Importance of Cation Species during Sulfate Resistance Tests for Alkali-Activated FA/GGBFS Blended Mortars
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Mix Proportions
2.3. Test Methods
3. Results and Discussion
3.1. Change in Mass
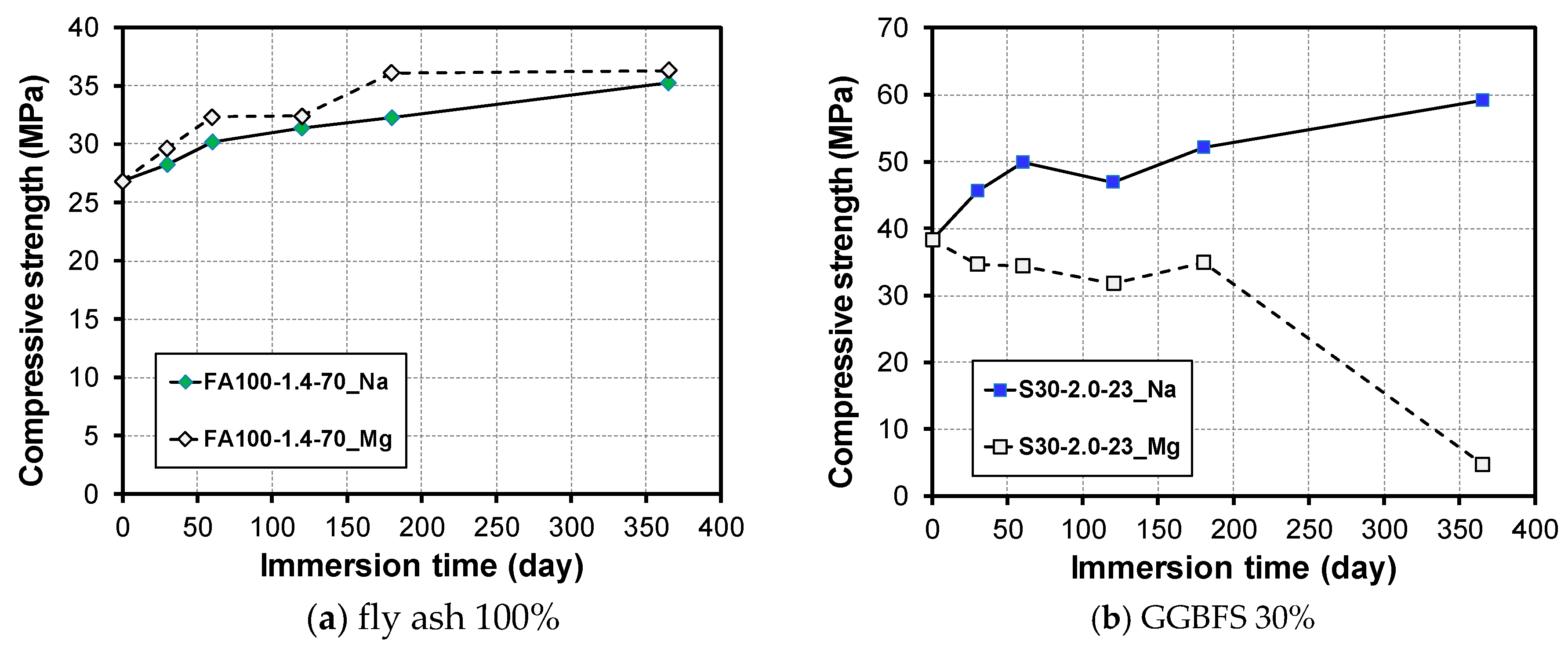
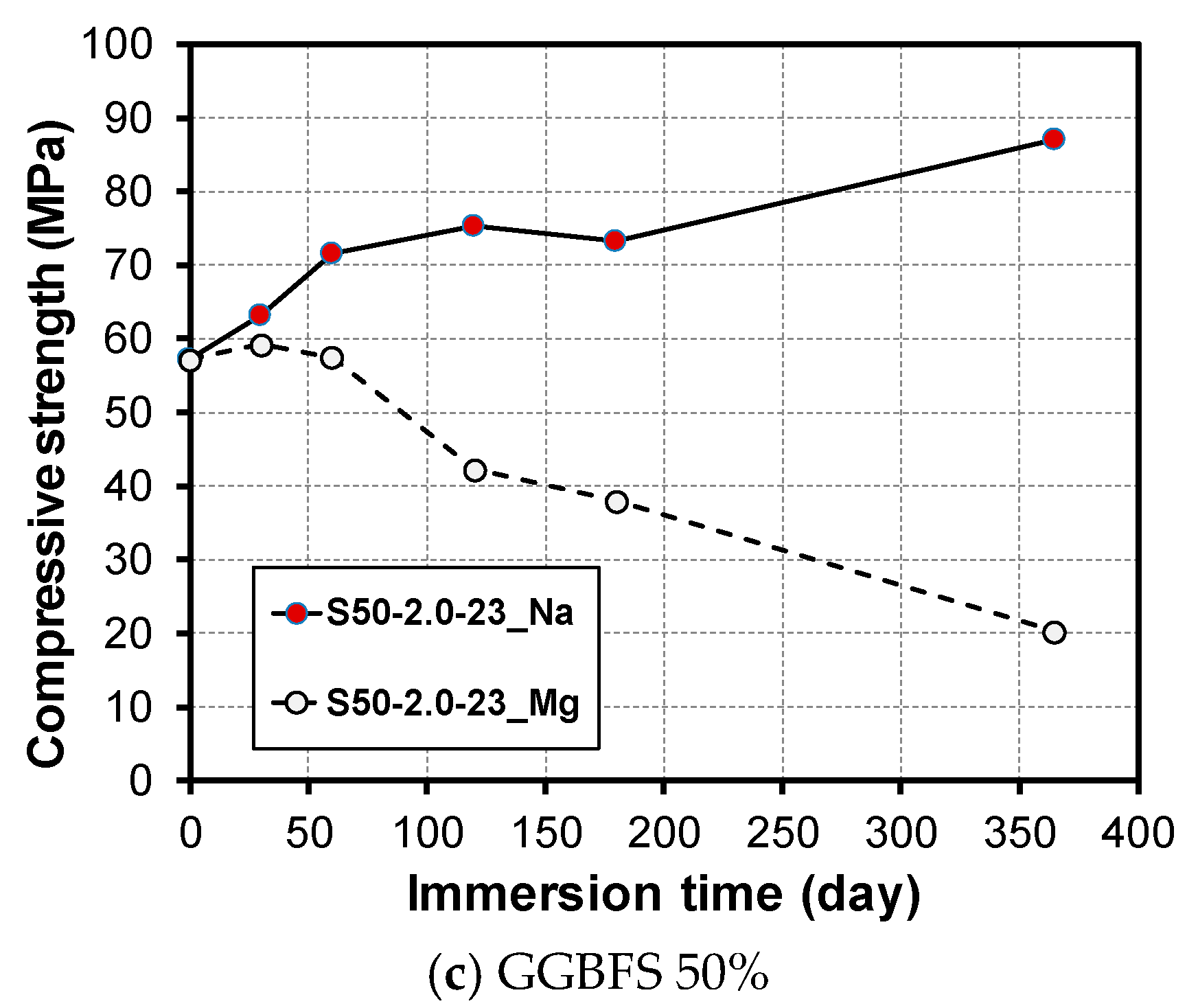
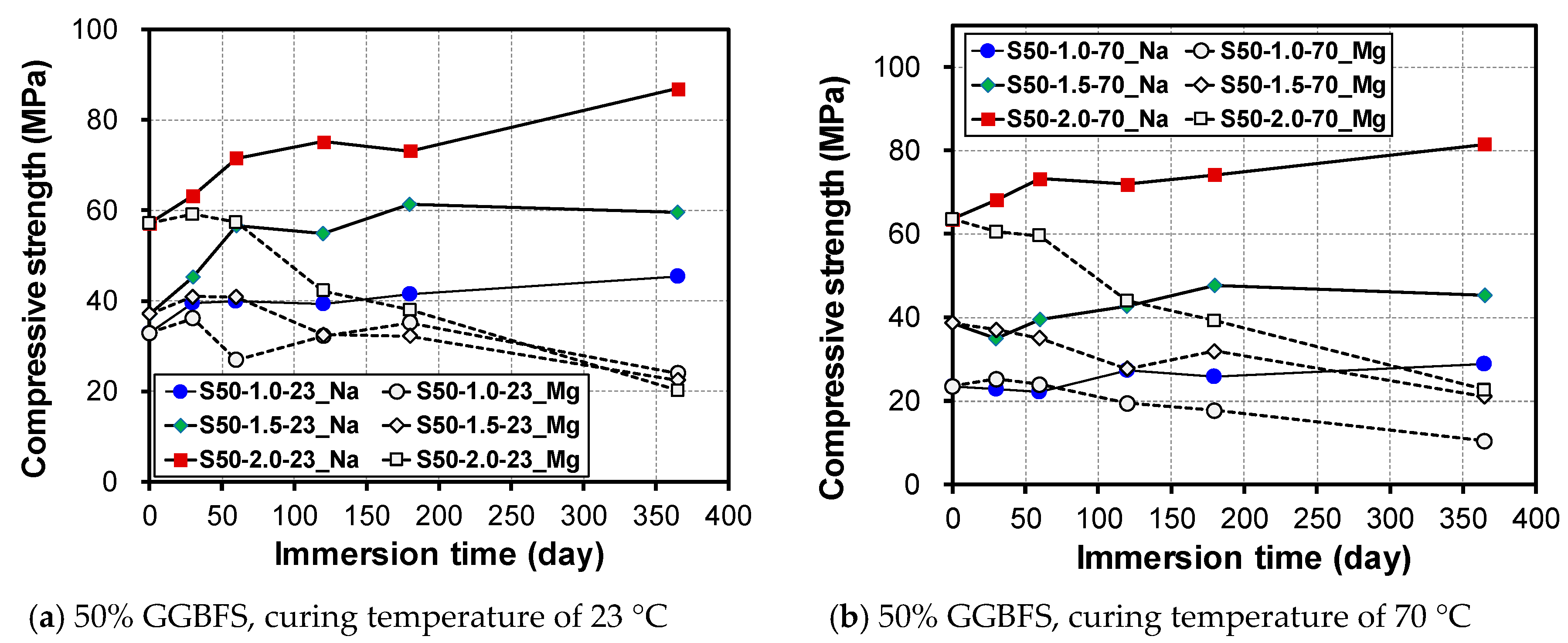
3.2. Change in Compressive Strength
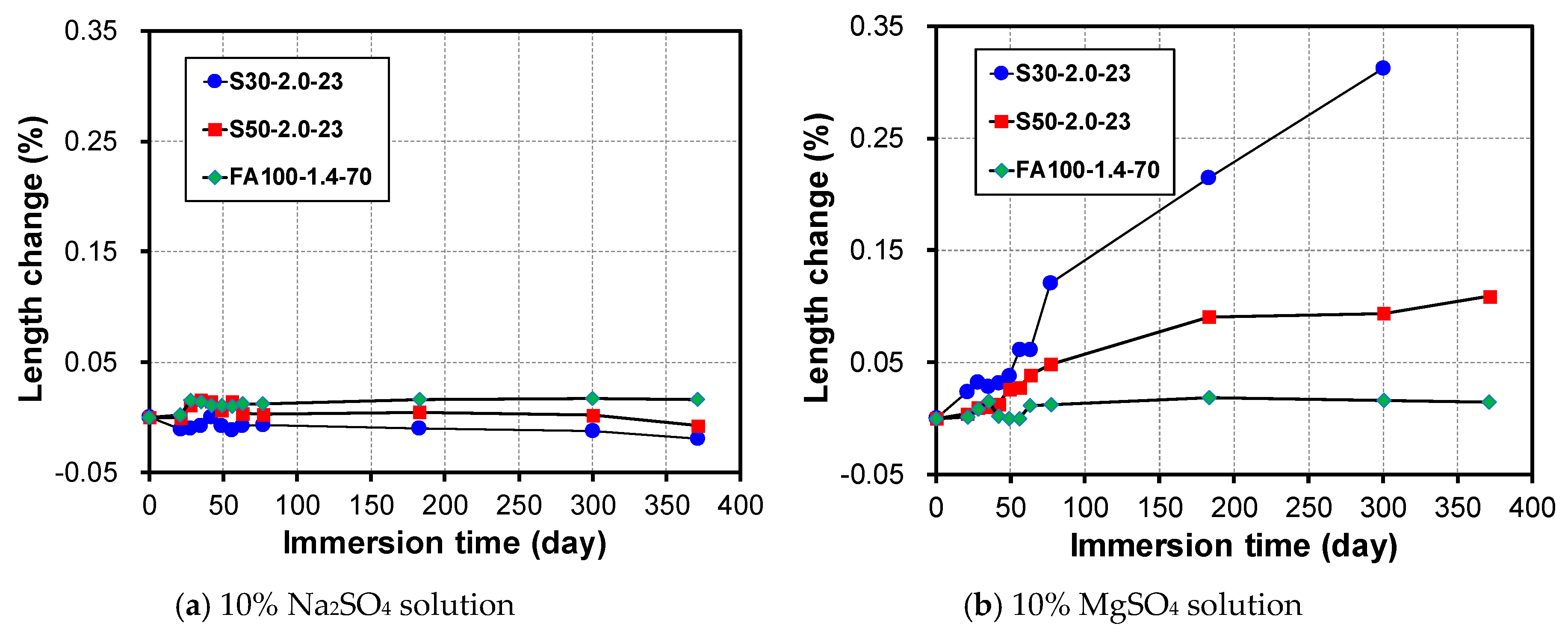
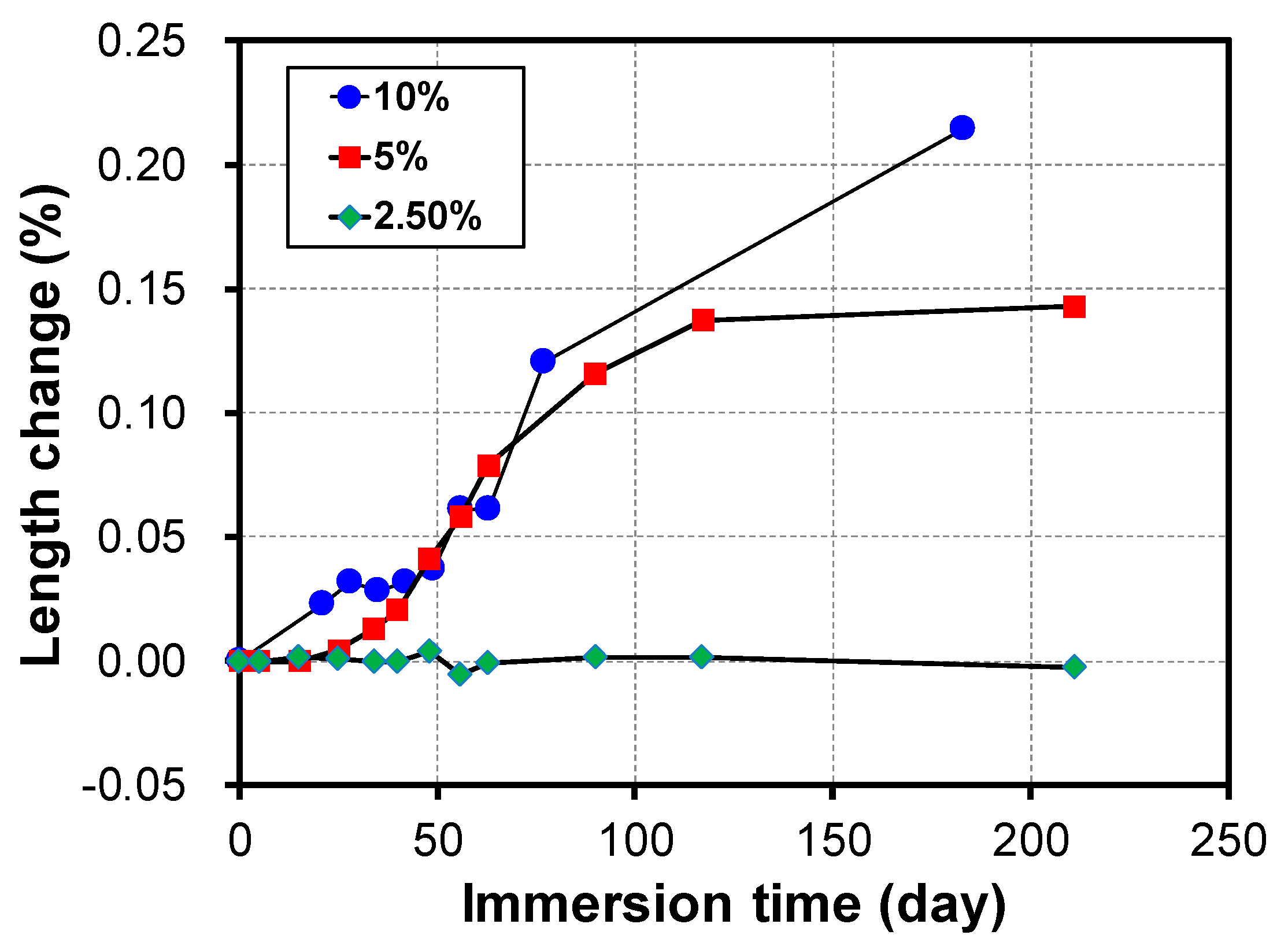
3.3. Change in Length
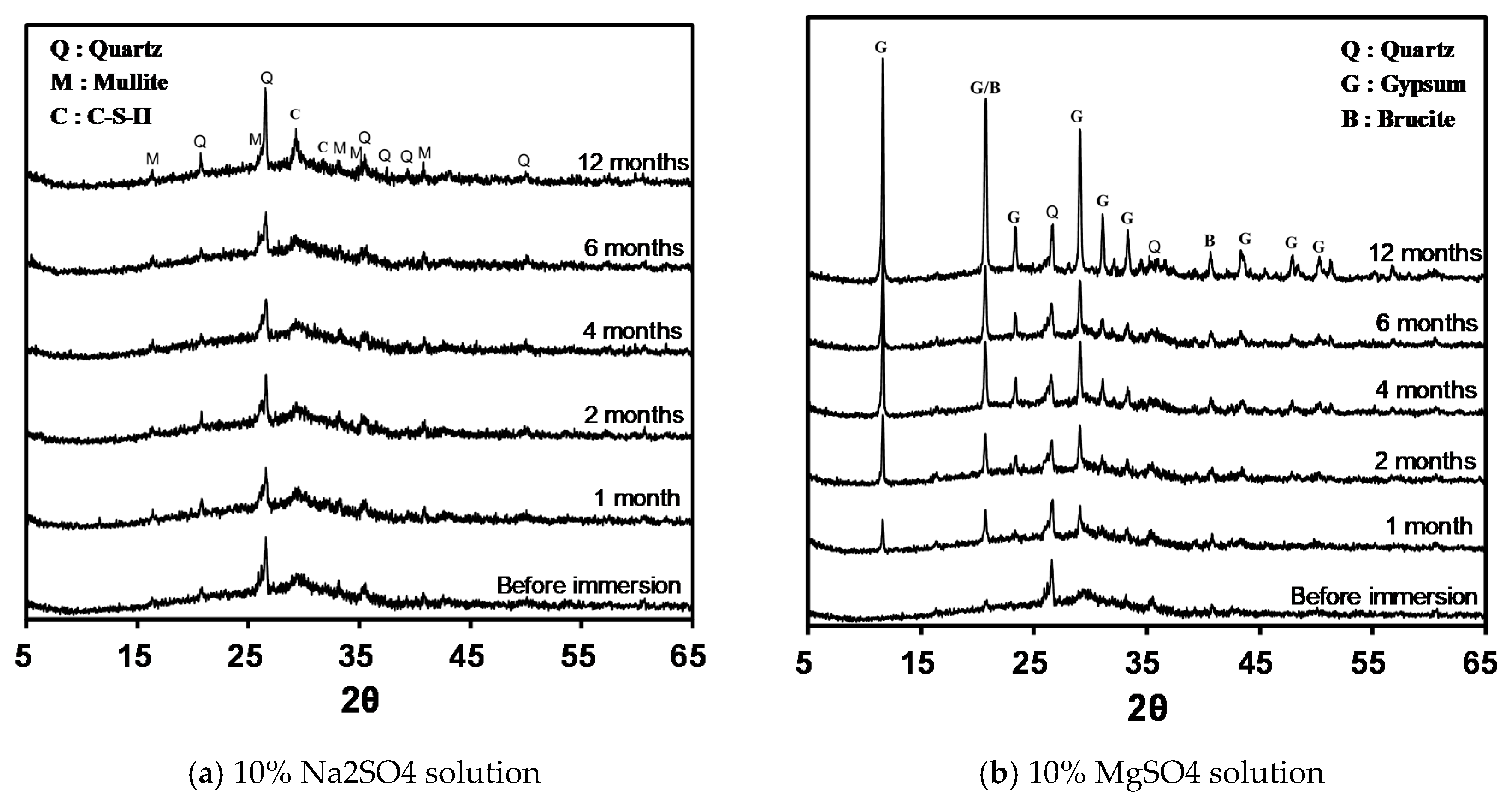
3.4. Changes in Mineral Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hemalatha, M.S.; Santhanam, M. Characterizing supplementary cementing materials in blended mortars. Constr. Build. Mater. 2018, 191, 440–459. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Gazzaniga, G.; Pastore, T. An empathetic added sustainability index (EASI) for cementitious based construction materials. J. Clean. Prod. 2019, 220, 475–482. