Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis
Abstract
:1. Introduction
2. Materials and Methods
2.1. HVSFS Coating Process
2.2. Sample Characterization
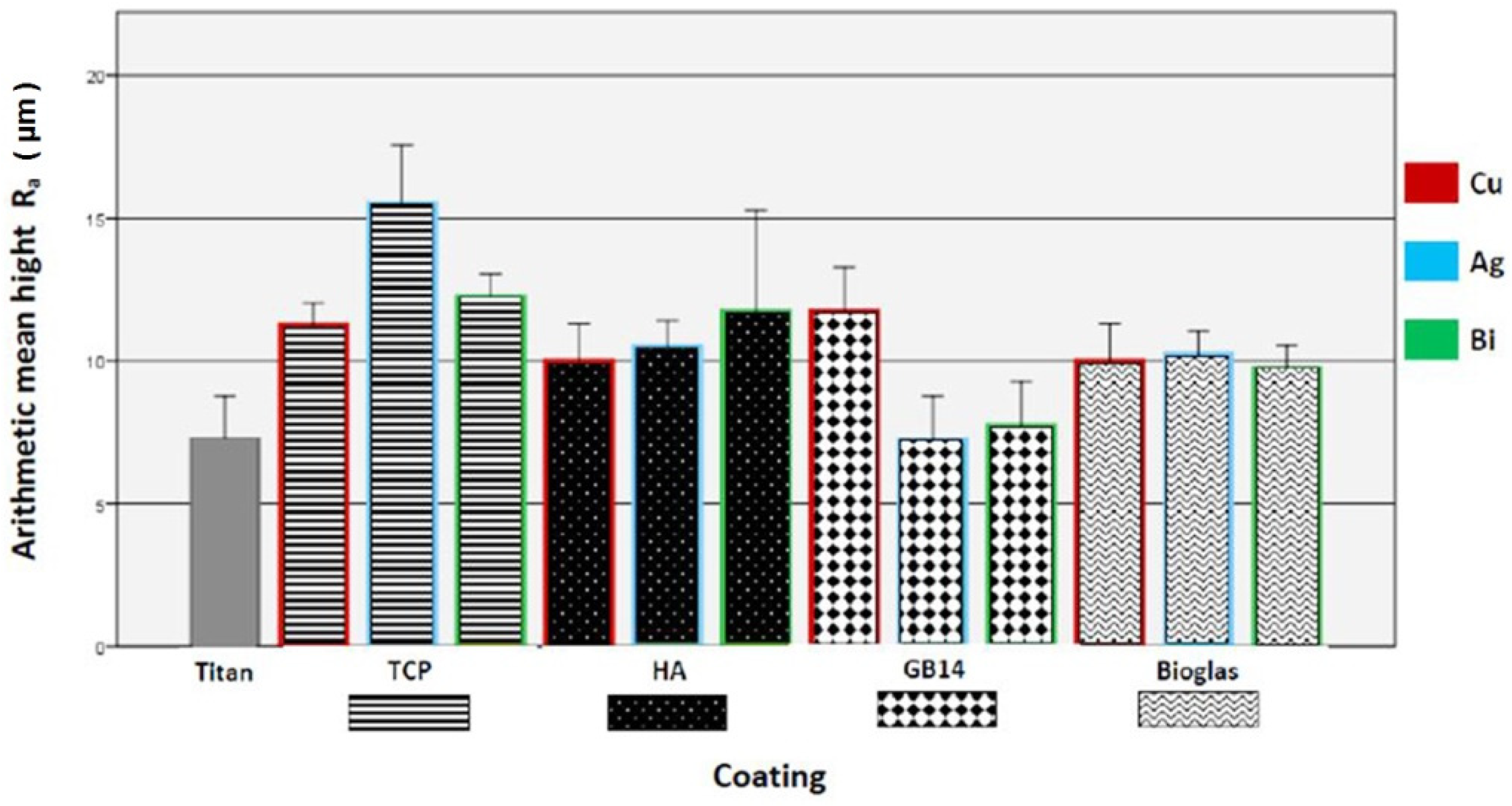
2.2.1. Surface Roughness
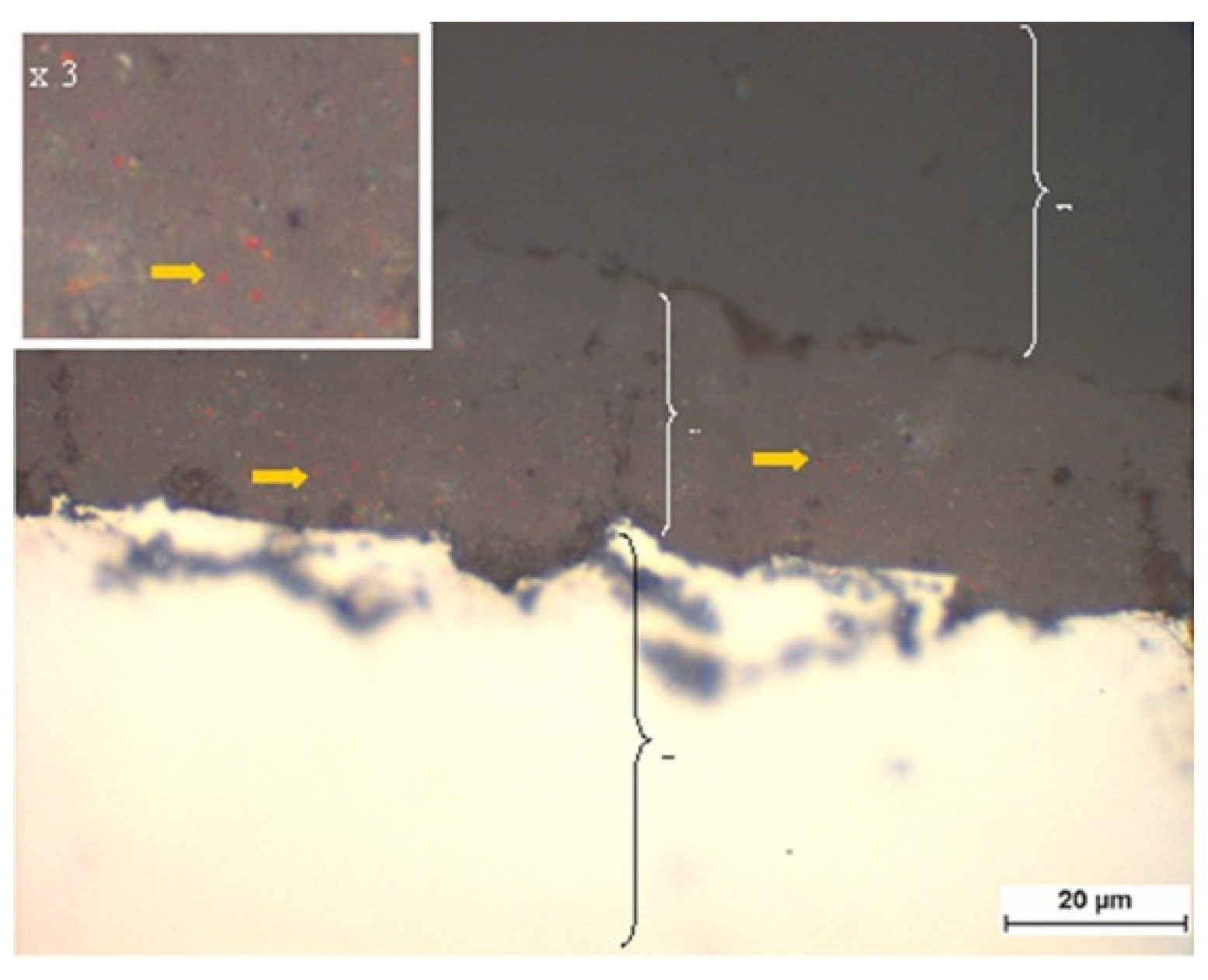
2.2.2. Brightfield Microscopy of the Coatings
2.2.3. XRD-Analysis for Definition of the Phase Composition of the Layers
2.2.4. Raman Spectroscopy for Determination of the Phase Composition of the Metals
2.2.5. SEM for Verification Metal Contained in the Coatings
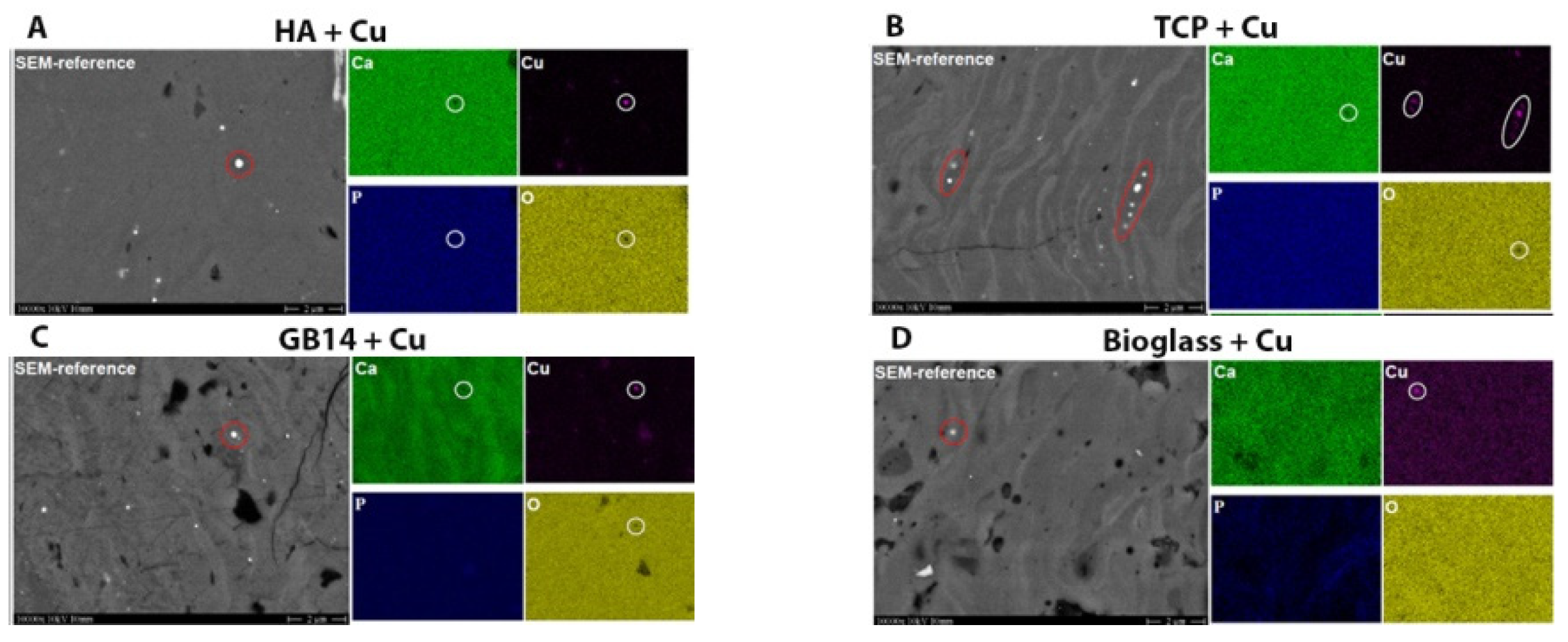
2.2.6. Element-Mapping for Cu-Content Verification
2.2.7. Release Kinetics for Detection of Metal Ions and Ceramic Components of the Coatings
2.3. Biocompatibility Testing in Vitro
2.3.1. Cell Culture
2.3.2. Cell Viability
2.3.3. WST-1-Kit Was Used to Quantify Cell Proliferation and Viability
2.3.4. Lactate Dehydrogenase (LDH) Test to Determine Cytotoxicity
2.3.5. In Vivo Bone Biocompatibility via Simulated Body Fluid (SBF) Experiment
2.4. Antimicrobial Testing
2.5. Statistical Analysis
- -
- Time of data collection (3, 7, 14, 21 days or 24, 48, 72 h)
- -
- Ceramic component (TCP, HA, GB14, bioglass, titanium)
- -
- Metal doping (Cu, Ag, Bi, no doping)
3. Results
3.1. Sample Characterization
3.1.1. Brightfield Microscopy of the Coatings
3.1.2. XRD Analysis
3.1.3. Raman Spectroscopy Phase Composition Determination of the Metals
3.1.4. SEM for Verification of Metal Doping Content of the Coatings
3.1.5. Element-Mapping for Cu-Content Verification
3.1.6. Release Kinetics of Coating Contained Metal Ions and Ceramic Components into Water
3.2. Biocompatibility
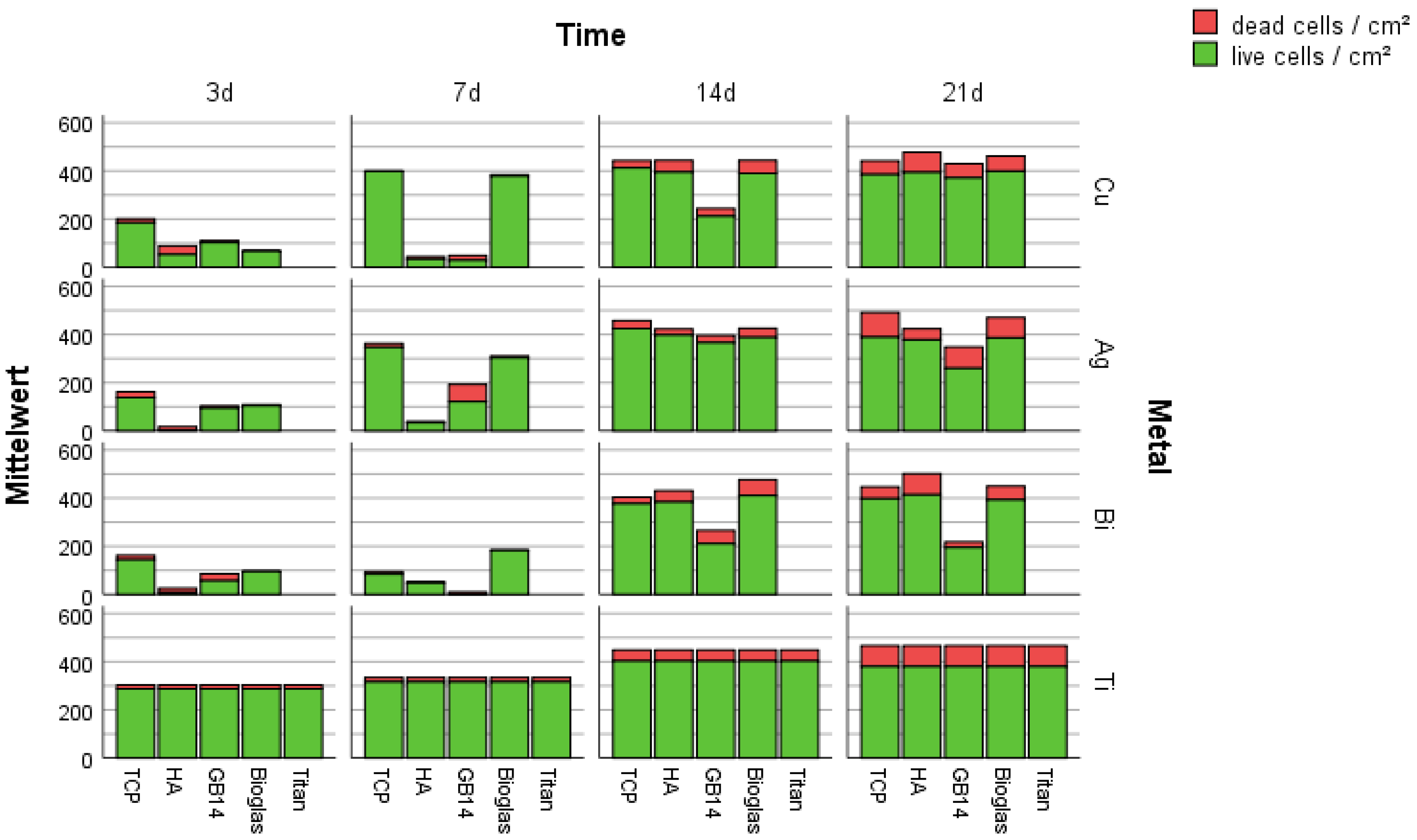
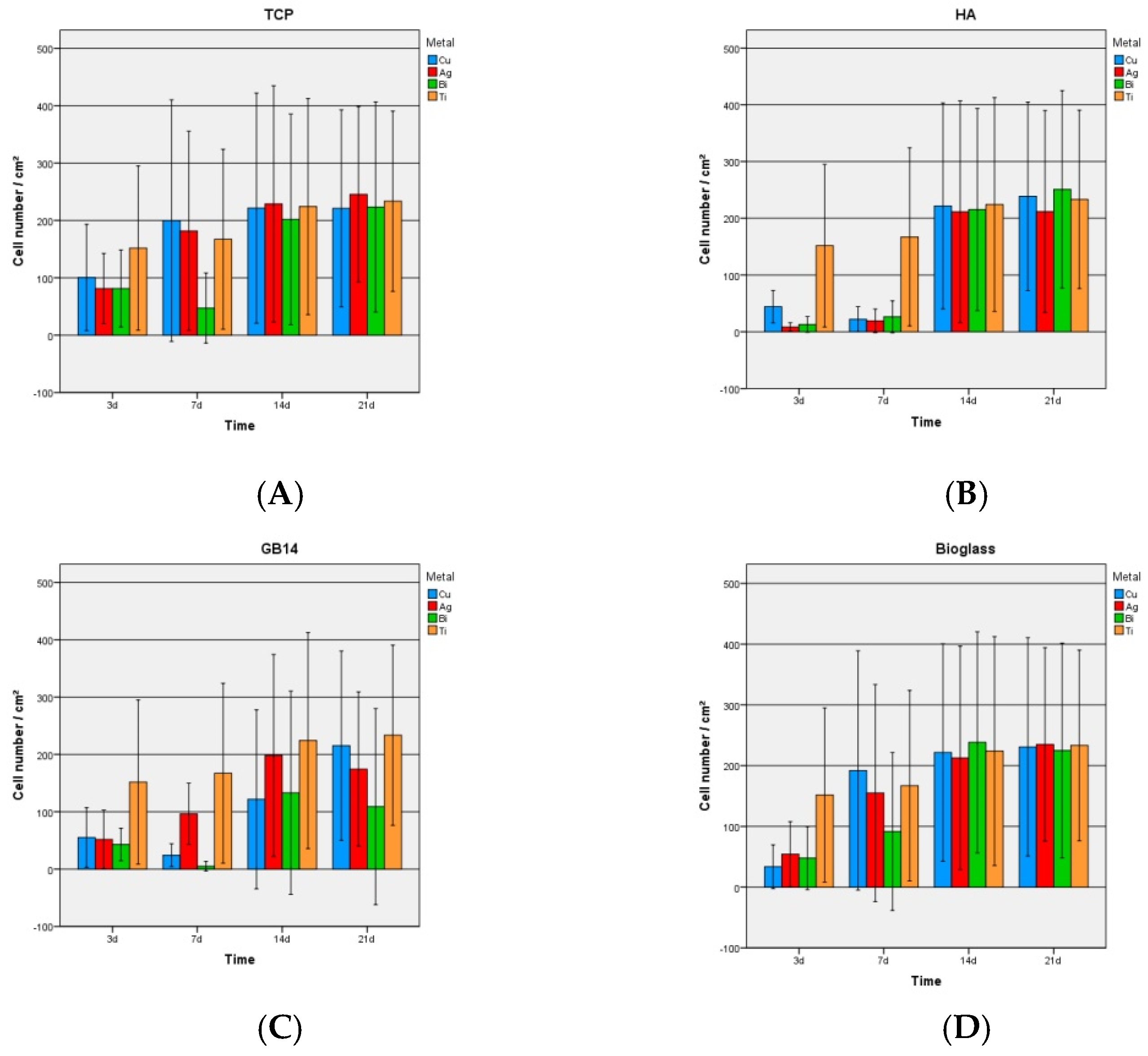
3.2.1. Live/Dead-Assay and Cell Count
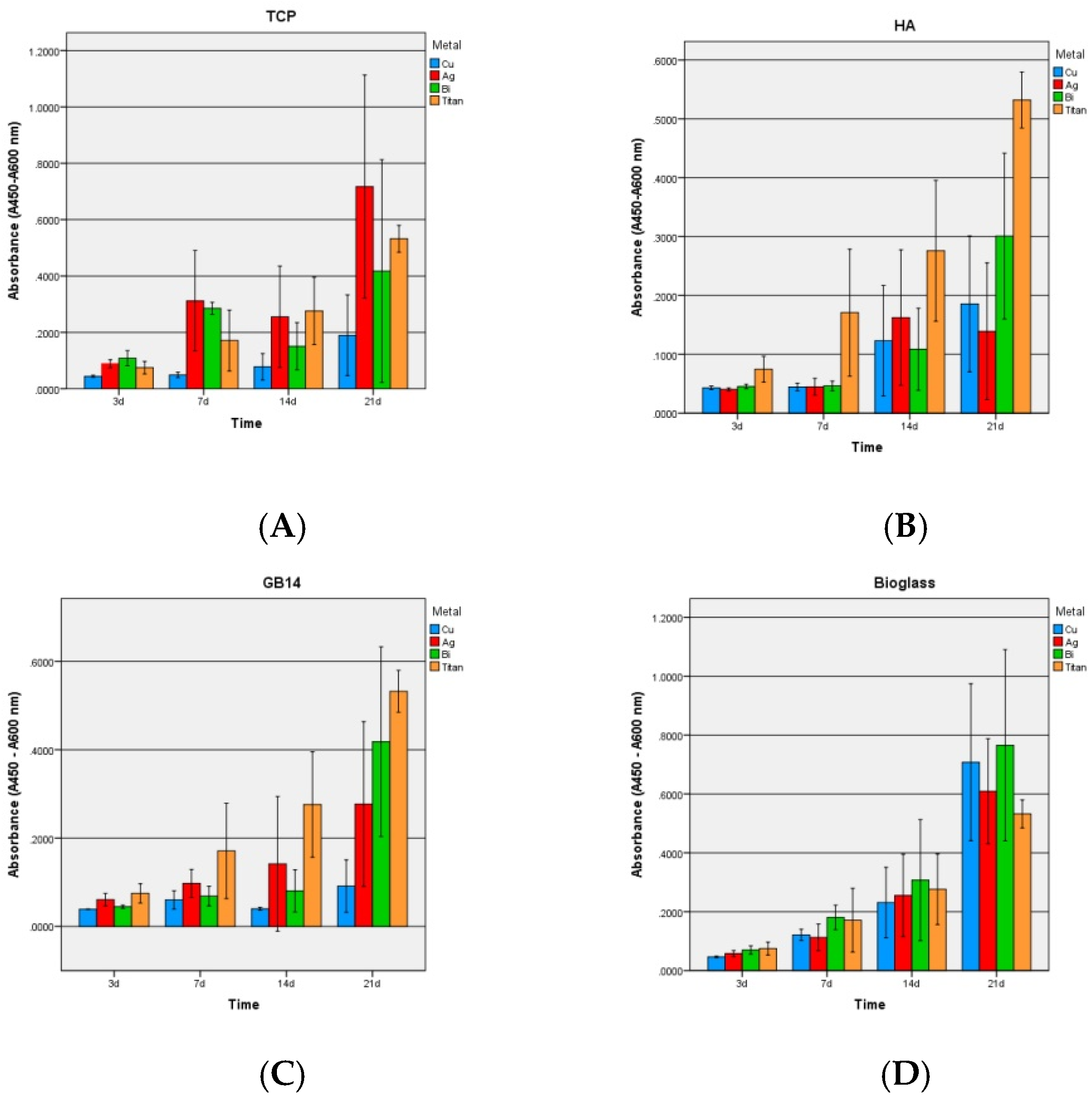
3.2.2. Proliferation
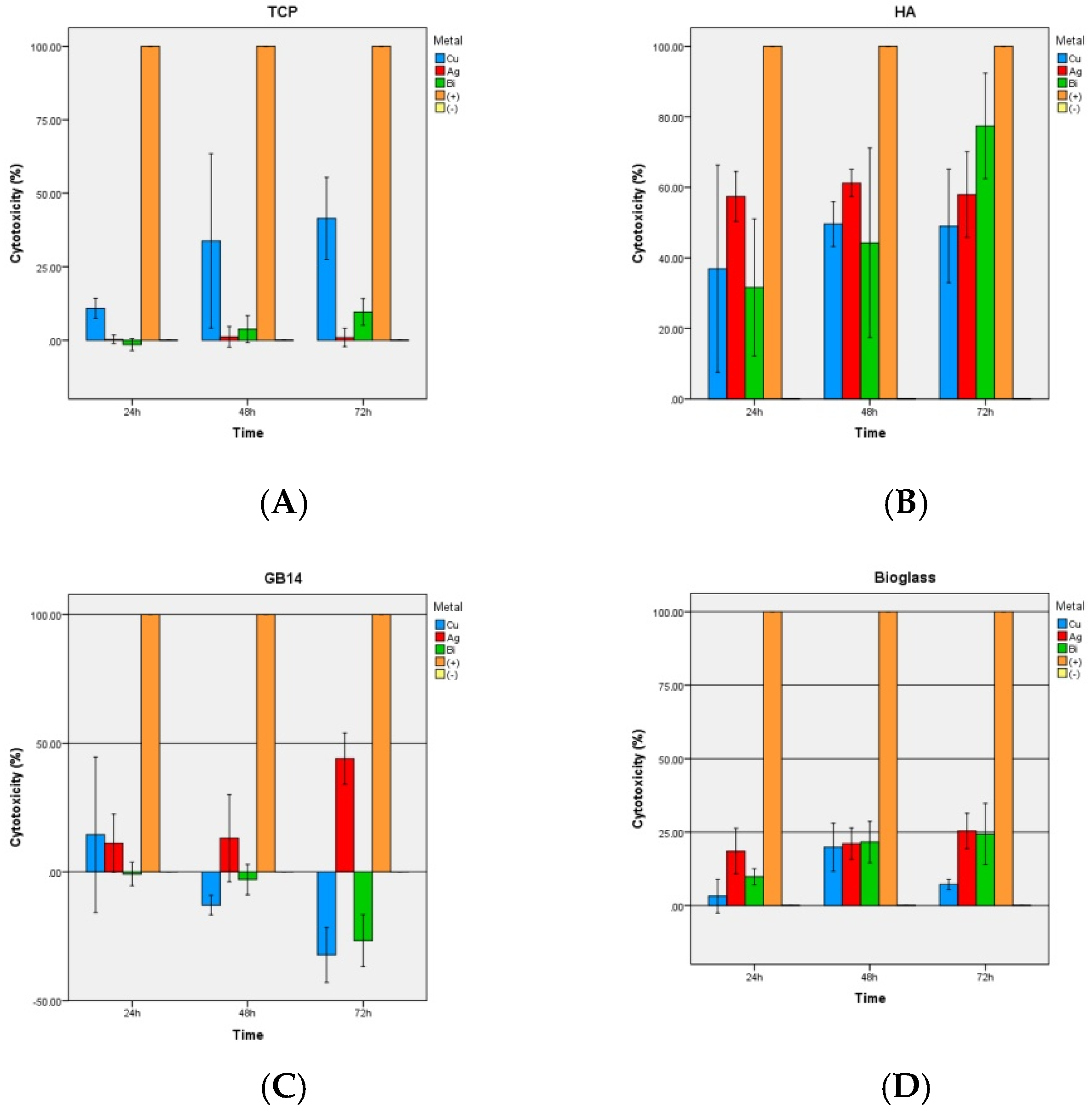
3.2.3. LDH-Kit
3.2.4. SBF-Experiment
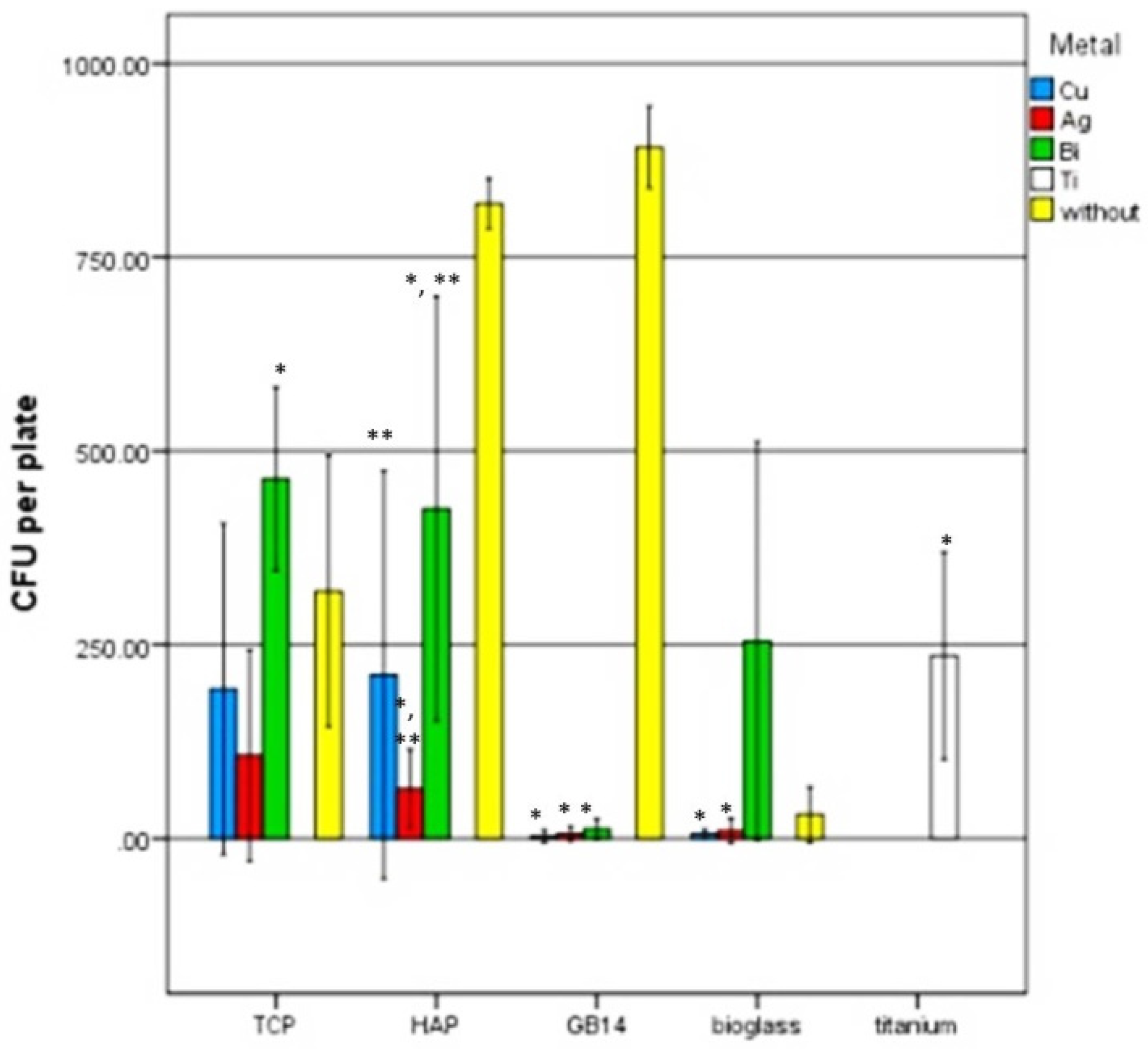
3.3. Antimicrobial Testing with Safe Airborne Antibacterial Assay
4. Discussion
4.1. Live/Dead-Assay
4.2. WST-1 Assay
4.3. LDH-Assay
4.4. SBF
4.5. Safe Airborne Antibacterial Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.; Osman, K.; Green, G.; Haddad, F.S. The epidemiology of failure in total knee arthroplasty: Avoiding your next revision. Bone Jt. J. 2016, 98-B, 105–112. [Google Scholar] [CrossRef]
