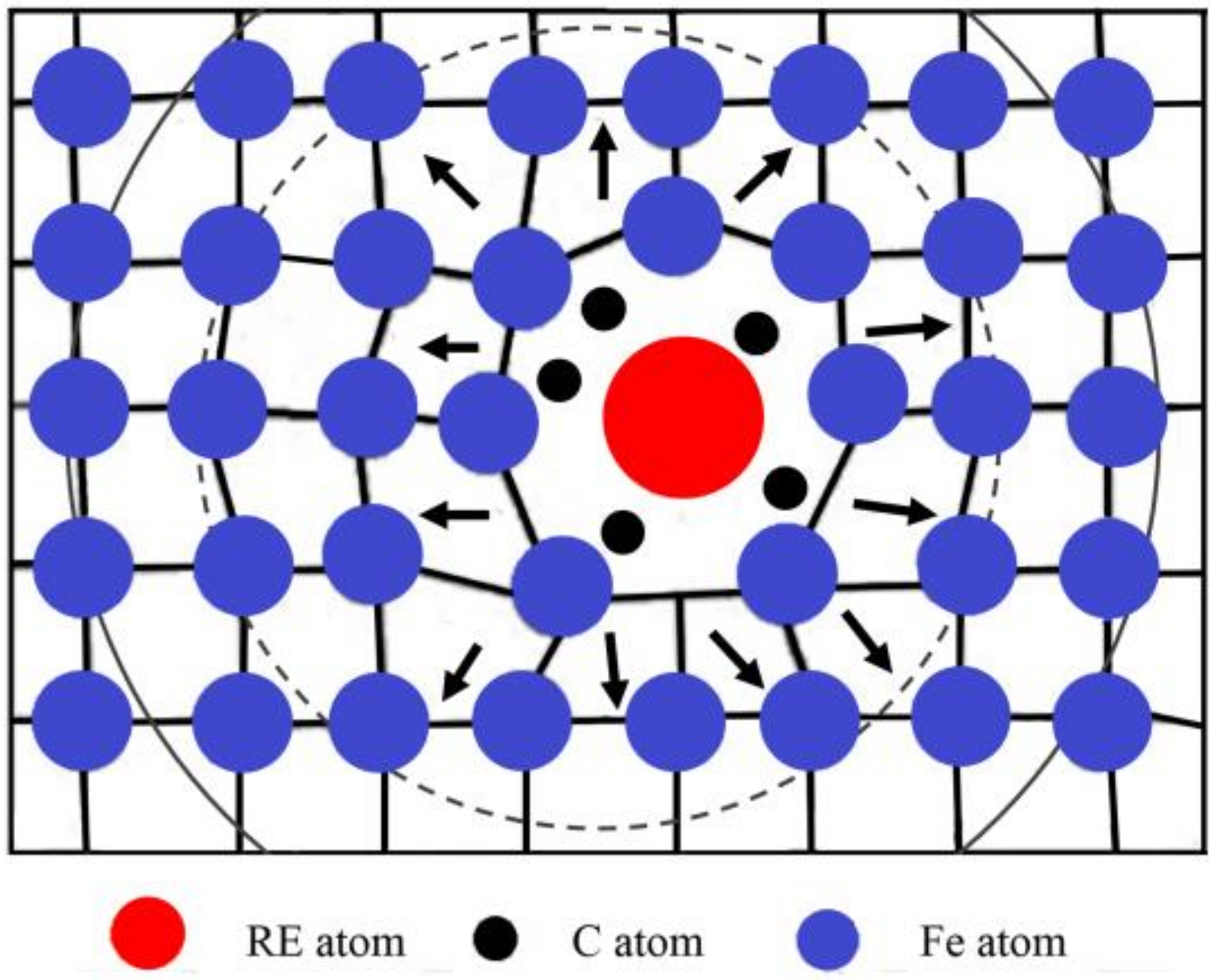
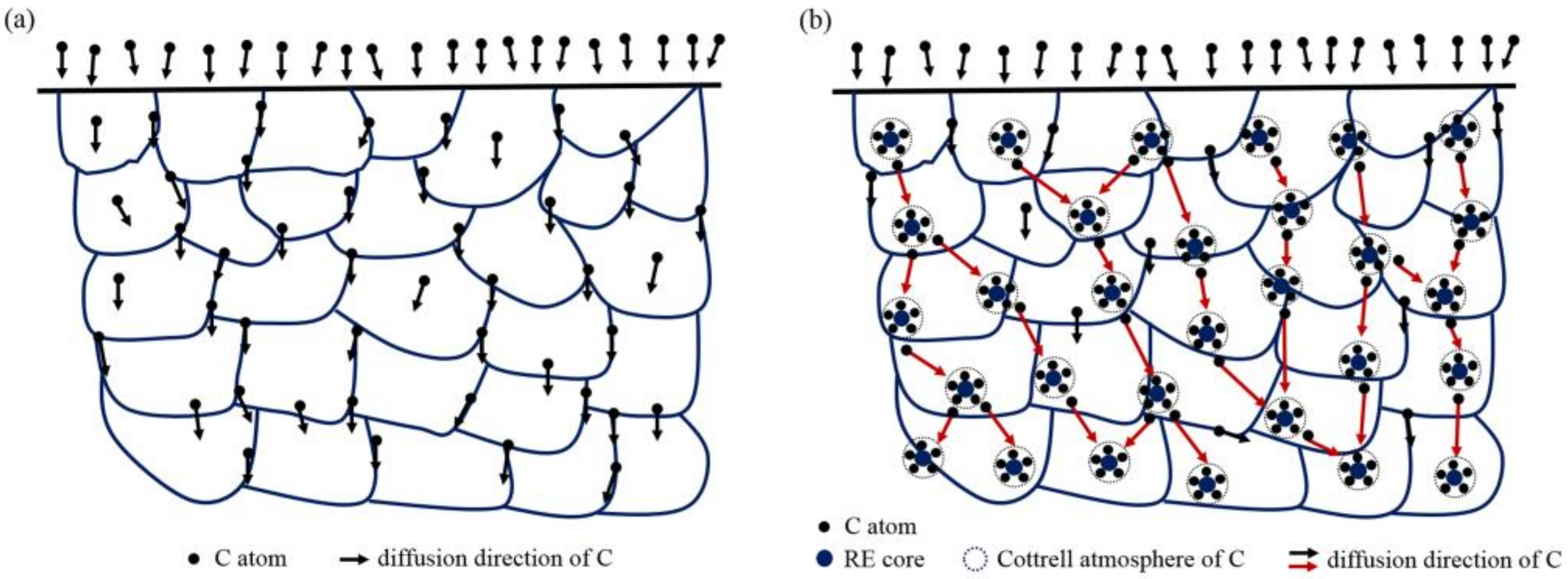
4.1. Effect of RE on Carbon Diffusion
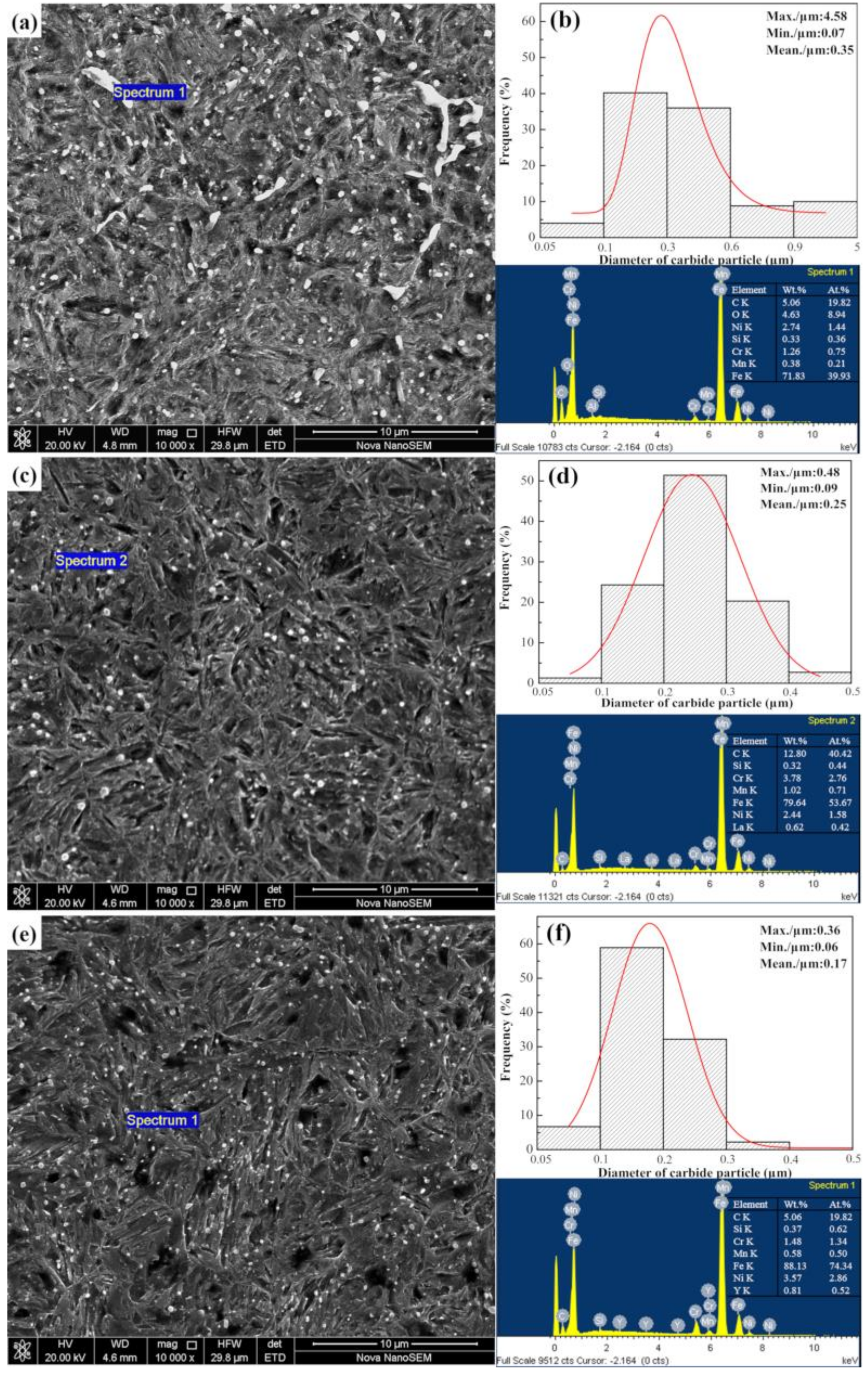
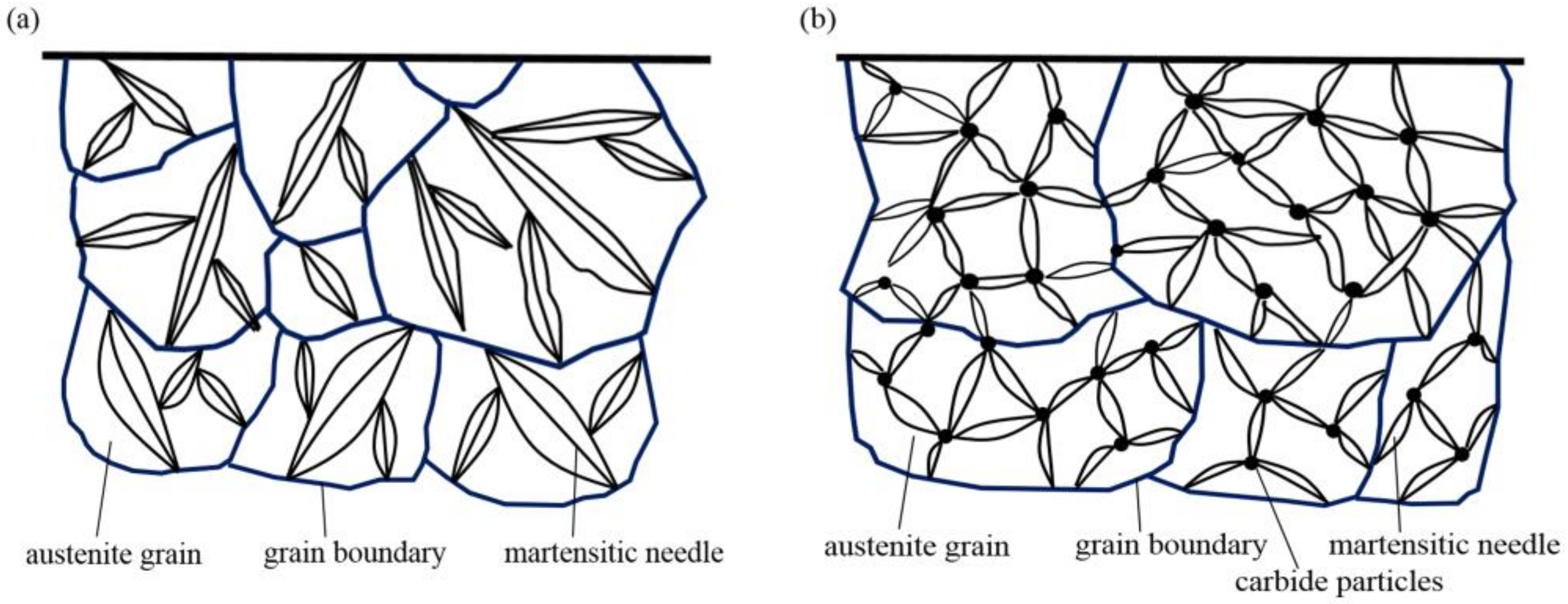
4.2. Effect of REs on the Microstructure and Hardness of the Carburized Layer
5. Conclusions
- La and Y ion implantation can result in the appearance of La2O3, Y2O3 and Y (0). In the implanted layer, the maximum concentration of La and Y occurred at 15 and 20 nm, respectively, and the concentration of both ions showed a normal distribution.
- During the carburization process, the high density of dislocation defects and the Cottrell atmosphere that formed on the surface of the steel by the RE ion implantation became the diffusion channels for the carbon atoms. During the carburization process at 920 °C and with a 1.2% carbon potential, the La ion implantation increased and the carbon diffusion coefficient was 1.12 times higher than that of the non-implanted steel, which was beneficial. Y ion implantation increased the carbon diffusion coefficient, which was 1.17 times higher than that of the non-implanted steel, which was also beneficial. The effective hardening depth of the carburized layer increased by 0.08 mm and 0.11 mm after the La and Y ion implantation, respectively.
- RE ion implantation pretreatment could change the transformation mode of the martensite and could significantly refine the microstructure of the carburized layer. Most of the fine dispersed carbides have diameters ranging from 0.1 mm to 0.2 mm and the maximum diameters were less than 0.32 mm. The maximum diameter and minimum diameter of the carbides on the surface of the carburized layer after Y ion implantation were 