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.; Moon, J. Effect of calcium carbonate fineness on calcium sulfoaluminate-belite cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Sharp, J.H.; Lawrence, C.D.; Yang, R. Calcium sulfoaluminate cements—Low-energy cements, special cements or what? Adv. Cem. Res. 1999, 11, 3–13. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–156. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Kang, H.; Chun, S.-C.; Moon, J. The effect of elevated curing temperatures on high ye’elimite calcium sulfoaluminate cement mortars. Materials 2019, 12, 1072. [Google Scholar] [CrossRef] [PubMed]
- Palomo, A.; Grutzeck, M.W.; Blanco, M.T. Alkali-activated fly ashes: A cement for the future. Cem. Concr. Res. 1999, 29, 1323–1329. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Roy, D.M. Alkali-activated cements Opportunities and challenges. Cem. Concr. Res. 1999, 29, 249–254. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L. Designing precursors for geopolymer cements. J. Am. Ceram. Soc. 2008, 91, 3864–3869. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.; Lukey, G.; Palomo, A.; Van Deventer, J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Saravanan, G.; Jeyasehar, C.; Kandasamy, S. Fly ash based geopolymer concrete—A state of the art review. J. Eng. Sci. Tech. Rev. 2013, 6, 25–32. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Provis, J.L. Discussion of C. Li et al., “A review: The comparison between alkali-activated slag (Si+Ca) and metakaolin (Si+Al) cements”. Cem. Concr. Res. 2010, 40, 1766–1767. [Google Scholar] [CrossRef]
- Yip, C.K.; Lukey, G.C.; van Deventer, J.S.J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Oh, J.E.; Monteiro, P.J.M.; Jun, S.S.; Choi, S.; Clark, S.M. The evolution of strength and crystalline phases for alkali-activated ground blast furnace slag and fly ash-based geopolymers. Cem. Concr. Res. 2010, 40, 189–196. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Monzó, M.; Vicent, M.; Barba, A.; Palomo, A. Alkaline activation of metakaolin-fly ash mixtures: Obtain of Zeoceramics and Zeocements. Micropor. Mesopor. Mat. 2008, 108, 41–49. [Google Scholar] [CrossRef]
