3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Egg Yolk Plasma (EYP) or Egg White (EW) Isolation and Heat Treatment
2.2. Well Insert Technology Design and Fabrication
2.3. HuSG-Fibro Isolation and Standard Culture of Both HuSG-Fibro and NS-SV-AC
2.4. Cell Culture in 3D-Cryo Well Insert Technology
2.5. Cryosectioning, Chemical Staining, and Immunohistochemistry Protocols
2.6. Gelation of Egg Yolk Plasma (EYP) and Biomaterial Testing
2.7. NS-SV-AC in Gelled EYP (GEYP) + Egg White (EW) Cell Interface Model
2.8. 3D-Printing the ball and socket, Speeds and Nuzzles
2.9. Addition of Cells to Gelled EYP (GEYP) and Manual Extrusion
2.10. Imaging
2.11. Statistical Analysis
3. Results
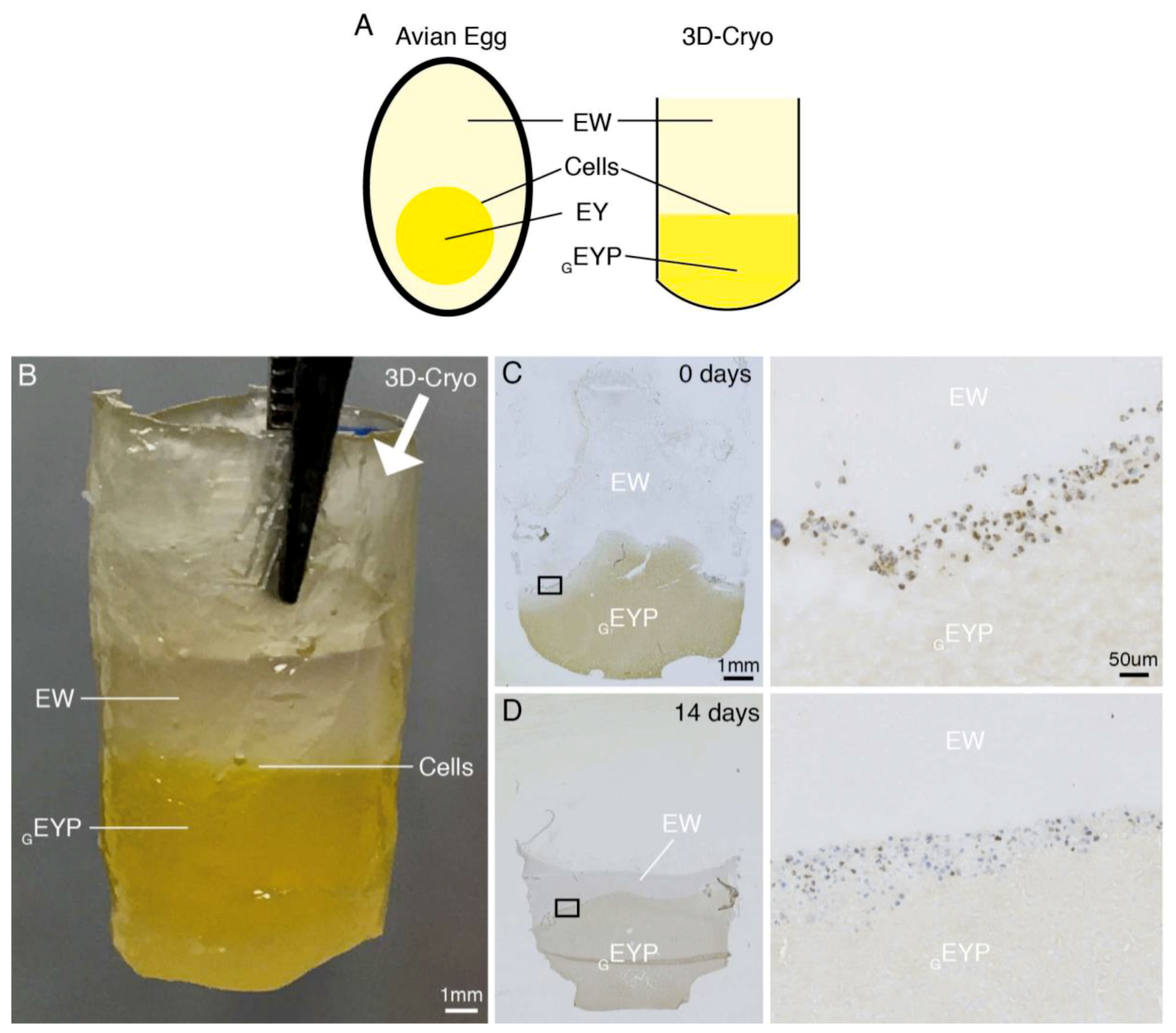
3.1. NS-SV-AC Cells’ Distribution in Egg Yolk Plasma (EYP) Mixtures
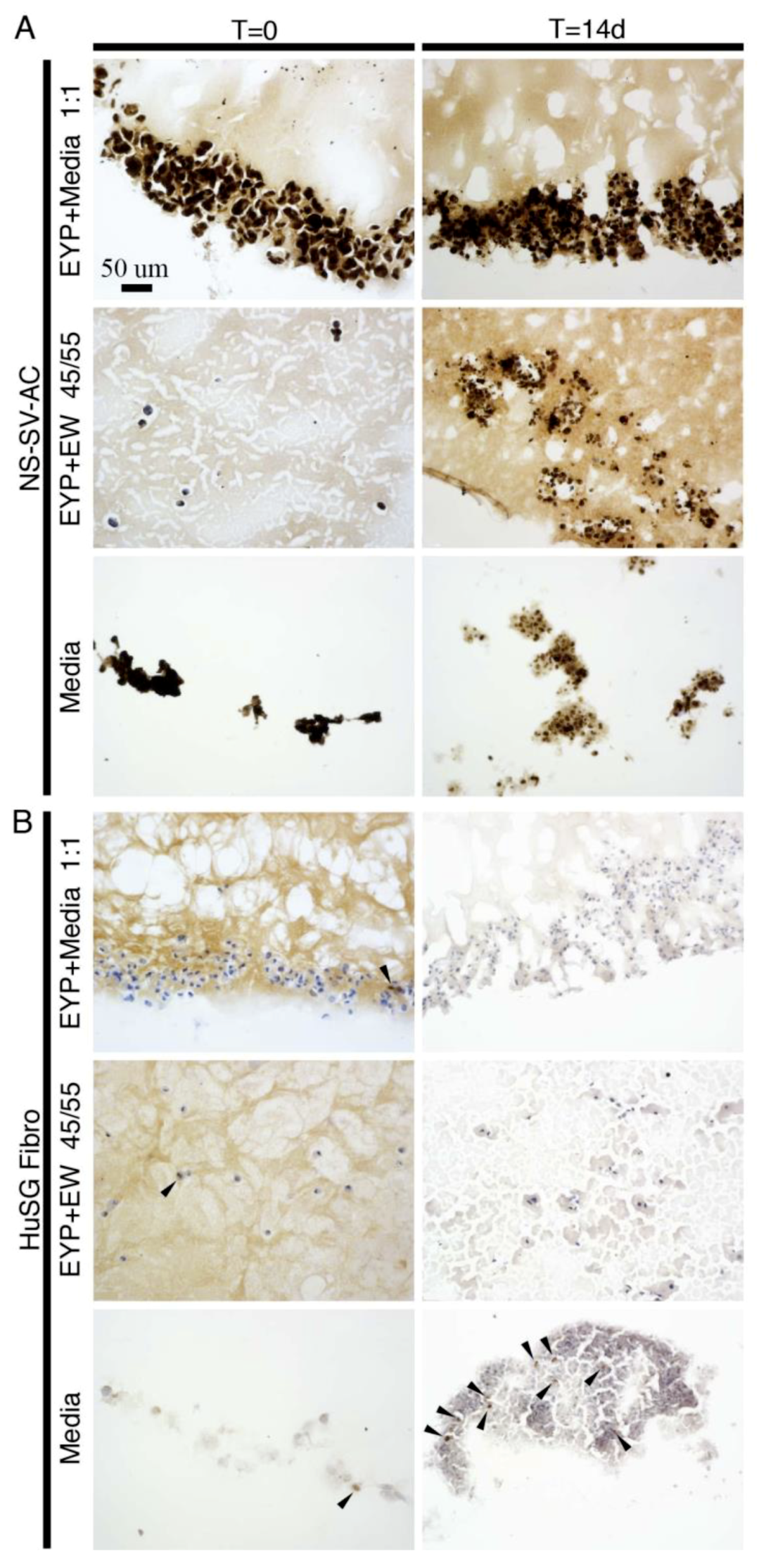
3.2. NS-SV-AC’s and HuSG-Fibro’s Ki-67 Expression in Egg Yolk Plasma (EYP) + Media or EYP + Egg White (EW)
3.3. Freeze and 37 °C Thaw Gelation of Egg Yolk Plasma (EYP)
3.4. Gelled EYP (GEYP) + Egg White (EW) Cell Interface Culture
3.5. Gelled EYP (GEYP)’s Potential for 3D-printing Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miranda-Rius, J.; Brunet-Llobet, L.; Lahor-Soler, E.; Farré, M. Salivary Secretory Disorders, Inducing Drugs, and Clinical Management. Int. J. Med. Sci. 2015, 12, 811–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borkent, D.; Moharamzadeh, K. 20-Tissue engineering of salivary glands. In Biomaterials for Oral and Dental Tissue Engineering; Tayebi, L., Moharamzadeh, K., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 337–351. [Google Scholar]
- Ozdemir, T.; Fowler, E.W.; Liu, S.; Harrington, D.A.; Witt, R.L.; Farach-Carson, M.C.; Pradhan-Bhatt, S.; Jia, X. Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D. Acs Biomater. Sci. Eng. 2016, 2, 2217–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shubin, A.D.; Felong, T.J.; Schutrum, B.E.; Joe, D.S.L.; Ovitt, C.E.; Benoit, D.S.W. Encapsulation of primary salivary gland cells in enzymatically degradable poly(ethylene glycol) hydrogels promotes acinar cell characteristics. Acta Biomater. 2017, 50, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Joraku, A.; Sullivan, C.A.; Yoo, J.J.; Atala, A. Tissue Engineering of Functional Salivary Gland Tissue. Laryngoscope 2005, 115, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Maria, O.M.; Liu, Y.; El-Hakim, M.; Zeitouni, A.; Tran, S.D. The role of human fibronectin- or placenta basement membrane extract-based gels in favouring the formation of polarized salivary acinar-like structures. J. Tissue Eng. Regen. Med. 2017, 11, 2643–2657. [Google Scholar] [CrossRef]
