Evaluation of the Microstructure and Mechanical Properties of a New Modified Cast and Laser-Melted AA7075 Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
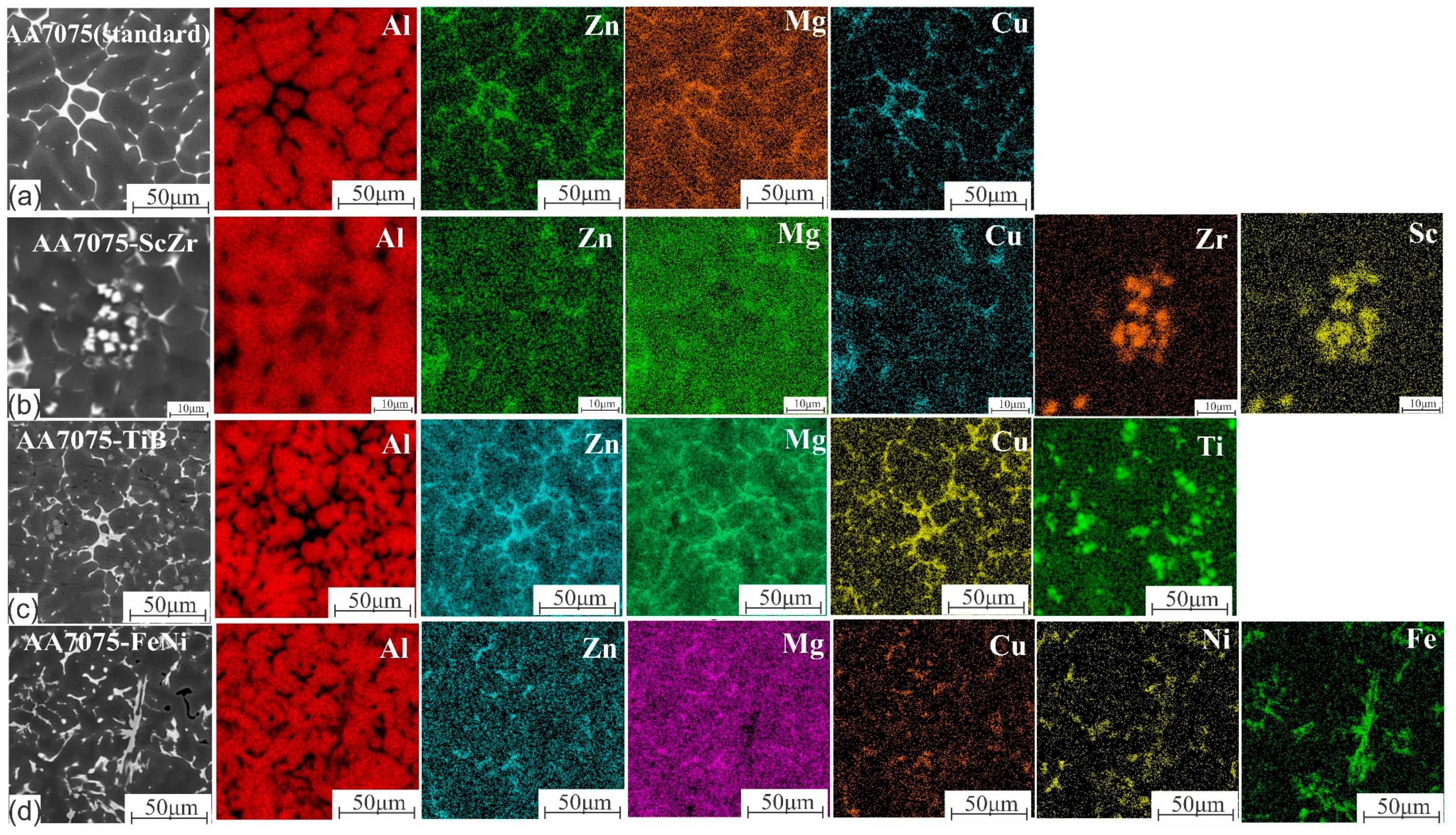
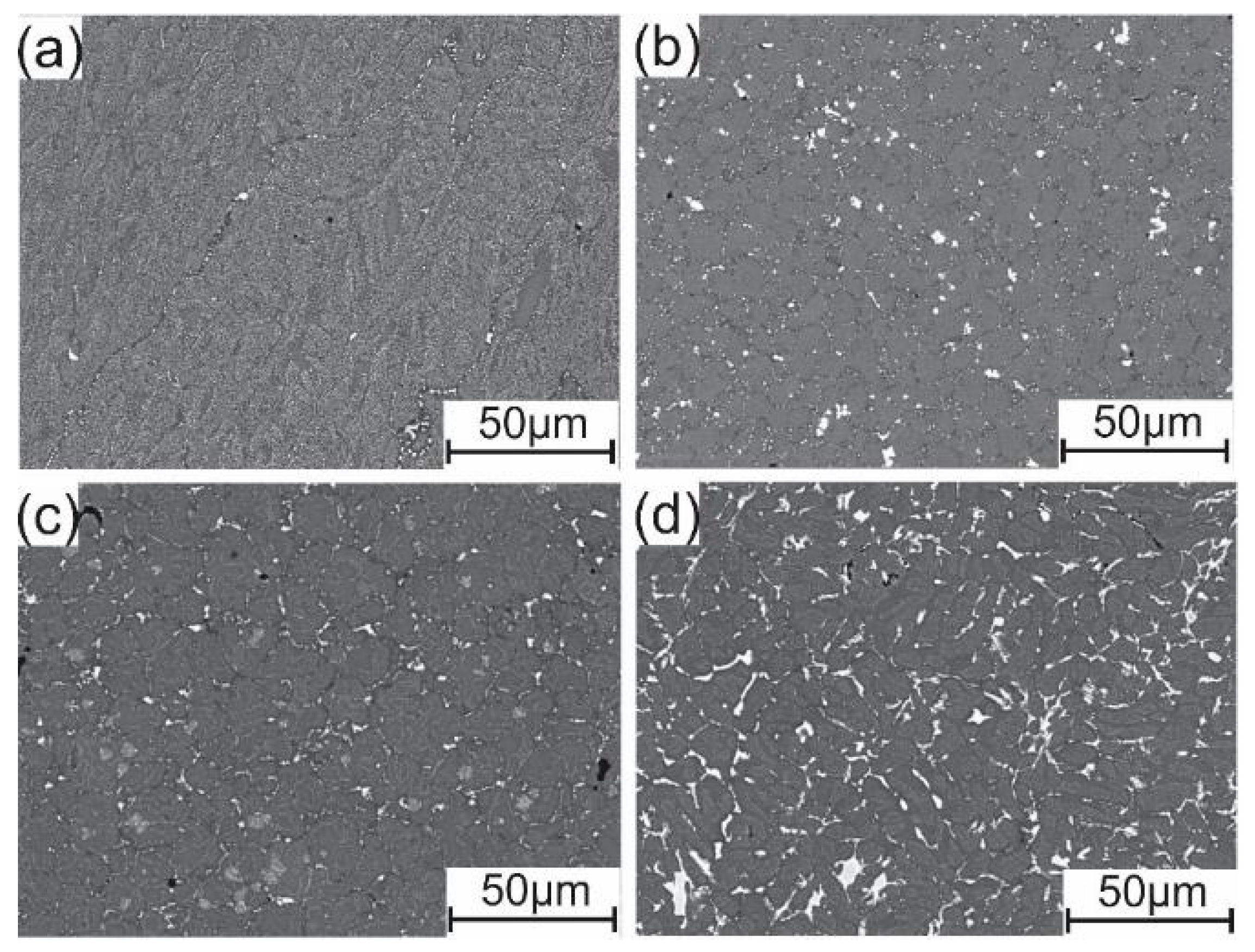
3.1. As-Cast Microstructure
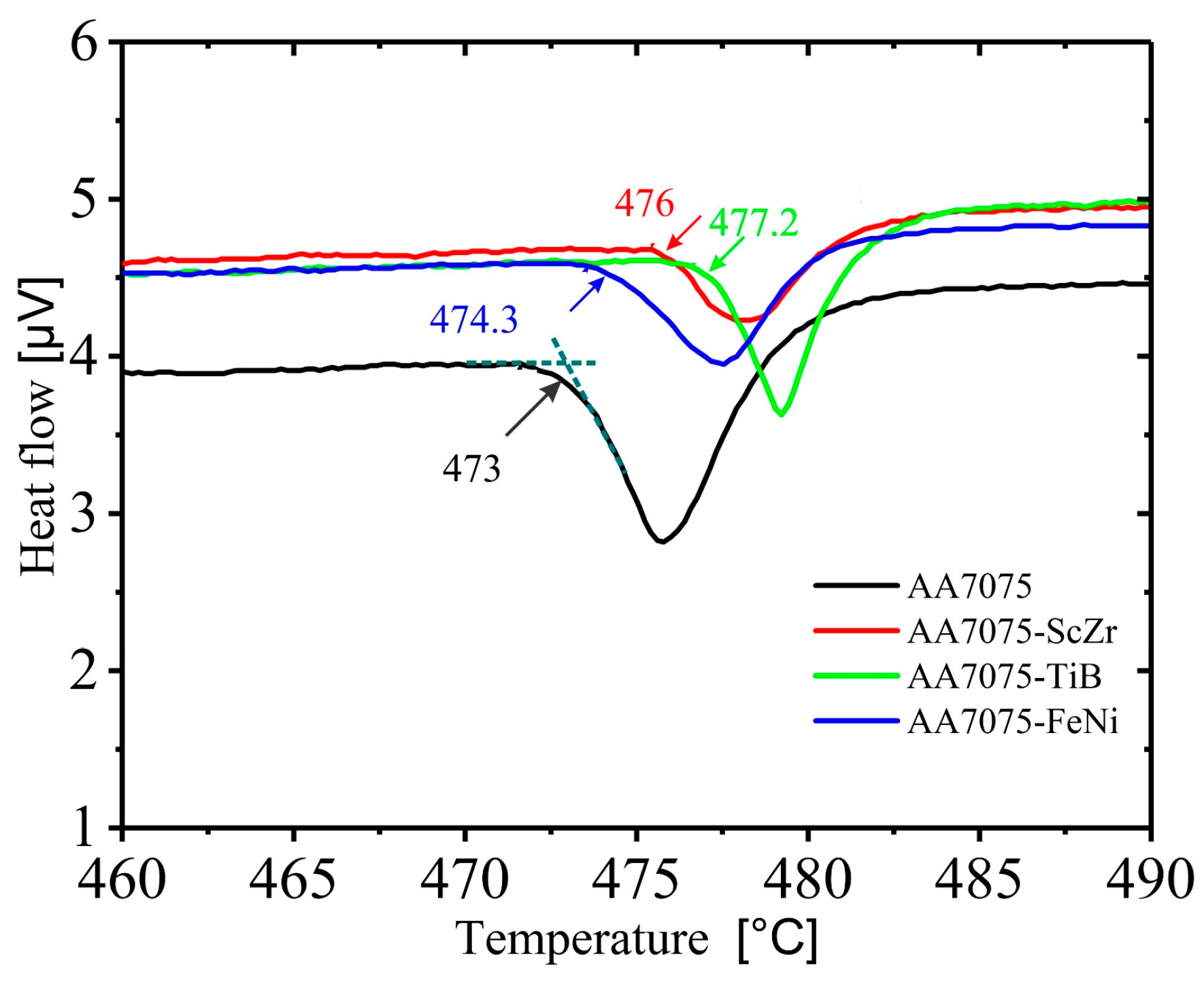
3.2. Microstructures after Homogenization Annealing
3.3. Mechanical Properties in As-Cast and Homogenized Annealing Conditions
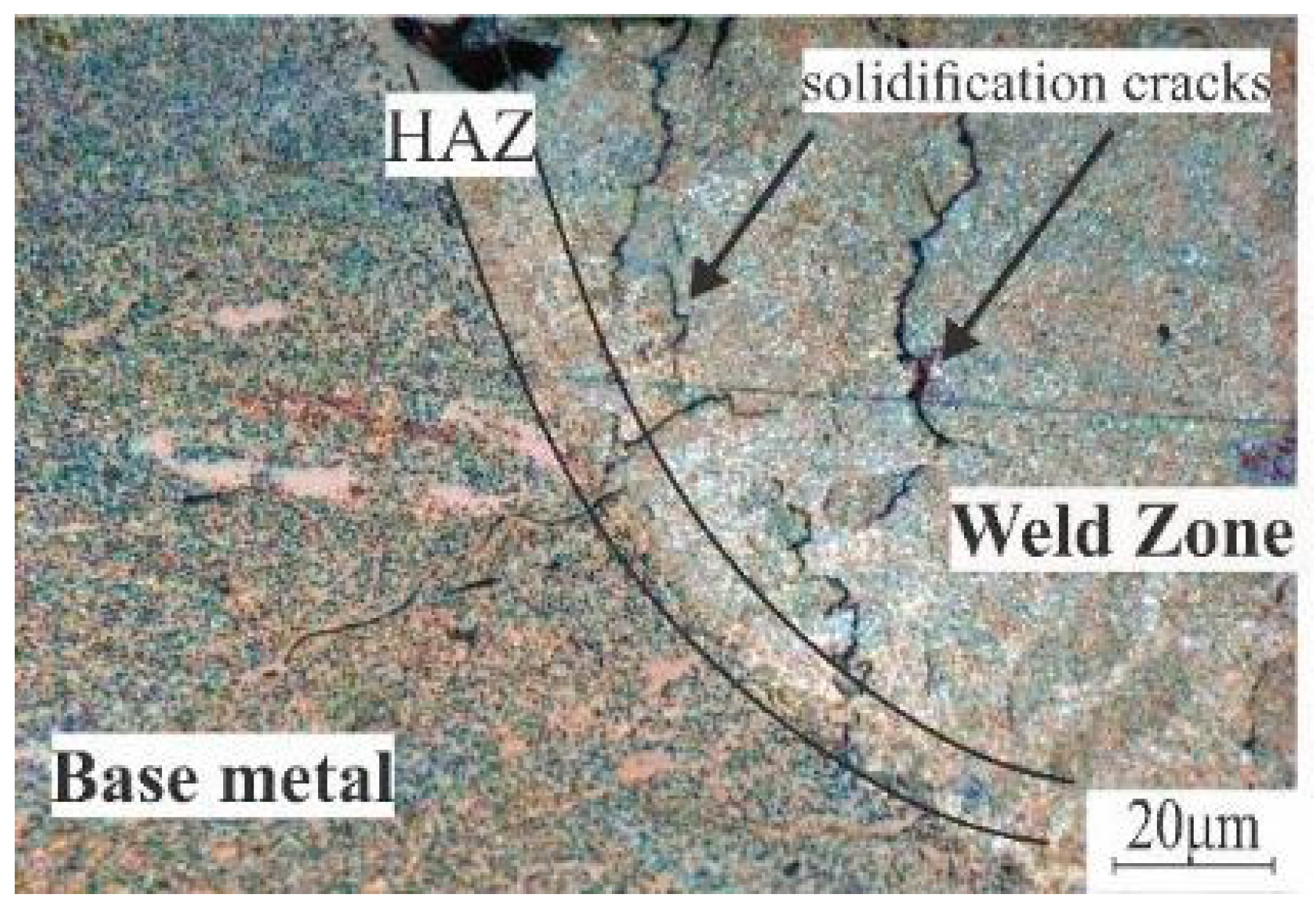
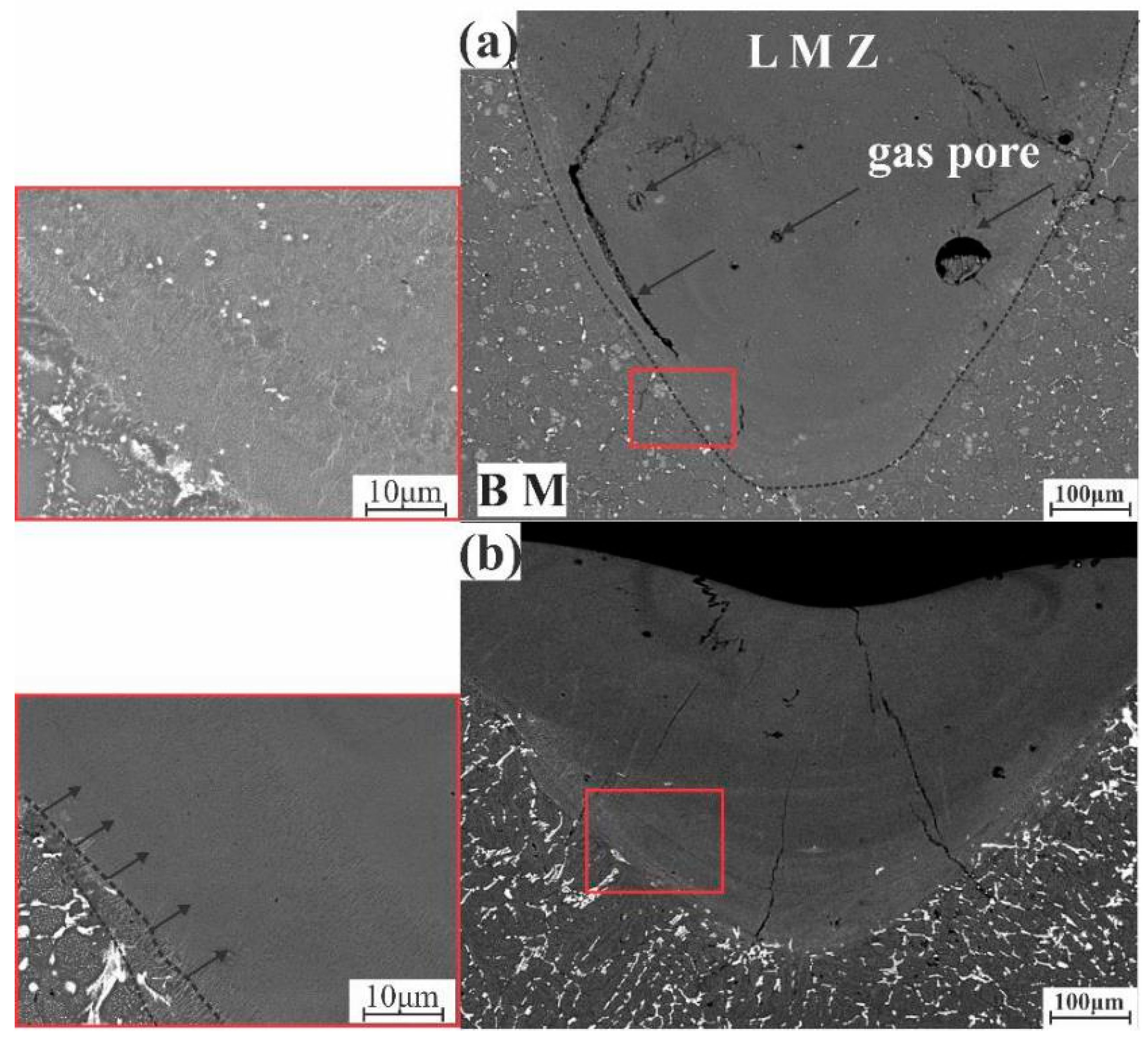
3.4. Laser Melt Zone Microstructure
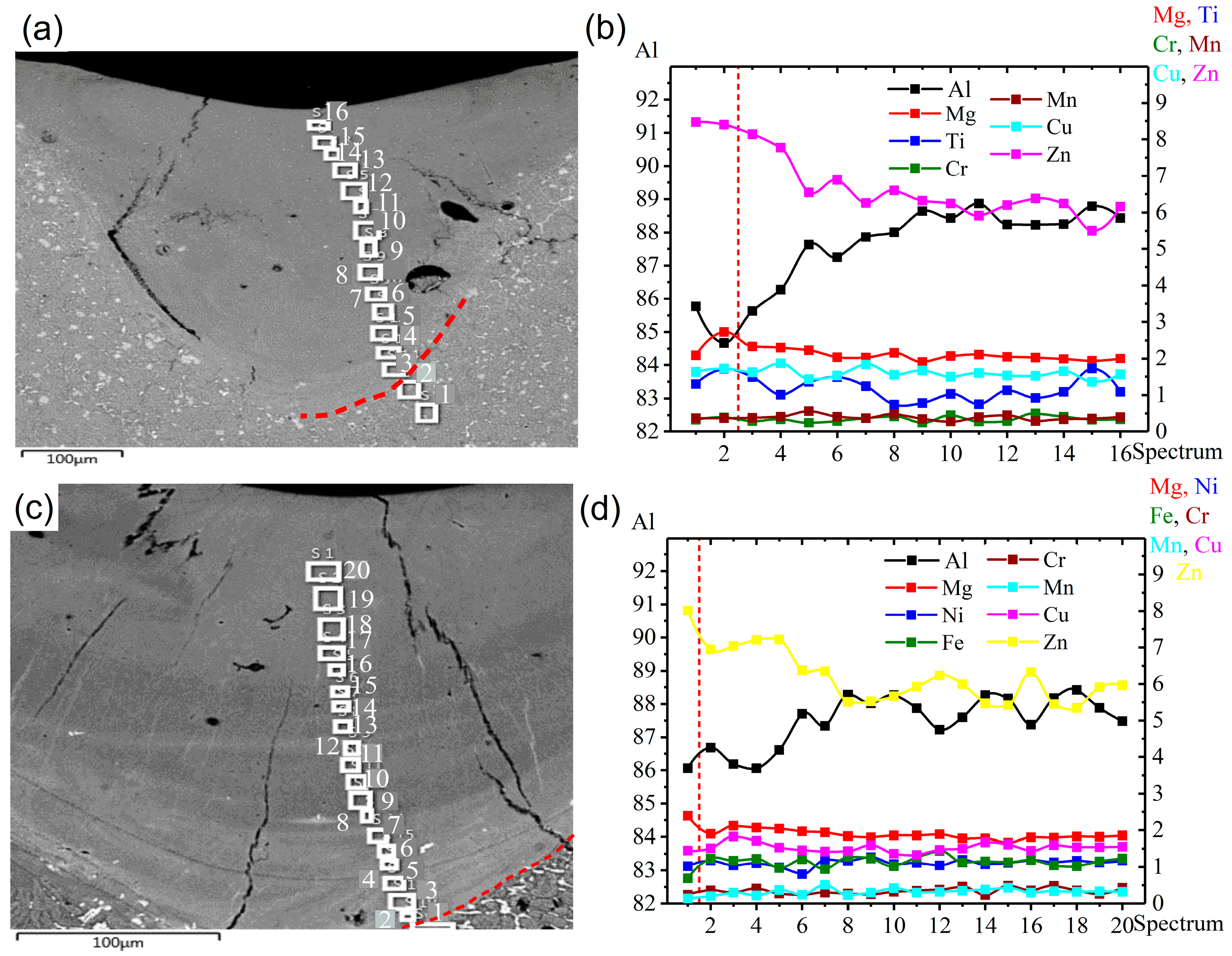
3.5. Elemental Distribution in the LMZ
3.6. Microhardness Results
3.7. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakai, M.; Eto, T. New aspect of development of high strength aluminum alloys for aerospace applications. Mater. Sci. Eng. A 2000, 285, 62–68. [Google Scholar] [CrossRef]
- Wu, Y.L.; Froes, F.H.; Alvarez, A.; Li, C.G.; Liu, J. Microstructure and properties of a new super-high-strength Al-Zn-Mg-Cu alloy C912. Mater. Des. 1997, 18, 211–215. [Google Scholar] [CrossRef]