- Shi, C.; Roy, D.; Krivenko, P. Alkali-Activated Cements and Concretes; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Jeong, Y.; Yum, W.S.; Jeon, D.; Oh, J.E. Strength development and microstructural characteristics of barium hydroxide-activated ground granulated blast furnace slag. Cem. Concr. Compos. 2017, 79, 34–44. [Google Scholar] [CrossRef]
- Jeong, Y.; Kang, S.-H.; Du, Y.; Moon, J. Local Ca-structure variation and microstructural characteristics on one-part activated slag system with various activators. Cem. Concr. Compos. 2019, 102, 1–13. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Coppola, L.; Coffetti, D.; Crotti, E.; Marini, A.; Passoni, C.; Pastore, T. Lightweight cement-free alkali-activated slag plaster for the structural retrofit and energy upgrading of poor quality masonry walls. Cem. Concr. Compos. 2019, 104, 103341. [Google Scholar] [CrossRef]
- Mohamed, O.A. A review of durability and strength characteristics of alkali-activated slag concrete. Materials 2019, 12, 1198. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, J.; Wang, J.; Wang, C.; Huang, P.; Fang, C. Sulfate resistance of recycled aggregate concrete with GGBS and fly ash-based geopolymer. Materials 2019, 12, 1247. [Google Scholar] [CrossRef]
- Arbi, K.; Nedeljković, M.; Zuo, Y.; Ye, G. A review on the durability of alkali-activated fly ash/slag systems: Advances, issues, and perspectives. Ind. Eng. Chem. Res. 2016, 55, 5439–5453. [Google Scholar] [CrossRef]
- Menashi, D.C.; Bryant, M. Sulfate attack on concrete: Research needs. ACI Mater. J. 1981, 88, 62–69. [Google Scholar]
- Mehta, P.K. Mechanism of sulfate attack on portland cement concrete—Another look. Cem. Concr. Res. 1983, 13, 401–406. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials; McGraw-Hill: New York, NY, USA, 2006; Volume 3. [Google Scholar]
- Rasheeduzzafar; Al-Amoudi, O.S.B.; Abduljauwad, S.N.; Maslehuddin, M. Magnesium-sodium sulfate attack in plain and blended cements. J. Mater. Civ. Eng. 1994, 6, 201–222. [Google Scholar] [CrossRef]
- Komljenović, M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. External sulfate attack on alkali-activated slag. Constr. Build. Mater. 2013, 49, 31–39. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Sulfate attack on alkali-activated slag concrete. Cem. Concr. Res. 2002, 32, 211–216. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Bhutta, M.A.R.; Hussin, W.M.; Azreen, M.; Tahir, M.M. Sulphate resistance of geopolymer concrete prepared from blended waste fuel ash. J. Mater. Civ. Eng. 2014, 26, 04014080. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; van Deventer, J.S.J. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Baščarević, Z.; Komljenović, M.; Miladinović, Z.; Nikolić, V.; Marjanović, N.; Petrović, R. Impact of sodium sulfate solution on mechanical properties and structure of fly ash based geopolymers. Mater. Struct. 2015, 48, 683–697. [Google Scholar] [CrossRef]
- Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete; ASTM C 618; American Society for Testing and Materials: West Conshohocken, PA, USA, 2019.