0.36 mm and 0.06 mm, respectively. The surface hardness of the carburized layer after ion implantation of lanthanum and yttrium was 60.5 HRC and 61.8 HRC, respectively. The yttrium-implanted carburized layer had a higher surface hardness than the non-implanted carburized layer, showing an increase of 3.5 HRC. Therefore, the results showed that compared to lanthanum, yttrium has a better strengthening effect.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, F.F.; Zhou, C.G.; Zheng, L.J.; Zhang, H. Improvement of the corrosion and tribological properties of CSS-42L aerospace bearing steel using carbon ion implantation. Appl. Surf. Sci. 2017, 392, 305–311. [Google Scholar] [CrossRef]
- Dong, M.L.; Cui, X.F.; Jin, G.; Wang, H.D.; Cai, Z.B.; Song, S.Q. Improved microstructure and properties of 12Cr2Ni4A alloy steel by vacuum carburization and Ti + N co-implantation. Appl. Surf. Sci. 2018, 440, 660–668. [Google Scholar] [CrossRef]
- Paulson, N.R.; Golmohammadi, Z.; Walvekar, A.A.; Sadeghi, F.; Mistry, K. Rolling contact fatigue in refurbished case carburized bearings. Tribol. Int. 2017, 115, 348–364. [Google Scholar] [CrossRef]
- Walvekar, A.A.; Sadeghi, F. Rolling contact fatigue of case carburized steels. Int. J. Fatigue 2017, 95, 264–281. [Google Scholar] [CrossRef]
- Guo, Y.B.; Zhang, Z.; Zhang, S.W. Advances in the application of biomimetic surface engineering in the oil and gas industry. Friction 2019, 7, 289–306. [Google Scholar] [CrossRef] [Green Version]
- Zajusz, M.; Tkacz-Śmiech, K.; Danielewski, M. Modeling of vacuum pulse carburizing of steel. Surf. Coat. Technol. 2014, 258, 646–651. [Google Scholar] [CrossRef]