- Peng, K.; Chen, W.; Zhang, H.; Qian, K.-W. Features of dynamic strain aging in high strength Al-Zn-Mg-Cu alloy. Mater. Sci. Eng. A 1997, 234, 138–141. [Google Scholar] [CrossRef]
- Mosleh, A.O.; Mahmoud, F.H.; Mahmoud, T.S.; Khalifa, T.A. Microstructure and static immersion corrosion behavior of AA7020-O Al plates joined by friction stir welding. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2016, 230, 1030–1040. [Google Scholar] [CrossRef]
- Norman, A.F.; Prangnell, P.B.; McEwen, R.S. The solidification behaviour of dilute aluminium—Scandium alloys. Acta Mater. 1998, 46, 5715–5732. [Google Scholar] [CrossRef]
- Glenn, A.M.; Russo, S.P.; Gorman, J.D.; Paterson, P.J.K. The effect of grain refining on the microsegregation of aluminium–magnesium alloy 5182. Micron 2001, 32, 841–850. [Google Scholar] [CrossRef]
- Kim, K.T.; Kim, J.M.; Sung, K.D.; Jun, J.H.; Jung, W.J. Effect of Alloying Elements on the Strength and Casting Characteristics of High Strength Al-Zn-Mg-Cu Alloys. Mater. Sci. Forum 2005, 475, 2539–2542. [Google Scholar] [CrossRef]