- Bauer, T.W.; Schils, J. The pathology of total joint arthroplasty.II. Mechanisms of implant failure. Skelet. Radiol. 1999, 28, 483–497. [Google Scholar] [CrossRef]
- Bauer, T.W.; Schils, J. The pathology of total joint arthroplasty. I. Mechanisms of implant fixation. Skelet. Radiol. 1999, 28, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W. Bone and Joint Infections: From Microbiology to Diagnostics and Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Roy, M.; Fielding, G.A.; Beyenal, H.; Bandyopadhyay, A.; Bose, S. Mechanical, in vitro antimicrobial and biological properties of plasma sprayed silver-doped hydroxyapatite coating. ACS Appl. Mater. Interfaces 2012, 4, 1341. [Google Scholar] [CrossRef] [PubMed]
- De Grado, G.F.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Zheng, Y.; Tang, L.; Qin, Y.X.; Zhu, D. Biofunctionalization of metallic implants by calcium phosphate coatings. Bioact. Mater. 2019, 4, 196–206. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive glasses and glass-ceramics for healthcare applications in bone regeneration and tissue engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.; Nobel, D.; Mayr, H.O.; Berger, G.; Gildenhaar, R.; Brandt, J. Histological and histomorphometric investigations on bone integration of rapidly resorbable calcium phosphate ceramics. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 84, 452–462. [Google Scholar] [CrossRef]
- Kluin, O.S.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin. Drug Deliv. 2013, 10, 341–351. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Xie, Y.; Ding, C.; Ruan, H.; Fan, C. Anti-bacterial and cytotoxic properties of plasma sprayed silver-containing ha coatings. J. Mater. Sci. Mater. Med. 2008, 19, 3603–3609. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Zhao, R.; Mao, H.; Yan, Y.; Pang, X. Antibacterial efficacy, corrosion resistance, and cytotoxicity studies of copper-substituted carbonated hydroxyapatite coating on titanium substrate. J. Mater. Sci. 2015, 50, 1688–1700. [Google Scholar] [CrossRef]
- Shanmugam, S.; Gopal, B. Antimicrobial and cytotoxicity evaluation of aliovalent substituted hydroxyapatite. Appl. Surf. Sci. 2014, 303, 277–281. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P. The molecular mechanisms of copper and silver ion disinfection of bacteria and viruses. Crit. Rev. Envron. Sci. Technol. 1988, 18, 295–315. [Google Scholar] [CrossRef]
