Fabrication of Hydrophobic Ni Surface by Chemical Etching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Characterization
3. Results and Discussion
3.1. Surface Wettability
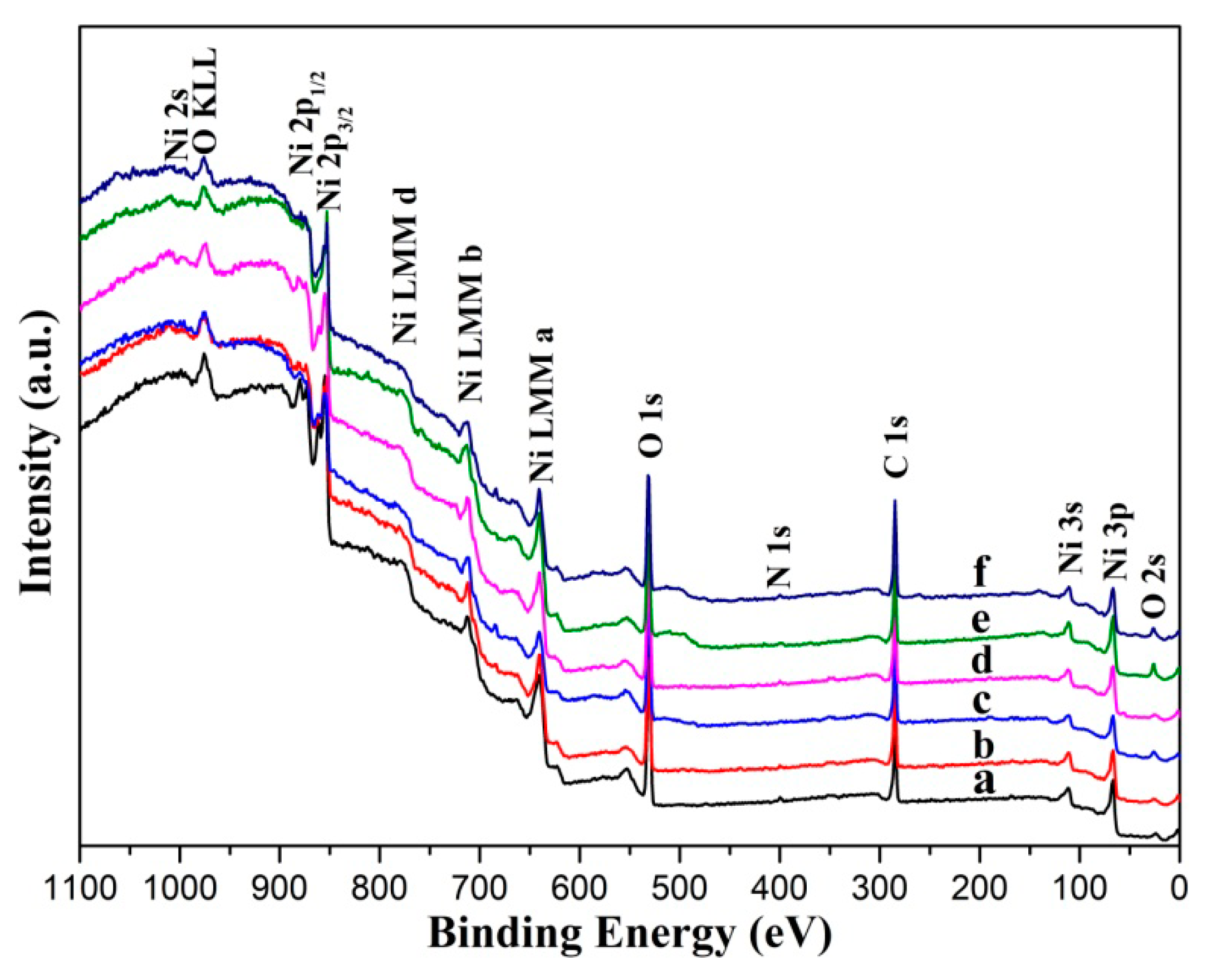
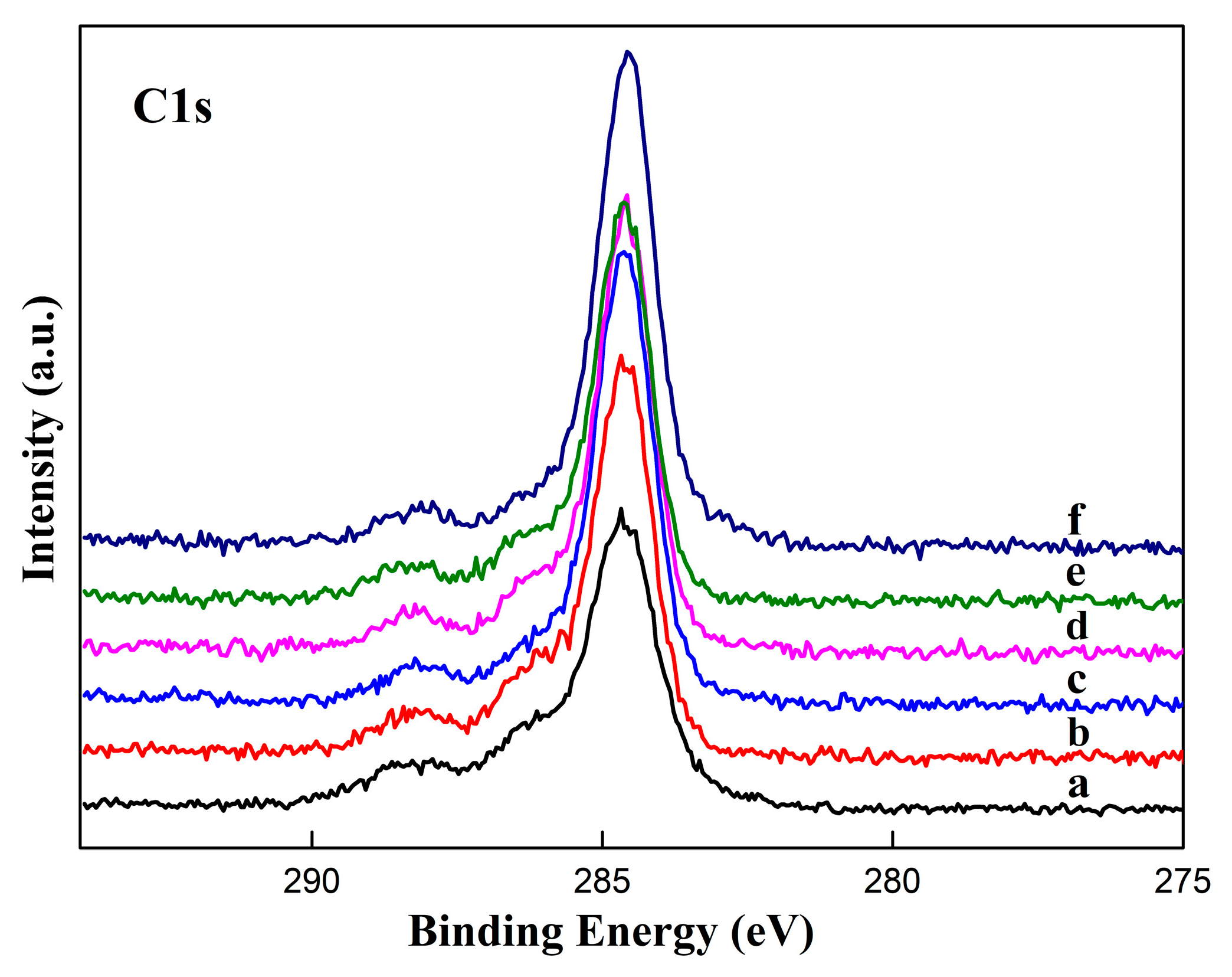
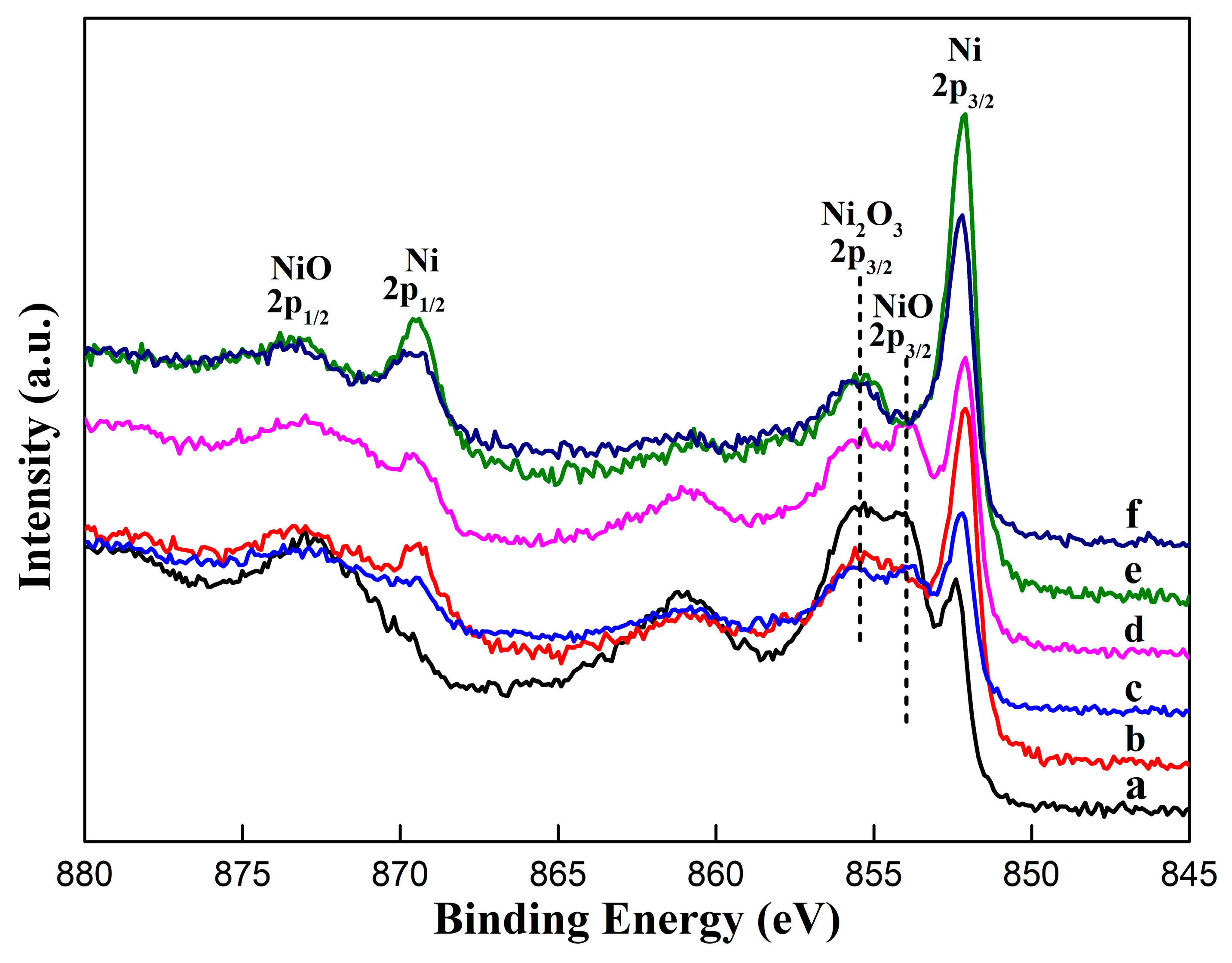
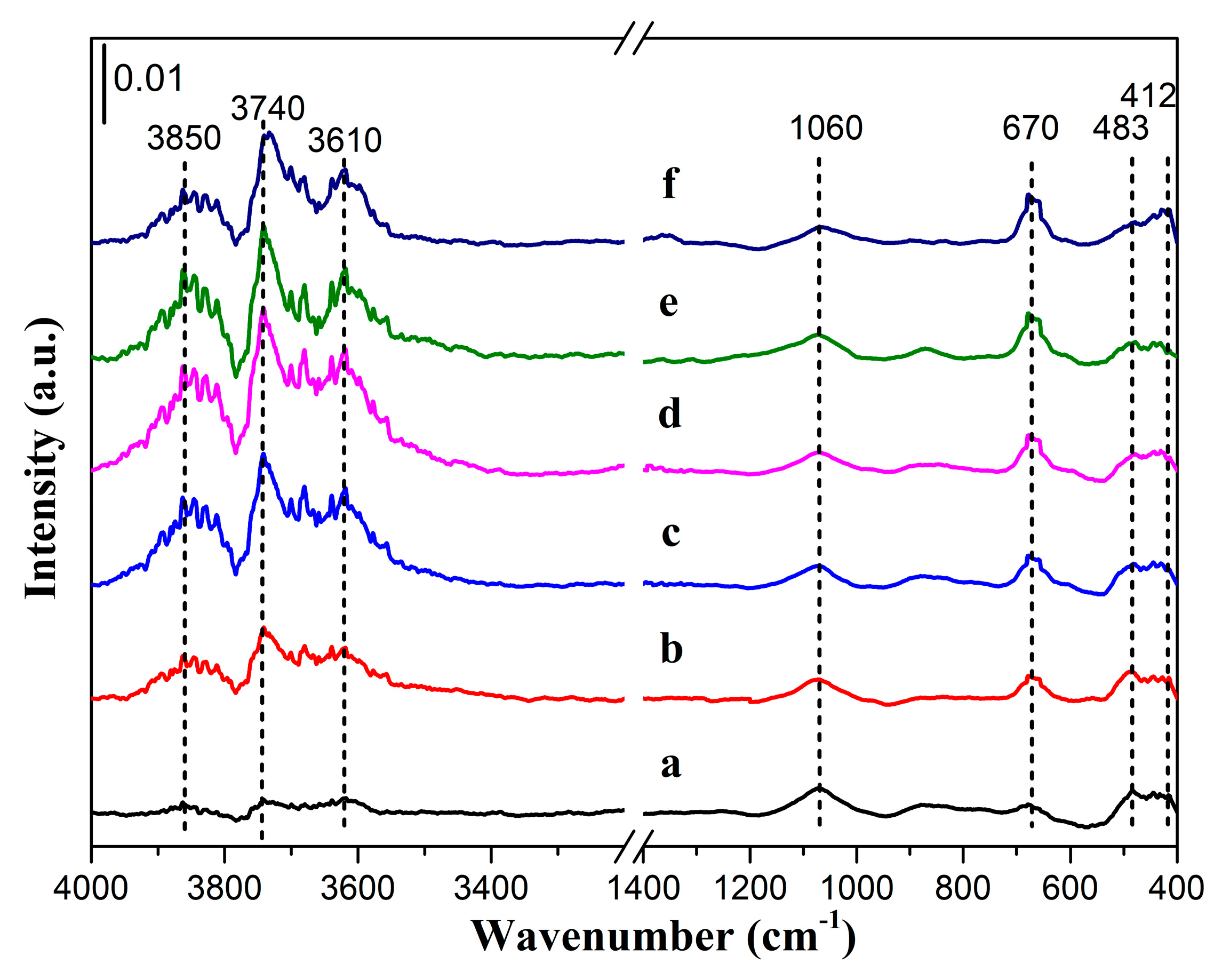
3.2. Surface Chemical Composition
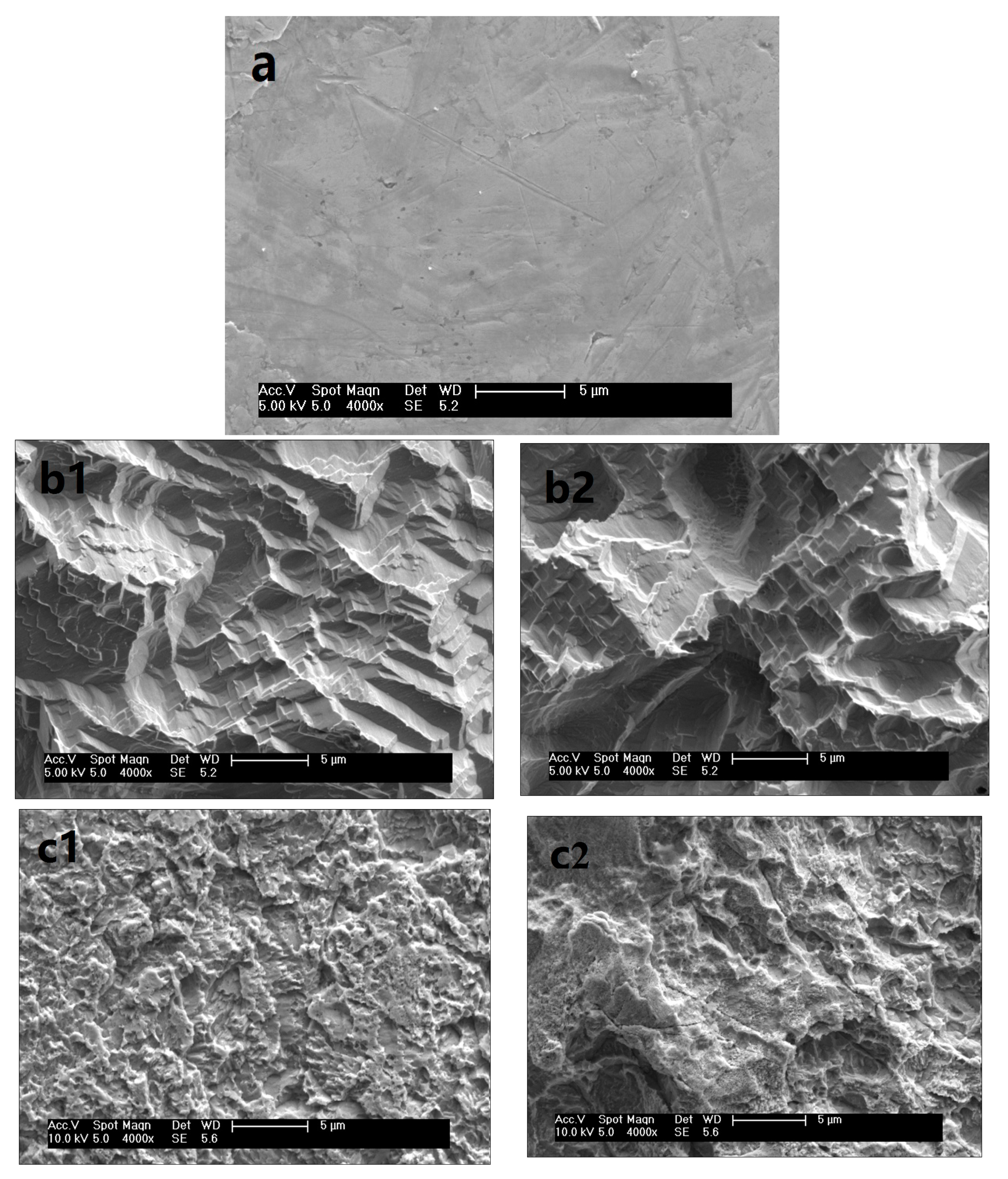
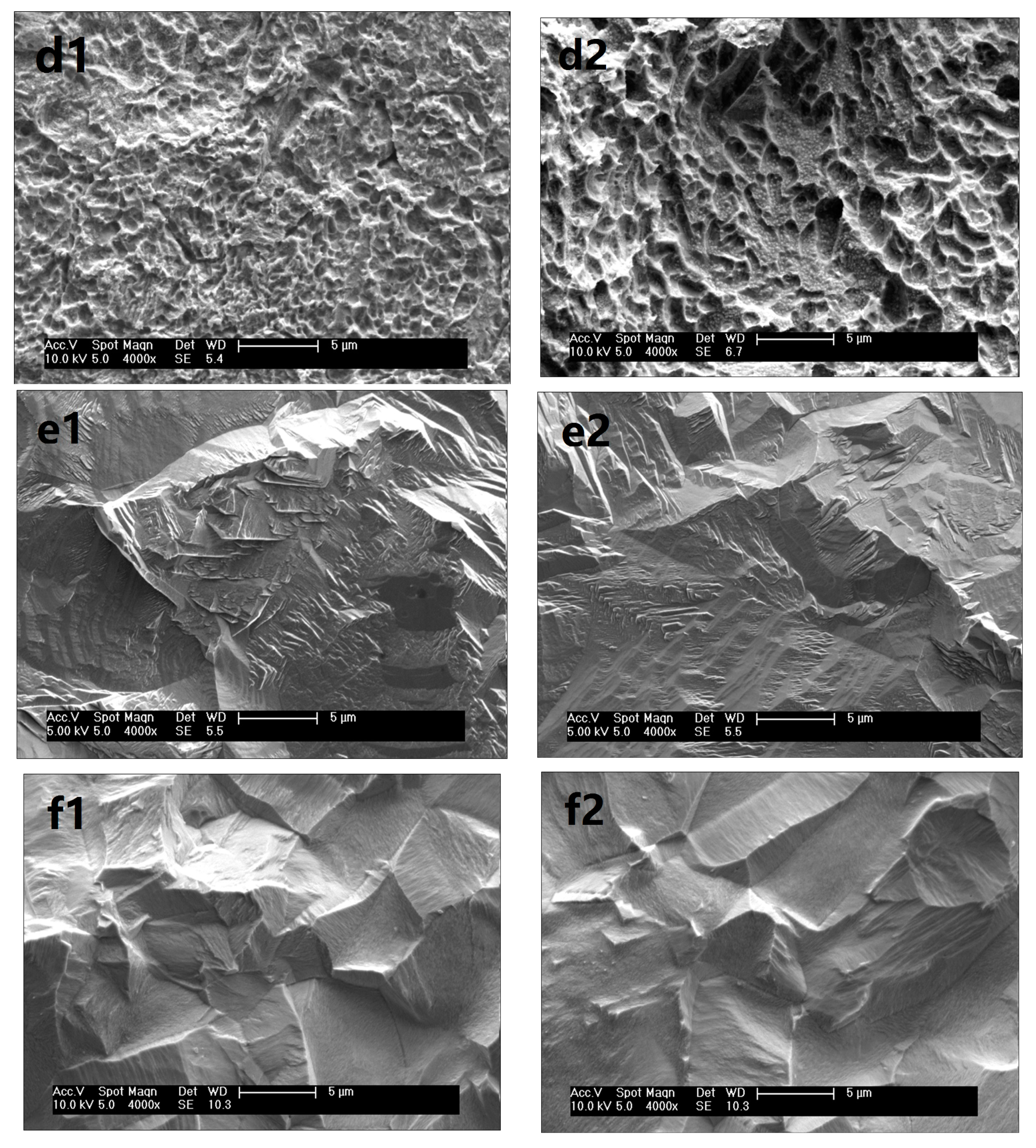
3.3. Surface Morphology
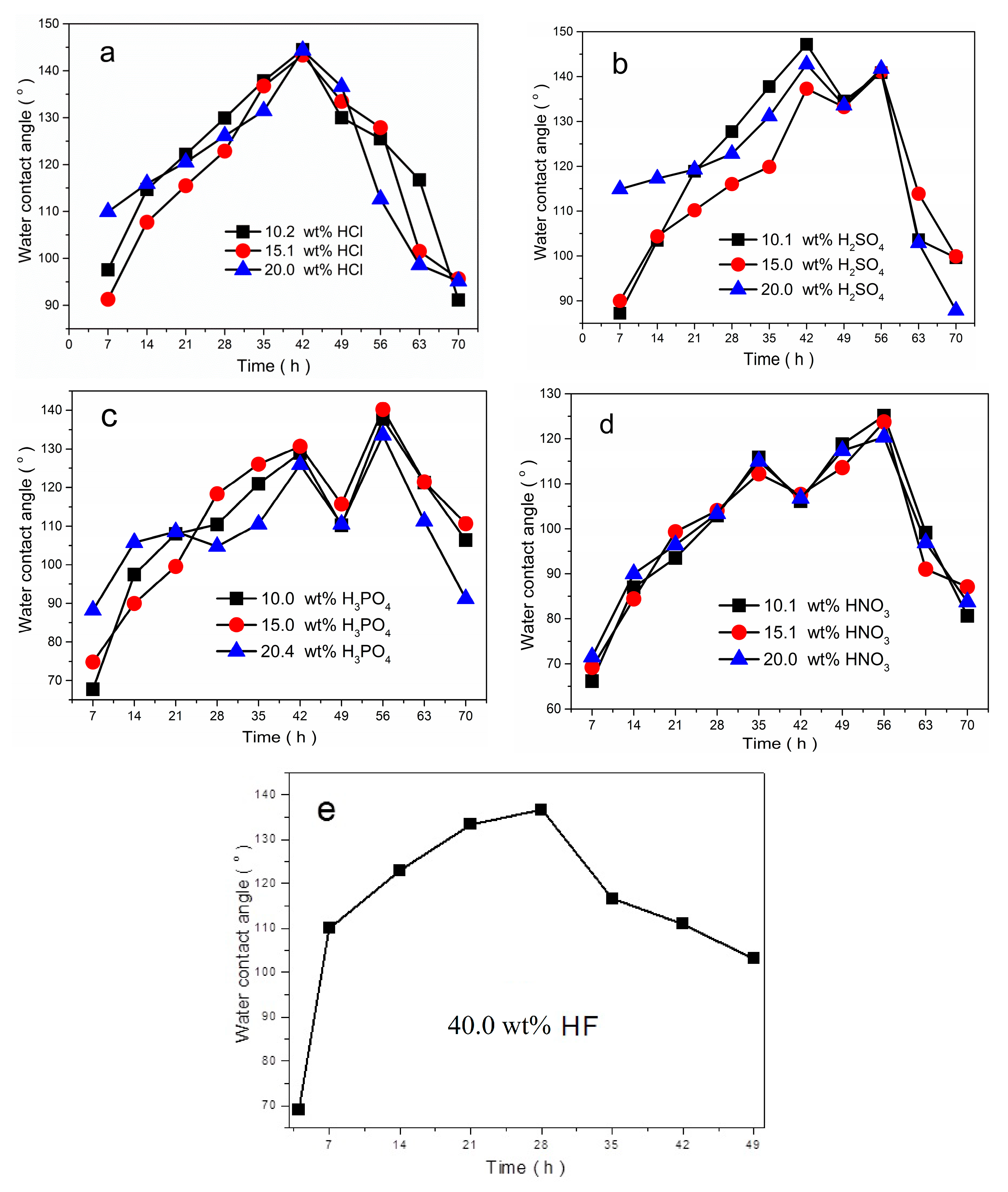
3.4. Effect of Etching Time and Etchants
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qian, B.T.; Shen, Z.Q. Fabrication of superhydrophobic surfaces by dislocation-selective chemical etching on aluminum, copper and zinc substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.L.; Mccarthy, T.J. The “lotus effect” explained: Two reasons why two length scales of topography are important. Langmuir 2006, 22, 2966–2967. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.L.; Coburn, C.E.; Dekoven, B.M.; Potter, G.E.; Meyers, G.F.; Fischer, D.A. Water-based non-stick hydrophobic coatings. Nature 1994, 368, 39–41. [Google Scholar] [CrossRef]
- Feng, L.; Li, S.H.; Li, Y.S.; Li, H.J.; Zhang, L.J.; Zhai, J.; Song, Y.L.; Liu, B.Q.; Jiang, L.; Zhu, D.B. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Guo, Z.G.; Liang, J.; Fang, J.; Guo, B.G.; Liu, W.M. A novel approach to the robust Ti6Al4V-based superhydrophobic surface with crater-like structure. Adv. Eng. Mater. 