- Atkinson, H.V.; Burke, K.; Vaneetveld, G. Recrystallisation in the semi-solid state in 7075 aluminium alloy. Mater. Sci. Eng. A 2008, 490, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Rao, T.S.; Reddy, G.M.; Rao, S.R.K. Studies on variations in microstructure and hardness of AA7075-T651 aluminum alloy friction stir welds. Metall. Ital. 2016, 108, 29–35. [Google Scholar]
- Enz, J. Laser Beam Welding of High-Alloyed Aluminium-Zinc Alloys. Doctoral Dissertation, Technische Universität Hamburg-Harburg, Hamburg, Germany, 2017. [Google Scholar]
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties, 1st ed.; BUTTER WORTHS: London, UK, 1979. [Google Scholar]
- Verhaeghe, I.G. Executive Summary Porosity Levels When Welding Thin And Thick-Section Aluminium Using Fibre-Delivered Lasers. Doctoral Dissertation, University of Warwick, Coventry, England, 2008. [Google Scholar]
- Zhou, W. Problems in Welding of High Strength Aluminium Alloys Porosity in Weld. Singap. Weld. Soc. Newsl. 1999, 1, 1–6. [Google Scholar]
- McCartney, D.G. Grain refining of aluminium and its alloys using inoculants. Int. Mater. Rev. 1989, 34, 247–260. [Google Scholar] [CrossRef]
- Campbell, J. Effects of vibration during solidification. Int. Met. Rev. 1981, 26, 71–108. [Google Scholar] [CrossRef]