- Gasqueres, C.; Schneider, G.; Nusko, R.; Maier, G.; Dingeldein, E.; Eliezer, A. Innovative antibacterial coating by anodic spark deposition. Surf. Coat. Technol. 2012, 206, 3410–3414. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Xiong, G.Y.; Liang, H.; Raman, S.; He, F.; Huang, Y. Modification of medical metals by ion implantation of copper. Appl. Surf. Sci. 2007, 253, 9426–9429. [Google Scholar] [CrossRef]
- George, N.; Faoagali, J.; Muller, M. Silvazine (tm) (silver sulfadiazine and chlorhexidine) activity against 200 clinical isolates. Burns 1997, 23, 493–495. [Google Scholar] [CrossRef]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef]
- Clement, J.L.; Jarrett, P.S. Antibacterial silver. Met. Based Drugs 1994, 1, 467–482. [Google Scholar] [CrossRef]
- Guggenbichler, J.P.; Boswald, M.; Lugauer, S.; Krall, T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection 1999, 27, S16–S23. [Google Scholar] [CrossRef]
- Gosheger, G.; Hardes, J.; Ahrens, H.; Streitburger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Kemper, F.H.; Winkelmann, W.; von Eiff, C. Silver-coated megaendoprostheses in a rabbit model—An analysis of the infection rate and toxicological side effects. Biomaterials 2004, 25, 5547–5556. [Google Scholar] [CrossRef] [PubMed]
- Hardes, J.; Ahrens, H.; Gebert, C.; Streitbuerger, A.; Buerger, H.; Erren, M.; Gunsel, A.; Wedemeyer, C.; Saxler, G.; Winkelmann, W.; et al. Lack of toxicological side-effects in silver-coated megaprostheses in humans. Biomaterials 2007, 28, 2869–2875. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.; Sitthisak, S.; Sengupta, M.; Johnson, M.; Jayaswal, R.K.; Morrissey, J.A. Copper stress induces a global stress response in staphylococcus aureus and represses sae and agr expression and biofilm formation. Appl. Environ. Microb. 2010, 76, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Patenge, N.; Arndt, K.; Eggert, T.; Zietz, C.; Kreikemeyer, B.; Bader, R.; Nebe, B.; Stranak, V.; Hippler, R.; Podbielski, A. Evaluation of antimicrobial effects of novel implant materials by testing the prevention of biofilm formation using a simple small scale medium-throughput growth inhibition assay. Biofouling 2012, 28, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.J.; Tsai, M.T.; Shieh, T.M.; Huang, H.L.; Hsu, J.T.; Ko, Y.C.; Fuh, L.J. In vitro antibacterial activity and cytocompatibility of bismuth doped micro-arc oxidized titanium. J. Biomater. Appl. 2013, 27, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Coomaraswamy, K.S.; Lumley, P.J.; Hofmann, M.P. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic portland cement-based (mta-like) system. J. Endodont. 2007, 33, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Liu, C.S.; Mao, Y.H. Bismuth-doped injectable calcium phosphate cement with improved radiopacity and potent antimicrobial activity for root canal filling. Acta Biomater. 2010, 6, 3199–3207. [Google Scholar] [CrossRef]
- Geesink, R.G.T.; de Groot, K.; Klein, C.P.A. Chemical implant fixation using hydroxyl-apatite coatings: The development of a human total hip prosthesis for chemical fixation to bone using hydroxyl-apatite coatings on titanium substrates. Clin. Orthop. Relat. Res. 1987, 225, 147–170. [Google Scholar] [CrossRef]
- Geesink, R.G.T.; de Groot, K.; Klein, C.P.A. Bonding of bone to apatite-coated implants. J. Bone Jt. Surg. Br. Vol. 1988, 70, 17–22. [Google Scholar] [CrossRef]
- Ripamonti, U.; Roden, L.C.; Renton, L.F. Osteoinductive hydroxyapatite-coated titanium implants. Biomaterials 2012, 33, 3813–3823. [Google Scholar] [CrossRef]
- Filiaggi, M.; Coombs, N.; Pilliar, R. Characterization of the interface in the plasma-sprayed ha coating/ti-6al-4v implant system. J. Biomed. Mater. Res. 1991, 25, 1211–1229. [Google Scholar] [CrossRef] [PubMed]
- Geesink, R.G. Osteoconductive coatings for total joint arthroplasty. Clin. Orthop. Relat. Res. 2002, 395, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Mucalo, M. Hydroxyapatite (Hap) for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015; p. 404. [Google Scholar]
- Roche, A.S. Cytotoxicity Detection Kit (LDH); Version 08; Roche: Hamburg, Germany, 2011. [Google Scholar]
- Ohtsuki, C.; Kokubo, T.; Yamamuro, T. Mechanism of apatite formation on cao sio 2 p 2 o 5 glasses in a simulated body fluid. J. Non-Cryst. Solids 1992, 143, 84–92. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Zou, P.; Solarte, D.L.G.; Hellwig, E.; Steinberg, T.; Lienkamp, K. Development of a standardized and safe airborne antibacterial assay, and its evaluation on antibacterial biomimetic model surfaces. PLoS ONE 2014, 9, e111357. [Google Scholar] [CrossRef]
- Krieg, P.; Killinger, A.; Gadow, R.; Burtscher, S.; Bernstein, A. High velocity suspension flame spraying (hvsfs) of metal doped bioceramic coatings. Bioact. Mater. 2017, 2, 162–169. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Evans, E. Toxicity of hydroxyapatite in vitro: The effect of particle size. Biomaterials 1991, 12, 574–576. [Google Scholar] [CrossRef]