2007, 9, 316–321. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.M.; Ling, H.Q.; Li, M.; Mao, D.L. Super-hydrophobic nickel films with micro-nano hierarchical structure prepared by electrodeposition. Appl. Surf. Sci. 2010, 256, 2400–2404. [Google Scholar] [CrossRef]
- Yildirim, E.H.; Levent, D.A.; Yonca, A.; Olcay, M. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar]
- Chen, Z.J.; Guo, Y.B.; Fang, S.M. A facial approach to fabricate superhydrophobic aluminum surface. Surf. Interface Anal. 2010, 42, 1–6. [Google Scholar] [CrossRef]
- Zhai, L.; Cebeci, F.C.; Cohen, R.E.; Rubner, M.F. Stable superhydrophobic coatings from polyelectrolyte multilayers. Nano Lett. 2004, 4, 1349–1353. [Google Scholar] [CrossRef]
- Nishino, T.; Meguro, M.; Nakamae, K.; Matsushita, M.; Ueda, Y. The lowest surface free energy based on -CF3 alignment. Langmuir 1999, 15, 4321–4323. [Google Scholar] [CrossRef]
- Satyaprasad, A.; Jain, V.; Nema, S.K. Deposition of superhydrophobic nanostructured Teflon-like coating using expanding plasma arc. Appl. Surf. Sci. 2007, 253, 5462–5466. [Google Scholar] [CrossRef]
- Riekerink, M.B.O.; Terlingen, J.G.A.; Engbers, G.H.M.; Feijen, J. Selective etching of semicrystalline polymers: CF4 gas plasma treatment of poly(ethylene). Langmuir 1999, 15, 4847–4856. [Google Scholar] [CrossRef]
- Zheng, S.L.; Li, C.; Fu, Q.T.; Li, M.; Hu, W.; Wang, Q.; Du, M.P.; Liu, X.C.; Chen, Z. Fabrication of self-cleaning superhydrophobic surface on aluminum alloys with excellent corrosion resistance. Surf. Coat. Technol. 2015, 276, 341–348. [Google Scholar] [CrossRef]