- Burford-Mason, A.P.; Dardick, I.; Mackay, A. Collagen gel cultures of normal salivary gland: Conditions for continued proliferation and maintenance of major cell phenotypes in vitro. Laryngoscope 1994, 104, 335–340. [Google Scholar] [CrossRef]
- Maria, O.M.; Zeitouni, A.; Gologan, O.; Tran, S.D. Matrigel improves functional properties of primary human salivary gland cells. Tissue Eng. Part A 2011, 17, 1229–1238. [Google Scholar] [CrossRef]
- Szlávik, V.; Szabó, B.; Vicsek, T.; Barabás, J.; Bogdán, S.; Gresz, V.; Varga, G.; O’Connell, B.; Vág, J. Differentiation of Primary Human Submandibular Gland Cells Cultured on Basement Membrane Extract. Tissue Eng. Part A 2008, 14, 1915–1926. [Google Scholar] [CrossRef] [Green Version]
- Joraku, A.; Sullivan, C.A.; Yoo, J.; Atala, A. In-vitro reconstitution of three-dimensional human salivary gland tissue structures. Differentiation 2007, 75, 318–324. [Google Scholar] [CrossRef]
- Lilliu, M.A.; Seo, Y.J.; Isola, M.; Charbonneau, A.M.; Zeitouni, A.; El-Hakim, M.; Tran, S.D. Natural extracellular matrix scaffolds recycled from human salivary digests: A morphometric study. Oral Dis. 2016, 22, 313–323. [Google Scholar] [CrossRef]
- Miyajima, H.; Matsumoto, T.; Sakai, T.; Yamaguchi, S.; An, S.H.; Abe, M.; Wakisaka, S.; Lee, K.Y.; Egusa, H.; Imazato, S. Hydrogel-based biomimetic environment for in vitro modulation of branching morphogenesis. Biomaterials 2011, 32, 6754–6763. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.B.; Naim, N.; Nelson, D.A.; Mosier, A.P.; Cady, N.C.; Larsen, M. Biocompatible tissue scaffold compliance promotes salivary gland morphogenesis and differentiation. Tissue Eng. Part A 2014, 20, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Munguia-Lopez, J.G.; Flores-Torres, S.; Grant, J.; Vijayakumar, S.; Leon-Rodriguez, A.D.; Kinsella, J.M. Directing the Self-assembly of Tumour Spheroids by Bioprinting Cellular Heterogeneous Models within Alginate/Gelatin Hydrogels. Sci. Rep. 2017, 7, 4575. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, L.E.; Zegers, M.M.P.; Mostov, K.E. Building epithelial architecture: Insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 2002, 3, 531. [Google Scholar] [CrossRef]