- Dychtoń, K.; Rokicki, P.; Nowotnik, A.; Drajewicz, M.; Sieniawski, J. Process Temperature Effect on Surface Layer of Vacuum Carburized Low-Alloy Steel Gears. Solid State Phenom. 2015, 227, 425–428. [Google Scholar] [CrossRef]
- Wei, S.; Wang, G.; Zhao, X.; Zhang, X.; Rong, Y. Experimental Study on Vacuum Carburizing Process for Low-Carbon Alloy Steel. J. Mater. Eng. Perform. 2014, 23, 545–550. [Google Scholar] [CrossRef]
- Li, G.; Hua, J. The influence of additive rare earths on ion carburization. Surf. Coat. Technol. 1993, 59, 117–120. [Google Scholar] [CrossRef]
- Yan, M.F.; Liu, Z.R. Study on microstructure and microhardness in surface layer of 20CrMnTi steel carburised at 880 °C with and without RE. Mater. Chem. Phys. 2001, 72, 97–100. [Google Scholar] [CrossRef]
- Yan, M.F.; Pan, W.; Bell, T.; Liu, Z. Effect of rare earth catalyst on carburizing kinetics in a sealed quench furnace with endothermic atmosphere. Appl. Surf. Sci. 2001, 173, 91–94. [Google Scholar] [CrossRef]
- Yan, M.F.; Liu, Z.R.; Bell, T. Effect of Rare Earths on Diffusion Coefficient and Transfer Coefficient of Carbon during Carburizing. J. Rare Earth 2001, 19, 122–124. [Google Scholar]
- Dong, M.L.; Cui, X.F.; Zhang, Y.H.; Jin, G.; Yue, C.W.; Zhao, X.; Cai, Z.B.; Xu, B.S. Vacuum carburization of 12Cr2Ni4A low carbon alloy steel with lanthanum and cerium ion implantation. J. Rare Earth 2017, 35, 1164–1170. [Google Scholar] [CrossRef]
- Chen, X.H.; Soveja, A.; Chaussumier, M.; Zhang, P.Z.; Wei, D.B.; Ding, F. Effect of MEVVA ion implantation on fatigue properties of TC18 titanium alloy. Surf. Coat. Technol. 2018, 344, 572–578. [Google Scholar] [CrossRef]