- Zhang, F.Z.; Zhao, L.L.; Chen, H.Y.; Xu, S.L.; Evans, D.G.; Duan, X. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum. Angew. Chem. Int. Ed. 2008, 47, 2466–2469. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Feng, L.; Zhai, J.; Jiang, L.; Zhu, D.B. Reversible wettability of a chemical vapor deposition prepared ZnO film between superhydrophobicity and superhydrophilicity. Langmuir 2004, 20, 5659–5661. [Google Scholar] [CrossRef]
- Ishizaki, T.; Hieda, J.; Saito, N.; Saito, N.; Takai, O. Corrosion resistance and chemical stability of super-hydrophobic film deposited on magnesium alloy AZ31 by microwave plasma-enhanced chemical vapor deposition. Electrochim. Acta 2010, 55, 7094–7101. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- Gu, C.D.; Tu, J.P. One-step fabrication of nanostructured Ni film with lotus effect from deep eutectic solvent. Langmuir 2011, 27, 10132–10140. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, Q.; She, Z.X.; Chen, F.N.; Li, L.Q.; Zhang, X.X.; Zhang, P. Facile and fast fabrication of superhydrophobic surface on magnesium alloy. Appl. Surf. Sci. 2013, 271, 182–192. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Hu, J.; Tang, A.Q.; Wei, W.H.; He, J. Preparation and properties of super-hydrophobic coating on magnesium alloy. Appl. Surf. Sci. 2010, 257, 1666–1671. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.M.; Zhang, J.J.; Wang, Y.M.; Han, Z.W.; Ren, L.Q. Biomimetic hydrophobic surface fabricated by chemical etching method from hierarchically structured magnesium alloy substrate. Appl. Surf. Sci. 2013, 280, 845–849. [Google Scholar] [CrossRef]