- Sun, J.-S.; Liu, H.-C.; Chang, W.H.-S.; Li, J.; Lin, F.-H.; Tai, H.-C. Influence of hydroxyapatite particle size on bone cell activities: An in vitro study. J. Biomed. Mater. Res. 1998, 39, 390–397. [Google Scholar] [CrossRef]
- Ewence, A.E.; Bootman, M.; Roderick, H.L.; Skepper, J.N.; McCarthy, G.; Epple, M.; Neumann, M.; Shanahan, C.M.; Proudfoot, D. Calcium phosphate crystals induce cell death in human vascular smooth muscle cells a potential mechanism in atherosclerotic plaque destabilization. Circ. Res. 2008, 103, 28–34. [Google Scholar] [CrossRef]
- Hashimoto, S.; Fujiwara, K.; Fuwa, K. Determination of phosphate ion by gas chromatography with the phosphine generation technique. Anal. Chem. 1985, 57, 1305–1309. [Google Scholar] [CrossRef]
- Fishbein, L. Potential hazards of fumigant residues. Environ. Health Perspect. 1976, 14, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shintani, H. Ethylene oxide gas sterilization of medical devices. Biocontrol Sci. 2017, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Swenberg, J.A.; Ham, A.; Koc, H.; Morinello, E.; Ranasinghe, A.; Tretyakova, N.; Upton, P.B.; Wu, K.-Y. DNA adducts: Effects of low exposure to ethylene oxide, vinyl chloride and butadiene. Mutat. Res. Genet. Toxicol. Environ. Mutagenesis 2000, 464, 77–86. [Google Scholar] [CrossRef]
- Tan, E.L.; Cumming, R.B.; Hsie, A.W. Mutagenicity and cytotoxicity of ethylene oxide in the cho/hgprt system. Environ. Mol. Mutagenesis 1981, 3, 683–686. [Google Scholar] [CrossRef]
- Chaudhry, M. Phosphine resistance. Pestic. Outlook 2000, 11, 88–91. [Google Scholar] [CrossRef]
- Chaudhry, M. Review a review of the mechanisms involved in the action of phosphine as an insecticide and phosphine resistance in stored-product insects. Pest. Manag. Sci. 1997, 49, 213–228. [Google Scholar] [CrossRef]
- Nath, N.S.; Bhattacharya, I.; Tuck, A.G.; Schlipalius, D.I.; Ebert, P.R. Mechanisms of phosphine toxicity. J. Toxicol. 2011, 2011. [Google Scholar] [CrossRef]
- Rashedinia, M.; Jamshidzadeh, A.; Mehrabadi, A.R.; Niknahad, H. Prevention of phosphine-induced cytotoxicity by nutrients in hepG2 cells. Indian J. Med. Res. 2016, 144, 560. [Google Scholar] [CrossRef]
- Ignatius, A.A.; Schmidt, C.; Kaspar, D.; Claes, L.E. In vitro biocompatibility of resorbable experimental glass ceramics for bone substitutes. J. Biomed. Mater. Res. 2001, 55, 285–294. [Google Scholar] [CrossRef]