- Panin, S.V.; Vlasov, I.V.; Sergeev, V.P.; Maruschak, P.O.; Sunder, R.; Ovechkin, B.B. Fatigue life improvement of 12Cr1MoV steel by irradiation with Zr + ion beam. Int. J. Fatigue 2015, 76, 3–10. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhang, P.Z.; Wei, D.B.; Huang, X.; Adriana, S.; Michel, C.; Ding, F.; Li, F.K. Structures and properties of Ti-5Al-5Mo-5V-1Cr-1Fe after Nb implantation. Surf. Coat. Technol. 2019, 358, 676–687. [Google Scholar] [CrossRef]
- Wang, S.X.; Li, C.; Xiong, B.J.; Tian, X.B.; Yang, S.Q. Surface modification of hard alloy by Y ion implantation under different atmosphere. Appl. Surf. Sci. 2011, 257, 5826–5830. [Google Scholar] [CrossRef]
- Zhu, S.F.; Huang, N.; Shu, H.; Wu, Y.P.; Xu, L. Corrosion resistance and blood compatibility of lanthanum ion implanted pure iron by MEVVA. Appl. Surf. Sci. 2009, 256, 99–104. [Google Scholar] [CrossRef]
- Wang, X.M.; Zeng, X.Q.; Wu, G.S.; Yao, S.S. Yttrium ion implantation on the surface properties of magnesium. Appl. Surf. Sci. 2006, 253, 2437–2442. [Google Scholar] [CrossRef]
- Bennett, M.J.; Tuson, A.T. Improved high temperature oxidation behaviour of alloys by ion implantation. Mat. Sci. Eng. A Struct. 1989, 116, 79–87. [Google Scholar] [CrossRef]
- Xu, J.; Bai, X.D.; Jin, A.; Fan, Y.D. Effect of yttrium ion implantation on aqueous corrosion resistance of zircaloy-4. J. Mater. Sci. Lett. 2000, 19, 1633–1635. [Google Scholar] [CrossRef]
- Qian, W.; Bai, X.D.; Liu, X.Y.; Zhao, X. Studies on the corrosion behavior of lanthanum-implanted zircaloy. J. Mater. Sci. 2005, 40, 475–479. [Google Scholar] [CrossRef]
- Sebayang, D.; Khaerudini, D.S.; Saryanto, H.; Hasan, S.; Othman, M.A.; Untoro, P. Oxidation Resistance of Fe80Cr20 Alloys Treated by Rare Earth Element Ion Implantation. In Proceedings of the American Institute of Physics Conference Series; American Institute of Physics: Melville, NY, USA, 2011; Volume 1394, pp. 90–102. [Google Scholar]