- Jeon, D.; Jun, Y.; Jeong, Y.; Oh, J.E. Microstructural and strength improvements through the use of Na2CO3 in a cementless Ca(OH)2-activated Class F fly ash system. Cem. Concr. Res. 2015, 67, 215–225. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.-H.; Oh, J.E. Microstructural verification of the strength performance of ternary blended cement systems with high volumes of fly ash and GGBFS. Constr. Build. Mater. 2015, 95, 96–107. [Google Scholar] [CrossRef]
- Oh, J.E.; Jun, Y.; Jeong, Y.; Monteiro, P.J.M. The importance of the network-modifying element content in fly ash as a simple measure to predict its strength potential for alkali-activation. Cem. Concr. Compos. 2015, 57, 44–54. [Google Scholar] [CrossRef]
- Jeong, Y.; Oh, J.E.; Jun, Y.; Park, J.; Ha, J.-H.; Sohn, S.G. Influence of four additional activators on hydrated-lime [Ca(OH)2] activated ground granulated blast-furnace slag. Cem. Concr. Compos. 2016, 65, 1–10. [Google Scholar] [CrossRef]
- Jeong, Y.; Park, H.; Jun, Y.; Jeong, J.H.; Oh, J.E. Influence of slag characteristics on strength development and reaction products in a CaO-activated slag system. Cem. Concr. Compos. 2016, 72, 155–167. [Google Scholar] [CrossRef]
- Cement-Test Methods—Determination of Strength; ISO 679; International Organization for Standardization: Geneva, Switzerland, 2009.
- Choi, S.; Lee, K.-M. Influence of Na2O content and Ms (SiO2/Na2O) of alkaline activator on workability and setting of alkali-activated slag paste. Materials 2019, 12, 2072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Scherer, G.W. Comparison of methods for arresting hydration of cement. Cem. Concr. Res. 2011, 41, 1024–1036. [Google Scholar] [CrossRef]
- Standard Test Method for Length Change of Mortar and Concrete; KS F 2424; Korea Standards Association: Seoul, Korea, 2015.