- Guo, Z.G.; Zhou, F.; Hao, J.C.; Liu, W.M. Effects of system parameters on making aluminum alloy lotus. J. Colloid Interface Sci. 2006, 303, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.S.; Gu, Y.; Zhong, M.L.; Li, L.; Sezer, K.; Ma, M.X.; Liu, W.J. Fabrication of superhydrophobic Cu surfaces with tunable regular micro and random nano-scale structures by hybrid laser texture and chemical etching. J. Mater. Process. Technol. 2011, 211, 1234–1240. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, P.; Li, S.B.; Li, W.; Wu, Z.M.; Jiang, Y.-D. Black silicon with self-cleaning surface prepared by wetting processes. Nanoscale Res. Lett. 2013, 8, 351. [Google Scholar] [PubMed]
- Ahmad, N.A.; Leo, C.P.; Ahmad, A.L. Synthesis of superhydrophobic alumina membrane: Effects of sol-gel coating, steam impingement and water treatment. Appl. Surf. Sci. 2013, 284, 556–564. [Google Scholar] [CrossRef]
- Fan, Y.H.; Li, C.Z.; Chen, Z.; Chen, H. Study on fabrication of the superhydrophobic sol-gel films based on copper wafer and its anti-corrosive properties. Appl. Surf. Sci. 2012, 258, 6531–6536. [Google Scholar] [CrossRef]
- Kwon, M.H.; Shin, H.S.; Chu, C.N. Fabrication of a super-hydrophobic surface on metal using laser ablation and electrodeposition. Appl. Surf. Sci. 2014, 288, 222–228. [Google Scholar] [CrossRef]