- Berger, G.; Gildenhaar, R.; Ploska, U. Rapid resorbable, glassy crystalline materials on the basis of calcium alkali orthophosphates. Biomaterials 1995, 16, 1241–1248. [Google Scholar] [CrossRef]
- Wiltfang, J.; Merten, H.; Schlegel, K.; Schultze-Mosgau, S.; Kloss, F.; Rupprecht, S.; Kessler, P. Degradation characteristics of α and β tri-calcium-phosphate (tcp) in minipigs. J. Biomed. Mater. Res. 2002, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Studer, A.M.; Limbach, L.K.; Van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Cortizo, M.C.; De Mele, M.F.L. Cytotoxicity of copper ions released from metal. Biol. Trace Elem. Res. 2004, 102, 129–141. [Google Scholar] [CrossRef]
- Fahmy, B.; Cormier, S.A. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol. Vitr. 2009, 23, 1365–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ho, J.; Ooi, C.P. Antibacterial efficacy and cytotoxicity studies of copper (ii) and titanium (iv) substituted hydroxyapatite nanoparticles. Mater. Sci. Eng. C 2010, 30, 1137–1144. [Google Scholar] [CrossRef]
- Przyojski, J.A.; Myers, N.N.; Arman, H.D.; Prosvirin, A.; Dunbar, K.R.; Natarajan, M.; Krishnan, M.; Mohan, S.; Walmsley, J.A. Dinuclear and heptanuclear complexes of copper(ii) with 7-azaindole ligand: Synthesis, characterization, magnetic properties, and biological activity. J. Inorg. Biochem. 2013, 127, 175–181. [Google Scholar] [CrossRef]
- Popescu, R.; Magyari, K.; Vulpoi, A.; Trandafir, D.; Licarete, E.; Todea, M.; Ştefan, R.; Voica, C.; Vodnar, D.; Simon, S. Bioactive and biocompatible copper containing glass-ceramics with remarkable antibacterial properties and high cell viability designed for future in vivo trials. Biomater. Sci. 2016, 4, 1252–1265. [Google Scholar] [CrossRef]
- Semisch, A.; Hartwig, A. Copper ions interfere with the reduction of the water-soluble tetrazolium salt-8. Chem. Res. Toxicol. 2014, 27, 169–171. [Google Scholar] [CrossRef]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Cronholm, P.; Hedberg, Y.; Tornberg, M.; De Battice, L.; Svedhem, S.; Wallinder, I.O. Cell membrane damage and protein interaction induced by copper containing nanoparticles—Importance of the metal release process. Toxicology 2013, 313, 59–69. [Google Scholar] [CrossRef]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdörster, G. Validation of an ldh assay for assessing nanoparticle toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is sbf in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kim, H.