- Vikram Singh, A.; Hasan Dad Ansari, M.; Wang, S.; Laux, P.; Luch, A.; Kumar, A.; Patil, R.; Nussberger, S. The Adoption of Three-Dimensional Additive Manufacturing from Biomedical Material Design to 3D Organ Printing. Appl. Sci. 2019, 9, 811. [Google Scholar] [CrossRef]
- Goldstein, R.S.; Drukker, M.; Reubinoff, B.E.; Benvenisty, N. Integration and differentiation of human embryonic stem cells transplanted to the chick embryo. Dev. Dyn. 2002, 225, 80–86. [Google Scholar] [CrossRef]
- Goldstein, R.S. Transplantation of Human Embryonic Stem Cells and Derivatives to the Chick Embryo. In Human Embryonic Stem Cell Protocols; Turksen, K., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 367–385. [Google Scholar]
- Mousseau, Y.; Mollard, S.; Qiu, H.; Richard, L.; Cazal, R.; Nizou, A.; Vedrenne, N.; Remi, S.; Baaj, Y.; Fourcade, L.; et al. In vitro 3D angiogenesis assay in egg white matrix: Comparison to Matrigel, compatibility to various species, and suitability for drug testing. Lab. Investig. A J. Tech. Methods Pathol. 2014, 94, 340–349. [Google Scholar] [CrossRef]
- Kaipparettu, B.A.; Kuiatse, I.; Tak-Yee Chan, B.; Benny Kaipparettu, M.; Lee, A.V.; Oesterreich, S. Novel egg white-based 3-D cell culture system. Biotechniques 2008, 45, 165–168. [Google Scholar] [CrossRef]
- Rodil, A.; Laca, A.; Paredes, B.; Rendueles, M.; Meana, A.; Diaz, M. Gels prepared from egg yolk and its fractions for tissue engineering. Biotechnol. Prog. 2016, 32, 1577–1583. [Google Scholar] [CrossRef]
- Murakami, H.; Okazaki, Y.; Yamada, K.; Omura, H. Egg yolk lipoprotein, a new supplement for the growth of mammalian cells in serum-free medium. Cytotechnology 1988, 1, 159–169. [Google Scholar] [CrossRef]
- Fujii, D.K.; Gospodarowicz, D. Chicken Egg Yolk-Supplemented Medium and the Serum-Free Growth of Normal Mammalian Cells. In Vitro 1983, 19, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Yu, G.Y.; Xiao, J.; Yan, C.; Kurihara, H.; Li, Y.F.; So, K.F.; He, R.R. Fostering efficacy and toxicity evaluation of traditional Chinese medicine and natural products: Chick embryo as a high throughput model bridging in vitro and in vivo studies. Pharmacol. Res. 2018, 133, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Powrie, W.D.; Little, H.; Lopez, A. Gelation of Egg Yolk. J. Food Sci. 1963, 28, 38–46. [Google Scholar] [CrossRef]