- Glicksman, M.E. Principles of Solidification: An Introduction to Modern Casting and Crystal Growth Concepts; Glicksman, M.E., Ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Easton, M.; Stjohn, D. Grain refinement of aluminum alloys: Part I. The nucleant and solute paradigms—A review of the literature. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 1999, 30, 1613–1623. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, X. The effect of melting temperature and time on the TiC particles. J. Alloys Compd. 2009, 484, 95–101. [Google Scholar] [CrossRef]
- Li, P.; Kandalova, E.G.; Nikitin, V.I. Grain refining performance of Al–Ti master alloys with different microstructures. Mater. Lett. 2005, 59, 723–727. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Z.; Fu, H.; Gao, L.; Li, T. Grain refinement mechanism of pure aluminum by inoculation with Al–B master alloys. Mater. Sci. Eng. A 2012, 549, 136–143. [Google Scholar] [CrossRef]
- Wang, T.; Fu, H.; Chen, Z.; Xu, J.; Zhu, J.; Cao, F.; Li, T. A novel fading-resistant Al–3Ti–3B grain refiner for Al–Si alloys. J. Alloys Compd. 2012, 511, 45–49. [Google Scholar] [CrossRef]
- Xiangfa, L.; Zhenqing, W.; Zuogui, Z.; Xiufang, B. The relationship between microstructures and refining performances of Al–Ti–C master alloys. Mater. Sci. Eng. A 2002, 332, 70–74. [Google Scholar] [CrossRef]
- Li, B.; Pan, Q.; Huang, X.; Yin, Z. Microstructures and properties of Al–Zn–Mg–Mn alloy with trace amounts of Sc and Zr. Mater. Sci. Eng. A 2014, 616, 219–228. [Google Scholar] [CrossRef]
- Yang, M.; Pan, F.; Cheng, R.; Tang, A. Effects of Al-10Sr master alloys on grain refinement of AZ31 magnesium alloy. Trans. Nonferrous Met. Soc. China 2008, 18, 52–58. [Google Scholar] [CrossRef]
- Birol, Y. Production of Al–Ti–B master alloys from Ti sponge and KBF4. J. Alloys Compd. 2007, 440, 108–112. [Google Scholar] [CrossRef]
- Wróbel, T. Review of inoculation methods of pure aluminium primary structure. Arch. Mater. Sci. Eng. 2011, 50, 110–119. [Google Scholar]
- Pourkia, N.; Emamy, M.; Farhangi, H.; Ebrahimi, S.H.S. The effect of Ti and Zr elements and cooling rate on the microstructure and tensile properties of a new developed super high-strength aluminum alloy. Mater. Sci. Eng. A 2010, 527, 5318–5325. [Google Scholar] [CrossRef]