-M.; Himeno, T.; Kokubo, T.; Nakamura, T. Process and kinetics of bonelike apatite formation on sintered hydroxyapatite in a simulated body fluid. Biomaterials 2005, 26, 4366–4373. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Doostmohammadi, A. Bioactive glass nanopowder and bioglass coating for biocompatibility improvement of metallic implant. J. Mater. Process. Technol. 2009, 209, 1385–1391. [Google Scholar] [CrossRef]
- Clèries, L.; Fernández-Pradas, J.M.; Morenza, J.L. Behavior in simulated body fluid of calcium phosphate coatings obtained by laser ablation. Biomaterials 2000, 21, 1861–1865. [Google Scholar] [CrossRef]
- Stiegler, N.; Bellucci, D.; Bolelli, G.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A. High-velocity suspension flame sprayed (hvsfs) hydroxyapatite coatings for biomedical applications. J. Therm. Spray Technol. 2012, 21, 275–287. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Neo, M.; Kim, H.-M.; Kokubo, T.; Nakamura, T. A comparative study between in vivo bone ingrowth and in vitro apatite formation on Na2O–CaO–SiO2 glasses. Biomaterials 2003, 24, 1349–1356. [Google Scholar] [CrossRef]
- Xin, R.; Leng, Y.; Chen, J.; Zhang, Q. A comparative study of calcium phosphate formation on bioceramics in vitro and in vivo. Biomaterials 2005, 26, 6477–6486. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Relationship between in vitro apatite-forming ability measured using simulated body fluid and in vivo bioactivity of biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar] [CrossRef]
- Bohner, M.; Van Lenthe, G.; Grünenfelder, S.; Hirsiger, W.; Evison, R.; Müller, R. Synthesis and characterization of porous β-tricalcium phosphate blocks. Biomaterials 2005, 26, 6099–6105. [Google Scholar] [CrossRef]
- Gubler, M.; Brunner, T.; Zehnder, M.; Waltimo, T.; Sener, B.; Stark, W. Do bioactive glasses convey a disinfecting mechanism beyond a mere increase in PH? Int. Endod. J. 2008, 41, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Papastergiou, V.; Georgopoulos, S.D.; Karatapanis, S. Treatment of helicobacter pylori infection: Past, present and future. World J. Gastrointest. Pathophysiol. 2014, 5, 392–399. [Google Scholar] [CrossRef]
- Novick, R.P.; Roth, C. Plasmid-linked resistance to inorganic salts in staphylococcus aureus. J. Bacteriol. 1968, 95, 1335–1342. [Google Scholar] [PubMed]