- Jaax, S.; Travnicek, D. The Effect of Pasteurization, Selected Additives and Freezing Rate on the Gelation of Frozen-Defrosted Egg Yolk. Poult. Sci. 1968, 47, 1013–1022. [Google Scholar] [CrossRef]
- Mahadevan, S.; Satyanarayana, T.; Kumar, S.A. Physicochemical studies on the gelation of hen egg yolk; separation of gelling protein components from yolk plasma. J. Agric. Food Chem. 1969, 17, 767–771. [Google Scholar] [CrossRef]
- Palmer, H.H.; Ijichi, K.; Roff, H. Partial Thermal reversal of gelation in thawed egg yolk products. J. Food Sci. 1970, 35, 403–406. [Google Scholar] [CrossRef]
- Chang, C.H.; Powrie, W.D.; Fennema, O. Studies on the gelation of egg yolk and plasma upon freezing and thawing. J. Food Sci. 1977, 42, 1658–1665. [Google Scholar] [CrossRef]
- Wakamatu, T.; Sato, Y.; Saito, Y. Identification of the Components Responsible for the Gelation of Egg Yolk during Freezing. Agric. Biol. Chem. 1982, 46, 1495–1503. [Google Scholar]
- Au, C.; Acevedo, N.C.; Horner, H.T.; Wang, T. Determination of the Gelation Mechanism of Freeze–Thawed Hen Egg Yolk. J. Agric. Food Chem. 2015, 63, 10170–10180. [Google Scholar] [CrossRef]
- Lopez, A.; Fellers, C.A.; Powrie, W.D. Some Factors Affecting Gelation of Frozen Egg Yolk. J. Milk Food Technol. 1954, 17, 334–339. [Google Scholar] [CrossRef]
- Cecchini, M.P.; Parnigotto, M.; Merigo, F.; Marzola, P.; Daducci, A.; Tambalo, S.; Boschi, F.; Colombo, L.; Sbarbati, A. 3D-Printing of Rat Salivary Glands: The Submandibular–Sublingual Complex. Anat. Histol. Embryol. 2014, 43, 239–244. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D-Bioprinting. Sci. Rep. 2016, 6, 29977. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Niu, X.; Shao, L.; Zhou, L.; Lin, Z.; Sun, A.; Fu, J.; Chen, Z.; Hu, J.; Liu, Y.; et al. 3D-Printing of complex GelMA-based scaffolds with nanoclay. Biofabrication 2019, 11, 035006. [Google Scholar] [CrossRef] [PubMed]
- Charbonneau, A.M.; Al-Samadi, A.; Salo, T.; Tran, S.D. 3D Culture Histology Cryosectioned Well Insert Technology Preserves Structural Relationship Between Cells and Biomaterials for Time-Lapse Analysis of 3D Cultures. Biotechnol. J. 2019, e1900105. [Google Scholar] [CrossRef]