- Zhang, T.H.; Xie, J.D.; Ji, C.Z.; Chen, J.; Hong, X.; Li, J.; Sun, G.R.; Zhang, H.X. Influence of the structure of implanted steel with Y, Y + C and Y + Cr on the behaviors of wear, oxidation and corrosion resistance. Surf. Coat. Technol. 1995, 72, 93–98. [Google Scholar] [CrossRef]
- Dudognon, J.; Vayer, M.; Pineau, A.; Erre, R. Mo and Ag ion implantation in austenitic, ferritic and duplex stainless steels: A comparative study. Surf. Coat. Technol. 2008, 203, 180–185. [Google Scholar] [CrossRef]
- Shulga, V.I. Note on the artefacts in SRIM simulation of sputtering. Appl. Surf. Sci. 2018, 439, 456–461. [Google Scholar] [CrossRef]
- Chrobak, Ł.; Maliński, M. Properties of silicon implanted with Fe+, Ge+, Mn+ ions investigated using a frequency contactless modulated free-carrier absorption technique. Opt. Mater. 2018, 86, 484–491. [Google Scholar] [CrossRef]
- Peng, D.Q.; Bai, X.D.; Chen, B.S. Surface analysis and corrosion behavior of zirconium samples implanted with yttrium and lanthanum. Surf. Coat. Technol. 2005, 190, 440–447. [Google Scholar] [CrossRef]
- Jin, H.M.; Zhou, X.W.; Zhang, L.N. Effects of lanthanum ion-implantation on microstructure of oxide film formed on Co-Cr alloy. J. Rare Earth 2008, 26, 406–409. [Google Scholar] [CrossRef]
- Sharkeev, Y.P.; Kozlov, E.V. The long-range effect in ion implanted metallic materials: Dislocation structures, properties, stresses, mechanisms. Surf. Coat. Technol. 2002, 158, 219–224. [Google Scholar] [CrossRef]
- Dai, M.Y.; Li, C.Y.; Hu, J. The enhancement effect and kinetics of rare earth assisted salt bath nitriding. J. Alloys Compd. 2016, 688, 350–356. [Google Scholar] [CrossRef]