- Liu, Y.; Yin, X.M.; Zhang, J.J.; Yu, S.R.; Han, Z.W.; Ren, L.Q. A electro-deposition process for fabrication of biomimetic super-hydrophobic surface and its corrosion resistance on magnesium alloy. Electrochim. Acta 2014, 125, 395–403. [Google Scholar] [CrossRef]
- Shirtcliffe, N.J.; Hale, G.M.; Newton, M.I.; Chabrol, G.; Perry, C.C. Dual-scale roughness produces unusually water-repellent surfaces. Adv. Mater. 2004, 16, 1929–1932. [Google Scholar] [CrossRef]
- Guo, Y.G.; Wang, Q.H.; Wang, T.M. Facile fabrication of superhydrophobic surface with micro/nanoscale binary structures on aluminum substrate. Appl. Surf. Sci. 2011, 257, 5831–5836. [Google Scholar] [CrossRef]
- Qi, Y.; Cui, Z.; Liang, B.; Parnas, R.S.; Lu, H.F. A fast method to fabricate superhydrophobic surfaces on zinc substrate with ion assisted chemical etching. Appl. Surf. Sci. 2014, 305, 716–724. [Google Scholar] [CrossRef]
- Ding, S.B.; Xiang, T.F.; Li, C.; Zheng, S.L.; Wang, J.; Zhang, M.X.; Dong, C.D.; Chan, W.M. Fabrication of self-cleaning super-hydrophobic nickel/graphene hybrid film with improved corrosion resistance on mild steel. Mater. Des. 2017, 117, 280–288. [Google Scholar] [CrossRef]
- Onda, T.; Shibuichi, S.; Satoh, N.; Tsujii, K. Super water repellent fractal surfaces. Langmuir 1996, 12, 2125–2127. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.P.; Tian, Y.L. Fabrication of super-hydrophobic nickel film on copper substrate with improved corrosion inhibition by electrodeposition process. Colloids Surf. A 2019, 560, 205–212. [Google Scholar] [CrossRef]