- Hobman, J.L.; Crossman, L.C. Bacterial antimicrobial metal ion resistance. J. Med. Microbiol. 2015, 64, 471–497. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]



| Ceramic | Chemical Composition | Particle Size (µm) | Manufacturer |
|---|---|---|---|
| Hydroxyapatite | Ca10(PO4)6(OH)2 | 5.8 | CeramTec GmbH, Plochingen, Germany |
| β-Tricalcium phosphate | Ca3(PO4)2 | 3.8 | Chemische Fabrik, Budenheim, Germany |
| GB14 | Ca2KNa(PO4)2 | 17.4 | Bundesanstalt für Materialforschung und—Prüfung (BAM), Berlin, Germany |
| Bioglass | 47.3% SiO2, 28.6% CaO, 15.2% P2O5, 4.90% Na2O, 2.50% MgO 1.5% F | 8.0 | Centro Ricerche Colorabbia Consulting, Florence, Italy |
| Coating | Solids Content (wt.%) | Metal Content (Mass) Related on Solids Content (%) | Liquid Phase |
|---|---|---|---|
| HA + Cu | 10 | 1.75 | Iso/H2O |
| HA + Ag | 10 | 1.75 | Iso/H2O |
| HA + Bi | 10 | 1.75 | Iso |
| TCP + Cu | 10 | 1.75 | Iso/H2O |
| TCP + Ag | 10 | 1.75 | Iso/H2O |
| TCP + Bi | 10 | 1.75 | Iso |
| GB14 + Cu | 10 | 1.75 | H2O |
| GB14 + Ag | 10 | 1.75 | H2O |
| GB14 + Bi | 10 | 1.75 | H2O |
| Bioglass + Cu | 10 | 1.75 | Ethanol |
| Bioglass + Ag | 10 | 1.75 | Ethanol |
| Bioglass + Bi | 10 | 1.75 | Ethanol |
| Iso = Isopropanol, H2O = Water | |||
| Laser (nm) | 638, 532 | Number of Scans | 30–100 |
| Power (mW) | 2.5–12 | Aperture Time Per Scan (sec.) | 5 |
| Grid (gr/mm) | 1200 | Magnification (Microscope) | 10–50 |
| Cu(II) (from Cu(CH3COO)2) | 6.12 × 10−5 g | 3.37 × 10−7 mol |
| Ag (from AgNO3) | 7.06 × 10−5 g | 6.54 × 10−7 mol |
| Bi | 1.75 × 10−4 g | 8.40 × 10−7 mol |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burtscher, S.; Krieg, P.; Killinger, A.; Al-Ahmad, A.; Seidenstücker, M.; Latorre, S.H.; Bernstein, A. Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis. Materials 2019, 12, 3495. https://doi.org/10.3390/ma12213495
Burtscher S, Krieg P, Killinger A, Al-Ahmad A, Seidenstücker M, Latorre SH, Bernstein A. Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis. Materials. 2019; 12(21):3495. https://doi.org/10.3390/ma12213495
Chicago/Turabian StyleBurtscher, Sophie, Peter Krieg, Andreas Killinger, Ali Al-Ahmad, Michael Seidenstücker, Sergio Hernandez Latorre, and Anke Bernstein. 2019. "Thin Degradable Coatings for Optimization of Osteointegration Associated with Simultaneous Infection Prophylaxis" Materials 12, no. 21: 3495. https://doi.org/10.3390/ma12213495