- Azuma, M.; Tamatani, T.; Kasai, Y.; Sato, M. Immortalization of normal human salivary gland cells with duct-, myoepithelial-, acinar-, or squamous phenotype by transfection with SV40 ori- mutant deoxyribonucleic acid. Lab. Investig. A J. Tech. Methods Pathol. 1993, 69, 24–42. [Google Scholar]
- Azuma, M.; Sato, M. Morphogenesis of normal human salivary gland cells in vitro. Histol. Histopathol. 1994, 9, 781–790. [Google Scholar]
- Kiernan, J.A. Methods for Connective Tissue. In Histological and Histochemical Methods Theory and Practice, 4th ed.; Scion: Bloxham, UK, 2008; pp. 197–198. [Google Scholar]
- Scholzen, T.; Gerdes, J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Chen, D.X.B. Mechanical Properties of Native Tissues and Scaffolds. In Extrusion Bioprinting of Scaffolds for Tissue Engineering Applications; Springer International Publishing: Cham, Switzerland, 2019; pp. 49–90. [Google Scholar]
- Stevens, L. Egg proteins: What are their functions? Sci. Prog. 1996, 79, 65–87. [Google Scholar]
- Takashima, A. Establishment of Fibroblast Cultures. Curr. Protoc. Cell Biol. 1998, 2. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The hematoxylins and eosin. In Bancroft’s Theory and Practice of histological Techniques, 8th ed.; Suvarna, K.S., Layton, C., Bancroft, J.D., Eds.; Elsevier Limited: Amsterdam, The Netherlands, 2019; pp. 126–138. [Google Scholar]
- Nielsen, L.F.; Moe, D.; Kirkeby, S.; Garbarsch, C. Sirius red and acid fuchsin staining mechanisms. Biotech. Histochem. Off. Publ. Biol. Stain Comm. 1998, 73, 71–77. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Cossermelli, W.; Brentani, R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch. Histol. Jpn.(Nihon Soshikigaku Kiroku) 1978, 41, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Delellis, R.L.; Bowling, M.C. The use of sirius red and congo red staining in routine histopathology. Hum. Pathol. 1970, 1, 655. [Google Scholar] [CrossRef]
- Weatherford, T.W. Staining of collagenous and non-collagenous structures with picrosirius red F3BA. Ala. J. Med. Sci. 1972, 9, 383–388. [Google Scholar]
- Wiradjaja, F.; DiTommaso, T.; Smyth, I. Basement membranes in development and disease. Birth Defects Res. Part C Embryo Today Rev. 2010, 90, 8–31. [Google Scholar] [CrossRef]
- The Human Protein Atlas 18.1. Available online: https://www.proteinatlas.org/ENSG00000148773-MKI67/tissue/salivary+gland (accessed on 7 July 2019).