- Li, P.; Liu, S.; Zhang, L.; Liu, X. Grain refinement of A356 alloy by Al–Ti–B–C master alloy and its effect on mechanical properties. Mater. Des. 2013, 47, 522–528. [Google Scholar] [CrossRef]
- Limmaneevichitr, C.; Eidhed, W. Fading mechanism of grain refinement of aluminum–silicon alloy with Al–Ti–B grain refiners. Mater. Sci. Eng. A 2003, 349, 197–206. [Google Scholar] [CrossRef]
- Compton, D.N.; Cornish, L.A.; Witcomb, M.J. The effect of microstructure on hardness measurements in the aluminium-rich corner of the Al–Ni–Cr system. J. Alloys Compd. 2001, 317, 372–378. [Google Scholar] [CrossRef]
- Deng, Y.; Yin, Z.; Zhao, K.; Duan, J.; He, Z. Effects of Sc and Zr microalloying additions on the microstructure and mechanical properties of new Al–Zn–Mg alloys. J. Alloys Compd. 2012, 530, 71–80. [Google Scholar] [CrossRef]
- Loginova, I.; Khalil, A.; Pozdniakov, A.; Solonin, A.; Zolotorevskiy, V. Effect of Pulse Laser Welding Parameters and Filler Metal on Microstructure and Mechanical Properties of Al-4.7Mg-0.32Mn-0.21Sc-0.1Zr Alloy. Metals 2017, 7, 564. [Google Scholar] [CrossRef]