- Haslam, G.E.; Chin, X.Y.; Burstein, G.T. X-ray Photoelectron Spectroscopy of the Passive Surface of Nickel-Carbon Electrocatalysts after Polarisation in Sulfuric Acid. Electrochim. Acta 2014, 118, 157–162. [Google Scholar] [CrossRef]
- Wagner, C.D.; Riggs, W.M.; Davis, L.E.; Moulder, J.F.; Muilenberg, G.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1979. [Google Scholar]
- Mclntyre, N.S.; Cook, M.G. X-ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Yu, G.H.; Chai, C.L.; Zhu, F.W.; Xiao, J.M.; Lai, W.Y. XPS studies of chemical states of NiO/NiFe interface. J. Univ. Sci. Technol. B. 2001, 8, 270–273. [Google Scholar]
- Trigwell, S.; Hayden, R.D.; Nelson, K.F.; Selvaduray, G. Effects of Surface Treatment on the Surface Chemistry of NiTi Alloy for Biomedical Applications. Surf. Interface Anal. 1998, 26, 483–489. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Legrand, D.; Bancroft, G.M. Interpretation of Ni2p XPS spectra of Ni conductors and Ni insulators. Phys. Chem. Miner. 2000, 27, 357–366. [Google Scholar] [CrossRef]
- Seshadri, G.; Sarid, R.M.; Kelber, J.A. Combined Ultrahigh Vacuum–Electrochemistry Study of Aniline Oxidation at Oxidized Polycrystalline Nickel. Surf. Interface Anal. 1999, 27, 897–903. [Google Scholar] [CrossRef]
- Kashani-Motlagh, M.M.; Youzbashi, A.A.; Sabaghzadeh, L. Synthesis and characterization of nickel hydroxide/oxide nanoparticles by the complexation-precipitation method. Int. J. Phys. Sci. 2011, 6, 1471–1476. [Google Scholar]