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cem. Concr. Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Criado, M.; Fernández-Jiménez, A.; de la Torre, A.G.; Aranda, M.A.G.; Palomo, A. An XRD study of the effect of the SiO2/Na2O ratio on the alkali activation of fly ash. Cem. Concr. Res. 2007, 37, 671–679. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Design 2014, 62, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Venkatanarayanan, H.K.; Rangaraju, P.R. Evaluation of sulfate resistance of Portland cement mortars containing low-carbon rice husk ash. J. Mater. Civil. Eng. 2014, 26, 582–592. [Google Scholar] [CrossRef]
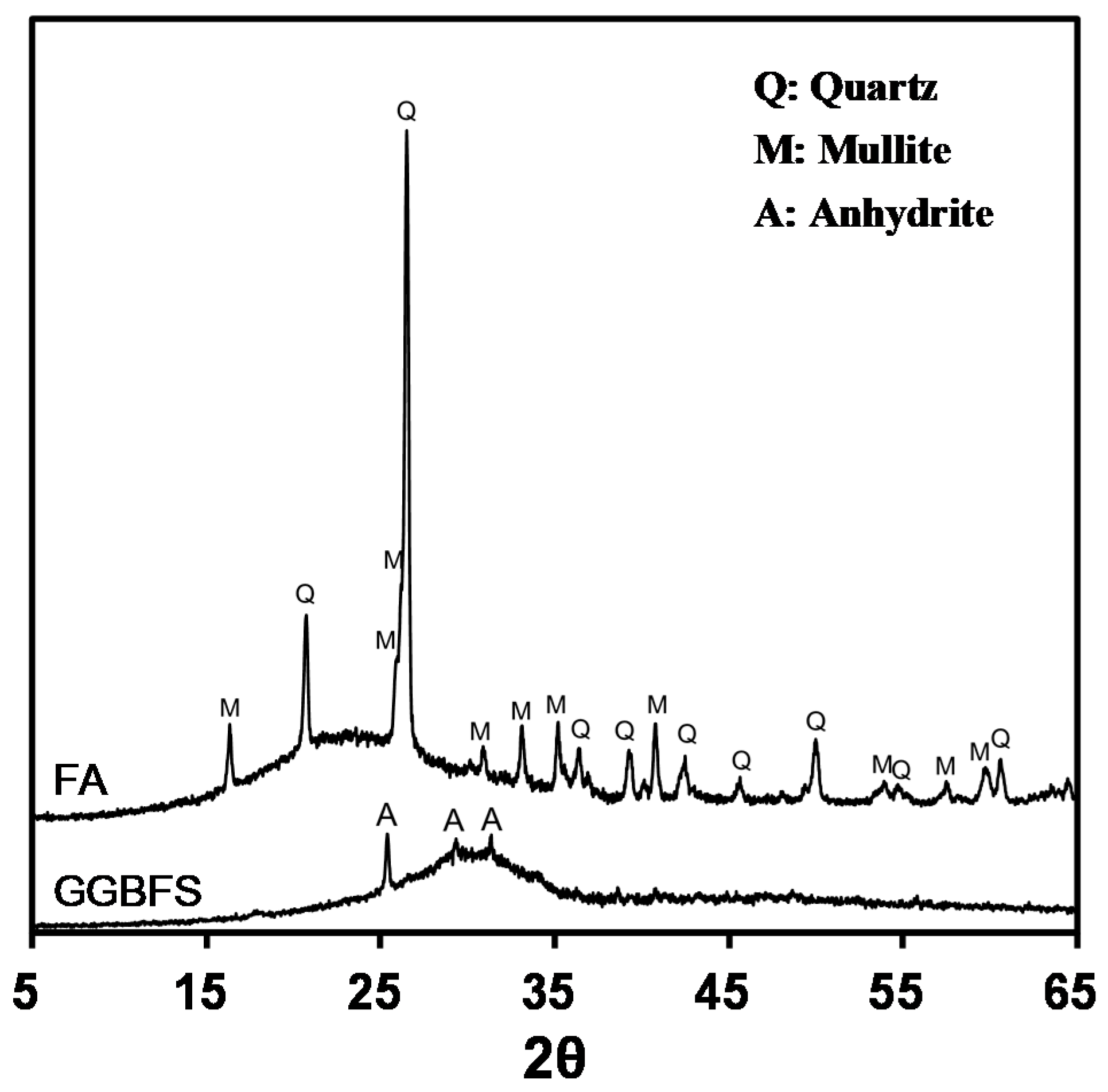



| Chemical Compositions (mass %) | Physical Properties | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | SO3 | LOI | Density (kg/m3) | Blaine (m2/kg) | |
| FA | 58.9 | 20.9 | 5.30 | 3.80 | 1.31 | 0.74 | 1.69 | 0.50 | 4.87 | 2210 | 381 |
| GGBFS | 34.3 | 14.2 | 0.47 | 43.0 | 2.71 | 0.50 | 0.20 | 3.64 | 0.01 | 2890 | 428 |
| Mix | Factors | Mass Proportions (g) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FA (%) | GGBFS (%) | Na2O (%) | Ms | FA | GGBFS | NaOH | Sodium Silicate Solution | Water | Sand | |
| FA100-1.4 | 100 | - | 8 | 1.4 | 450 | - | 25.4 | 175.8 | 114.4 | 1350 |
| S30-2.0 | 70 | 30 | 4 | 2.0 | 315 | 135 | 8.2 | 125.6 | 146.0 | 1350 |
| S50-1.0 | 50 | 50 | 4 | 1.0 | 225 | 225 | 15.7 | 62.8 | 185.5 | 1350 |
| S50-1.5 | 50 | 50 | 4 | 1.5 | 225 | 225 | 11.9 | 94.2 | 165.8 | 1350 |
| S50-2.0 | 50 | 50 | 4 | 2.0 | 225 | 225 | 8.2 | 125.6 | 146.0 | 1350 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.; Kim, J.H.; Jung, S.; Chung, Y.; Jeong, Y. Importance of Cation Species during Sulfate Resistance Tests for Alkali-Activated FA/GGBFS Blended Mortars. Materials 2019, 12, 3547. https://doi.org/10.3390/ma12213547
Cho Y, Kim JH, Jung S, Chung Y, Jeong Y. Importance of Cation Species during Sulfate Resistance Tests for Alkali-Activated FA/GGBFS Blended Mortars. Materials. 2019; 12(21):3547. https://doi.org/10.3390/ma12213547
Chicago/Turabian StyleCho, Youngkeun, Joo Hyung Kim, Sanghwa Jung, Yoonseok Chung, and Yeonung Jeong. 2019. "Importance of Cation Species during Sulfate Resistance Tests for Alkali-Activated FA/GGBFS Blended Mortars" Materials 12, no. 21: 3547. https://doi.org/10.3390/ma12213547