- Polmear, I.; Stjohn, D.; Nie, J.-F.; Qian, M. Light Alloys. Metallurgy of the Light Metals; Butterworth-Heinemann: Boston, MA, USA, 2017. [Google Scholar]
- Adisa, S.; Loginova, I.; Khalil, A.; Solonin, A. Effect of Laser Welding Process Parameters and Filler Metals on the Weldability and the Mechanical Properties of AA7020 Aluminium Alloy. J. Manuf. Mater. Process. 2018, 2, 33. [Google Scholar] [CrossRef]









| Alloy | Chemical Composition, wt % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | Zn | Mg | Cu | Cr | Mn | Ti | B | Fe | Ni | Zr | Sc | Other Elements | |
| AA7075-standard | Bal. | 6.8 | 2.2 | 1.4 | 0.3 | 0.3 | - | - | - | - | - | - | <0.1 |
| AA7075-ZrSc | Bal. | 6.8 | 2.2 | 1.4 | 0.3 | 0.3 | - | - | - | - | 0.5 | 0.3 | <0.1 |
| AA7075-TiB | Bal. | 6.8 | 2.2 | 1.4 | 0.3 | 0.3 | 1 | 0.2 | - | - | - | - | <0.1 |
| AA7075-FeNi | Bal. | 6.8 | 2.2 | 1.4 | 0.3 | 0.3 | - | - | 1 | 1 | - | - | <0.1 |
| Alloy | UTS, (MPa) | YS, (MPa) | Elongation, % * |
|---|---|---|---|
| As-Cast AA7075-standard | 161 ± 6 | 126 ± 3 | 1.2 |
| Homogenized AA7075-standard | 146 ± 1 | 81 ± 20 | 8.3 |