- Wesam, A.A.T. Spectral analysis of electronic transitions for low doping concentration of nickel ions in silica matrices at different phases. J. Non-Cryst. Solids 2013, 382, 45–51. [Google Scholar]
- Huberty, J.S.; Madix, R.J. An FTIR study of the bonding of methoxy on Ni (100): Effects of coadsorbed sulfur, carbon monoxide and hydrogen. Surf. Sci. 1996, 360, 144–156. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Kno1zinger, H.; Mihaylov, M. FTIR Study of CO Adsorption on Ni-ZSM-5. J. Phys. Chem. B. 2002, 106, 2618–2624. [Google Scholar] [CrossRef]
- Bhargava, S.K.; Akolekar, D.B. Adsorption of NO and CO over transition-metal-incorporated mesoporous catalytic materials. J. Colloid Interface Sci. 2005, 281, 171–178. [Google Scholar] [CrossRef]
- Maache, M.; Janin, A.; Lavalley, J.C.; Benazzi, E. FT infrared study of Brønsted acidity of H-mordenites: Heterogeneity and effect of dealumination. Zeolites 1995, 15, 507–516. [Google Scholar] [CrossRef]
- Oyama, S.T.; Gott, T.; Asakura, K.; Takakusagi, S.; Miyazaki, K.; Koike, Y.; Bando, K.K. In situ FTIR and XANES studies of thiophene hydrodesulfurization on Ni2P/MCM-41. J. Catal. 2009, 268, 209–222. [Google Scholar] [CrossRef]
- Mihaylov, M.; Hadjiivanov, K.; Panayotov, D. FTIR mechanistic studies on the selective catalytic reduction of NOx with methane over Ni-containing zeolites: Comparison between NiY and Ni-ZSM-5. Appl. Catal. B 2004, 51, 33–42. [Google Scholar] [CrossRef]