- Yin, Y.G.; Shen, M.H.; Tan, Z.Q.; Yu, S.J.; Liu, J.F.; Jiang, G.B. Particle coating-dependent interaction of molecular weight fractionated natural organic matter: Impacts on the aggregation of silver nanoparticles. Environ. Sci. Technol. 2015, 49, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Asi, O.; Can, A.Ç.; Pineault, J.; Belassel, M. The effect of high temperature gas carburizing on bending fatigue strength of SAE 8620 steel. Mater. Des. 2009, 30, 1792–1797. [Google Scholar] [CrossRef]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM—The stopping and range of ions in matter (2010). Nucl. Instrum. Meth. B 2010, 268, 1818–1823. [Google Scholar] [CrossRef]
- Cutroneo, M.; Torrisi, L.; Havranek, V.; Mackova, A.; Malinsky, P.; Torrisi, A.; Stammers, J.; Sofer, Z.; Silipigni, L.; Fazio, B.; et al. Characterization of graphene oxide film by implantation of low energy copper ions. Nucl. Instrum. Meth. B 2019. [Google Scholar] [CrossRef]
- Waseda, O.; Veiga, R.G.; Morthomas, J.; Chantrenne, P.; Becquart, C.S.; Ribeiro, F.; Jelea, A.; Goldenstein, H.; Perez, M. Formation of carbon Cottrell atmospheres and their effect on the stress field around an edge dislocation. Scr. Mater. 2017, 129, 16–19. [Google Scholar] [CrossRef]
- Liu, Z.R.; Yan, M.F.; Luo, Q.; Zheng, T.Q.; Chen, Y.J. Diffusion and micro-alloying mechanism of rare earth(RE) and carbon/nitrogen atoms permeated into surface layer of steel during RE-carburizing,-nitriding and-nitrocarburizing. Mater. Heat Treat. 2011, 32, 121–129. [Google Scholar]
- Wang, X.A.; Yan, M.F.; Liu, R.L.; Zhang, Y.X. Effect of rare earth addition on microstructure and corrosion behavior of plasma nitrocarburized M50NiL steel. J. Rare Earth 2016, 34, 1148–1155. [Google Scholar] [CrossRef]