- Ariza, A.; Mate, J.L.; Isamat, M.; Calatrava, A.; Fernandez-Vasalo, A.; Navas-Palacios, J.J. Overexpression of Ki-67 and cyclins A and B1 in JC virus-infected cells of progressive multifocal leukoencephalopathy. J. Neuropathol. Exp. Neurol. 1998, 57, 226–230. [Google Scholar] [CrossRef]
- Noiva, R.M.; Menezes, A.C.; Peleteiro, M.C. Influence of temperature and humidity manipulation on chicken embryonic development. BMC Vet. Res. 2014, 10, 234. [Google Scholar] [CrossRef]
- Bruzual, J.J.; Peak, S.D.; Brake, J.; Peebles, E.D. Effects of Relative Humidity during Incubation on Hatchability and Body Weight of Broiler Chicks from Young Breeder Flocks1. Poult. Sci. 2000, 79, 827–830. [Google Scholar] [CrossRef]
- Valverde, D.; Laca, A.; Estrada, L.N.; Paredes, B.; Rendueles, M.; Díaz, M. Egg yolk and egg yolk fractions as key ingredient for the development of a new type of gels. Int. J. Gastron. Food Sci. 2016, 3, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.A.; Mahadevan, S. Physicochemical studies on the gelation of hen’s egg yolk. Delipidation of yolk plasma by treatment with phospholipase-C and extraction with solvents. J. Agric. Food Chem. 1970, 18, 666–670. [Google Scholar] [CrossRef]
- Krouskop, T.A.; Wheeler, T.M.; Kallel, F.; Garra, B.S.; Hall, T. Elastic Moduli of Breast and Prostate Tissues under Compression. Ultrason. Imaging 1998, 20, 260–274. [Google Scholar] [CrossRef]
- Cotterill, O.J. Freezing Egg Products. In Egg Science and Technology; Stadelman, W.J., Cotterill, O.J., Eds.; Haworth Press: New York, NY, USA, 1994; pp. 269–270. [Google Scholar]
- Pillet, E.; Duchamp, G.; Batellier, F.; Beaumal, V.; Anton, M.; Desherces, S.; Schmitt, E.; Magistrini, M. Egg yolk plasma can replace egg yolk in stallion freezing extenders. Theriogenology 2011, 75, 105–114. [Google Scholar] [CrossRef] [PubMed]



| Figure | Cell Type | Biomaterials | Tools | Test Parameter |
|---|---|---|---|---|
| 1 | NS-SV-AC | EYP + Media EYP + EW | - Chemical stain Sirius Red - 3D-Cryo well insert | - 0 & 14 days - Color of biomaterial - Cell distribution |
| 2 | NS-SV-AC HuSG-Fibro | EYP + Media EYP + EW Media | - IHC (Anti-Ki67) - 3D-Cryo well insert | - 0 & 14 days - Cell proliferation |
| 3 | N/A | EYP → GEYP | - Rotational rheometer - −20 °C Freezer | - 0, 1, 4, 7, 11, 15, 30 days - Storage Modulus (G’) - Loss Modulus (G’’) |
| 4 | NS-SV-AC | GEYP + EW | - IHC (anti-Ki67) - 3D-Cryo well insert | - 0 & 14 days - Cell distribution - Cell proliferation |
| 5 | NS-SV-AC (CFSE) NS-SV-AC (Hoeschst) | GEYP | - 3D-Extrusion printer - Glass syringe - 3D-Cryo well insert | - Design & extrudability - Structural Maintenance - Cell Localization |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charbonneau, A.M.; Kinsella, J.M.; Tran, S.D. 3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma. Materials 2019, 12, 3480. https://doi.org/10.3390/ma12213480
Charbonneau AM, Kinsella JM, Tran SD. 3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma. Materials. 2019; 12(21):3480. https://doi.org/10.3390/ma12213480
Chicago/Turabian StyleCharbonneau, André M., Joseph M. Kinsella, and Simon D. Tran. 2019. "3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma" Materials 12, no. 21: 3480. https://doi.org/10.3390/ma12213480
APA StyleCharbonneau, A. M., Kinsella, J. M., & Tran, S. D. (2019). 3D Cultures of Salivary Gland Cells in Native or Gelled Egg Yolk Plasma, Combined with Egg White and 3D-Printing of Gelled Egg Yolk Plasma. Materials, 12(21), 3480. https://doi.org/10.3390/ma12213480