- Tansel, B.; Boglaienko, D. Characterization of aggregation and declustering tendency of hydrophobic fine particles in water. Granul. Matter 2019, 21, 33–44. [Google Scholar] [CrossRef]
- Rafael, S.K.; Nicole, R.D. Surface modification to control the water wettability of electrospun mats. Int. Mater. Rev. 2019, 64, 249–287. [Google Scholar]
- Quéré, D. Wetting and roughness. Annu. Rev. Mater. Res. 2008, 38, 71–99. [Google Scholar] [CrossRef]
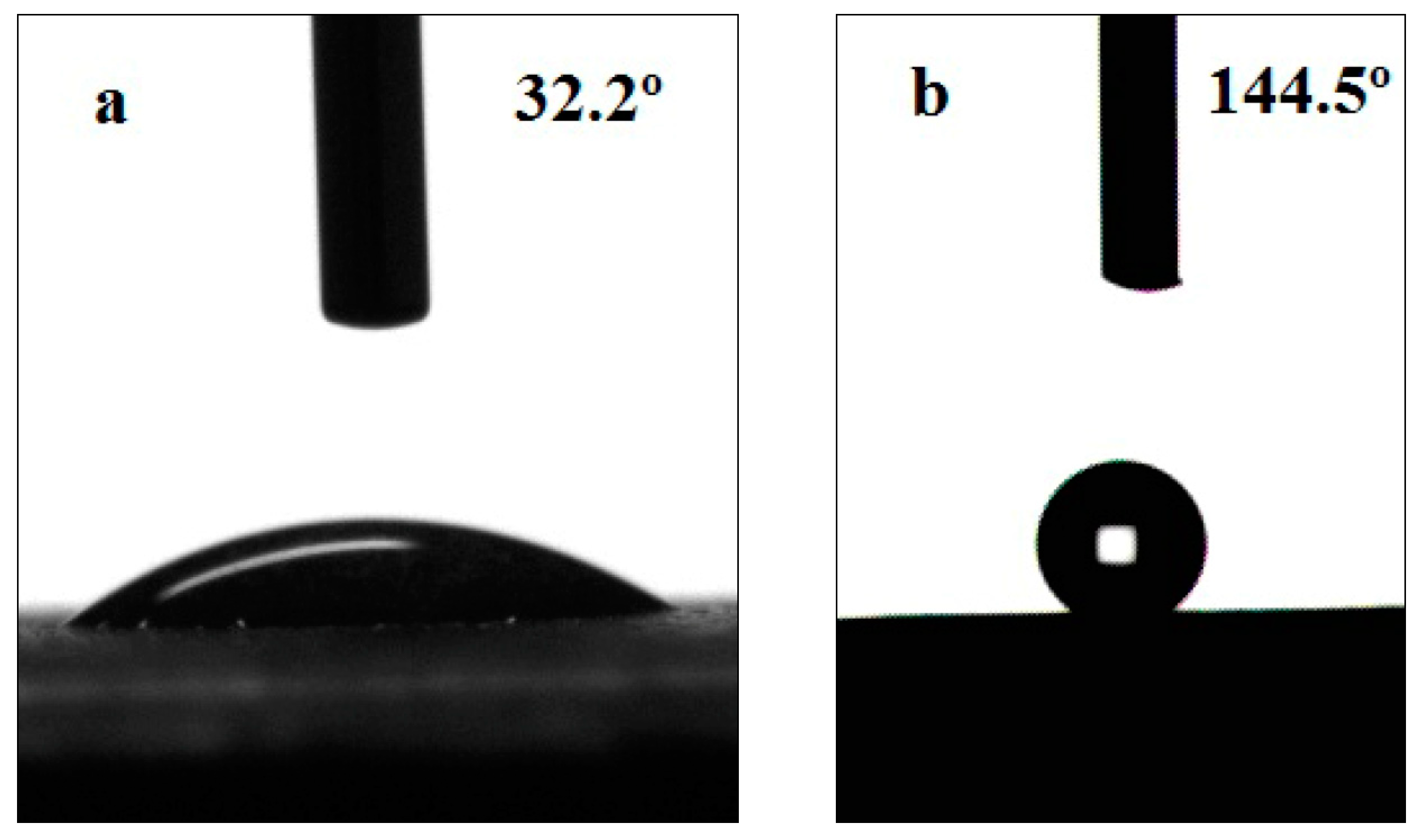
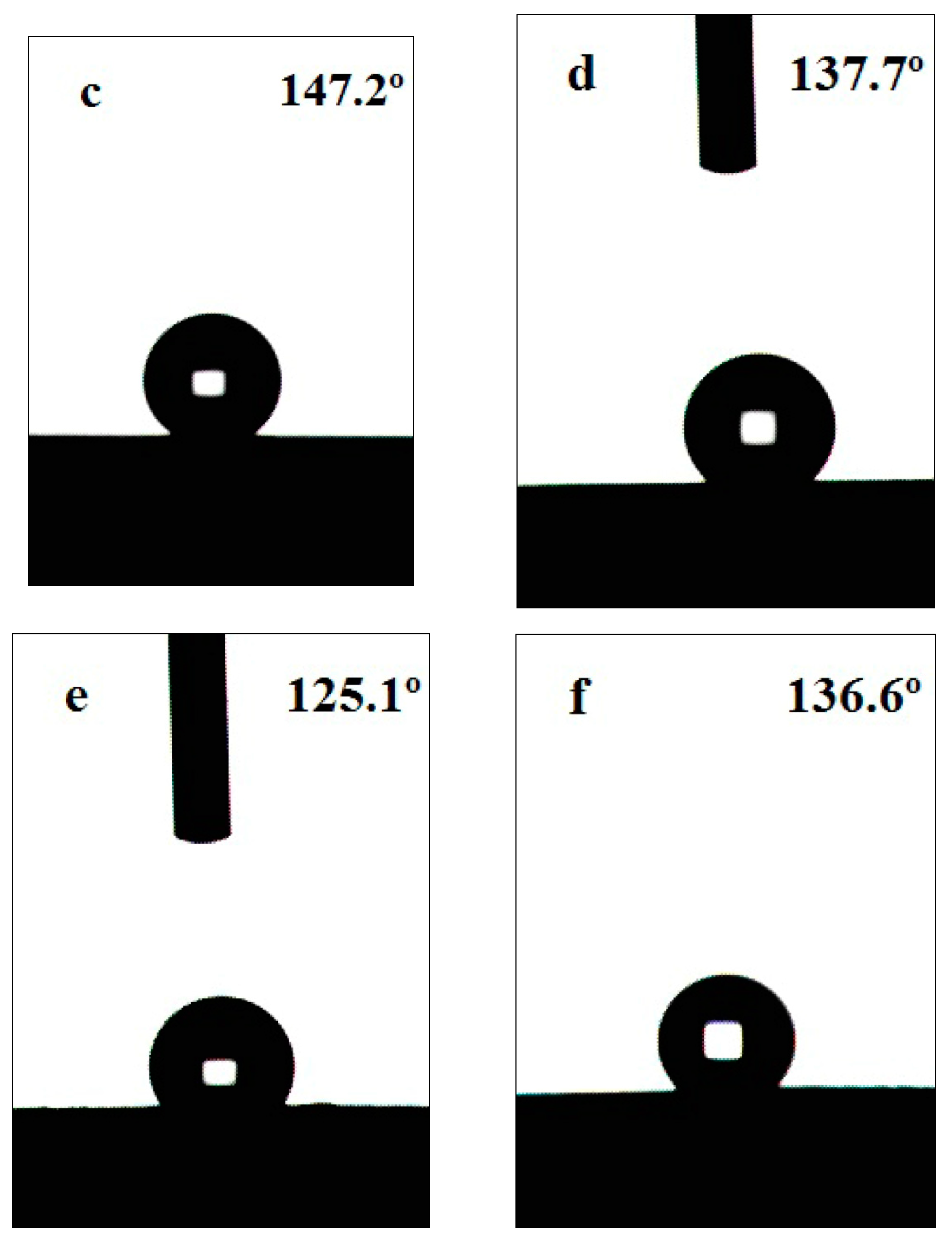

| Sample | SCA | Roughness (μm) | SCA | Roughness (μm) |
|---|---|---|---|---|
| untreated | 32.2° | 1.33 | ||
| 10.2 wt% HCl | 144.5° | 6.24 | 125.5° | 4.58 |
| 10.1 wt% H2SO4 | 147.2° | 6.59 | 135.5° | 5.70 |
| 10.0 wt% H3PO4 | 137.7° | 5.83 | 121.3° | 4.25 |
| 10.1 wt% HNO3 | 125.1° | 4.58 | 99.1° | 3.29 |
| 40.0 wt% HF | 136.6° | 5.75 | 116.6° | 3.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, X.; Tang, T.; Wang, H.; Chen, C.; Luo, J.; Luo, D. Fabrication of Hydrophobic Ni Surface by Chemical Etching. Materials 2019, 12, 3546. https://doi.org/10.3390/ma12213546
Qian X, Tang T, Wang H, Chen C, Luo J, Luo D. Fabrication of Hydrophobic Ni Surface by Chemical Etching. Materials. 2019; 12(21):3546. https://doi.org/10.3390/ma12213546
Chicago/Turabian StyleQian, Xiaojing, Tao Tang, Huan Wang, Changan Chen, Junhong Luo, and Deli Luo. 2019. "Fabrication of Hydrophobic Ni Surface by Chemical Etching" Materials 12, no. 21: 3546. https://doi.org/10.3390/ma12213546