- Liu, D.R.; Qi, Z.; Qin, Z.B.; Qin, L.; Wu, Z.; Liu, L. Tribological performance of surfaces enhanced by texturing and nitrogen implantation. Appl. Surf. Sci. 2016, 363, 161–167. [Google Scholar] [CrossRef]
- Yuan, Z.X.; Yu, Z.S.; Tan, P.; Song, S.H. Effect of rare earths on the carburization of steel. Mater. Sci. Eng. A Struct. 1999, 267, 162–166. [Google Scholar] [CrossRef]


| Sample | Implanted with La | Implanted with Y |
|---|---|---|
| Vacuum level (Pa) | 3.5 × 10−3 | 3.5 × 10−3 |
| Ion energy (Kev) | 100 | 105 |
| Implantation dose | 2 × 1017 ions/cm2 | 2 × 1017 ions/cm2 |
| Temperature (°C) | Room temperature | Room temperature |
| Element | La | Y |
| Sample | Non-Implanted | Lanthanum-Implanted | Yttrium-Implanted |
|---|---|---|---|
| Retained austenite | 16.7% | 15.6% | 14.2% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Li, C.; Xing, Z.; Wang, H.; Huang, Y.; Guo, W.; Liu, H. Study of the Catalytic Strengthening of a Vacuum Carburized Layer on Alloy Steel by Rare Earth Pre-Implantation. Materials 2019, 12, 3420. https://doi.org/10.3390/ma12203420
Li G, Li C, Xing Z, Wang H, Huang Y, Guo W, Liu H. Study of the Catalytic Strengthening of a Vacuum Carburized Layer on Alloy Steel by Rare Earth Pre-Implantation. Materials. 2019; 12(20):3420. https://doi.org/10.3390/ma12203420
Chicago/Turabian StyleLi, Guolu, Caiyun Li, Zhiguo Xing, Haidou Wang, Yanfei Huang, Weiling Guo, and Haipeng Liu. 2019. "Study of the Catalytic Strengthening of a Vacuum Carburized Layer on Alloy Steel by Rare Earth Pre-Implantation" Materials 12, no. 20: 3420. https://doi.org/10.3390/ma12203420