| As-Cast AA7075-ZrSc | 289 ± 3 | 224 ± 20 | 12.7 |
| Homogenized AA7075-ZrSc | 199 ± 10 | 122 ± 30 | 8.6 |
| As-Cast AA7075-TiB | 293 ± 1 | 232 ± 9 | 6.3 |
| Homogenized AA7075-TiB | 269 ± 6 | 169 ± 9 | 8.3 |
| As-Cast AA7075-FeNi | 224 ± 8 | 194 ± 19 | 1.7 |
| Homogenized AA7075-FeNi | 185 ± 6 | 161 ± 3 | 6.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, A.M.; Loginova, I.S.; Pozdniakov, A.V.; Mosleh, A.O.; Solonin, A.N. Evaluation of the Microstructure and Mechanical Properties of a New Modified Cast and Laser-Melted AA7075 Alloy. Materials 2019, 12, 3430. https://doi.org/10.3390/ma12203430
Khalil AM, Loginova IS, Pozdniakov AV, Mosleh AO, Solonin AN. Evaluation of the Microstructure and Mechanical Properties of a New Modified Cast and Laser-Melted AA7075 Alloy. Materials. 2019; 12(20):3430. https://doi.org/10.3390/ma12203430
Chicago/Turabian StyleKhalil, Asmaa M., Irina S. Loginova, Andrey V. Pozdniakov, Ahmed O. Mosleh, and Alexey N. Solonin. 2019. "Evaluation of the Microstructure and Mechanical Properties of a New Modified Cast and Laser-Melted AA7075 Alloy" Materials 12, no. 20: 3430. https://doi.org/10.3390/ma12203430








