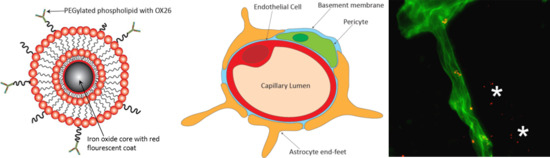
Evaluation of Targeted Delivery to the Brain Using Magnetic Immunoliposomes and Magnetic Force
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production, Purification, and Specificity Control of Anti-Transferrin Receptor (OX26) Monoclonal Antibodies
2.2. OX26-MAbs’ Binding to Rat Brain Endothelial Cells
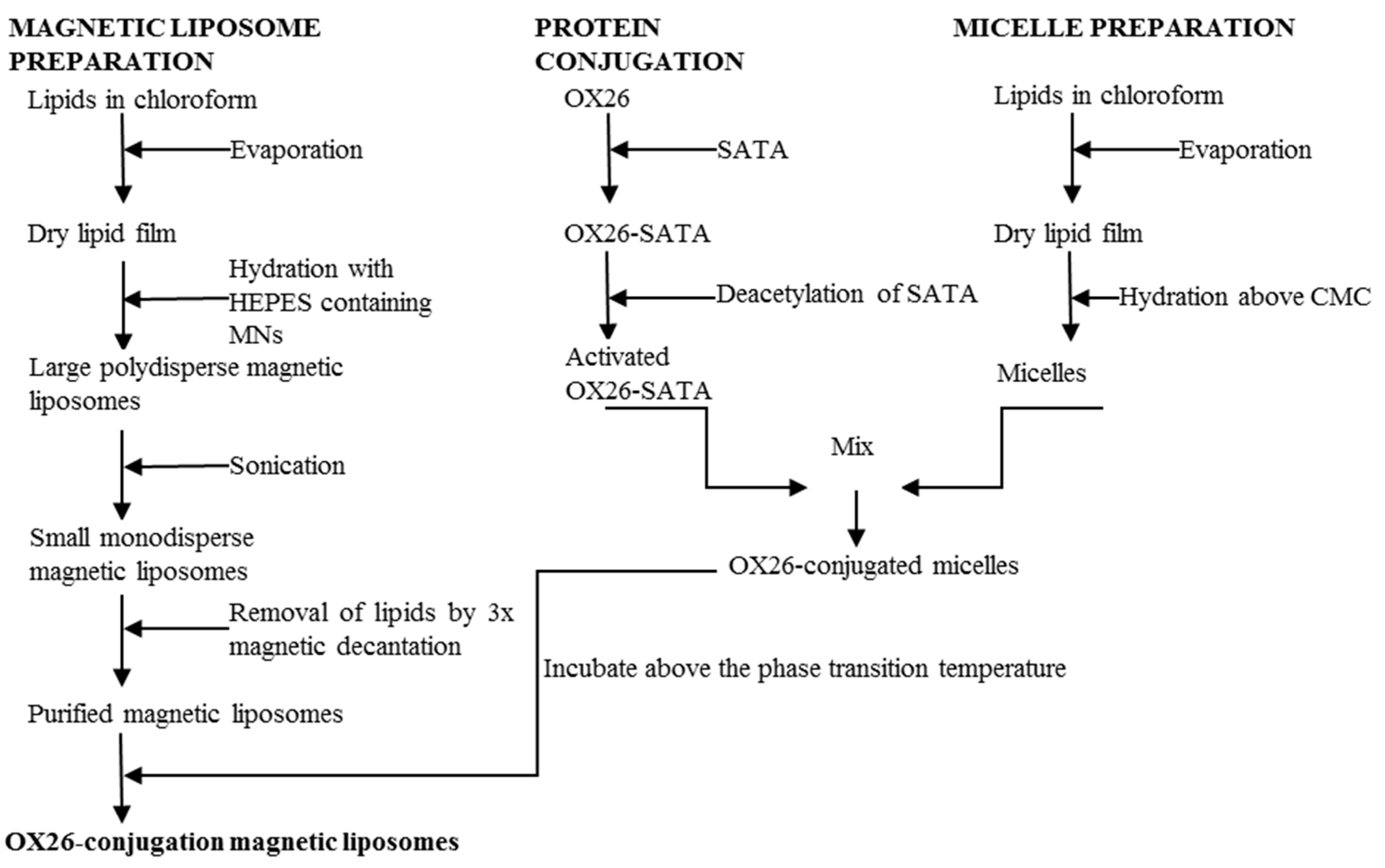
2.3. Preparation of Magnetic Liposomes
2.4. Synthesis of OX26-MAb-Conjugated Magnetic Immunotoliposomes Using the SATA Method
2.5. Determination of the Particle Concentration after Synthesis
2.6. Determination of the Size and Zeta-Potential, and Analysis with Transmission Electron Microscopy
2.7. Determination of the Antibody Concentration on the Nanoparticles
2.8. Binding and Uptake Magnetic Nanoparticles, Magnetic Liposomes, and OX26-Magnetic Immunoliposomes
2.9. Cell Viability
2.10. In Situ Brain Perfusion
2.11. Immunohistochemistry
2.12. Fluorescence and Confocal Microscopy
3. Results
3.1. Syntheses and Specificities of OX26-MABs
3.2. Particle Characterization
3.3. Cellular Uptake of Nanoparticles In Vitro
3.4. In Situ Brain Perfusion
4. Discussion
4.1. Methodological Considerations
4.2. Uptake and Transport of OX26-Magnetic Immunoliposomes at the BBB
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Caraglia, M.; Luongo, L.; Salzano, G.; Zappavigna, S.; Marra, M.; Guida, F.; Lusa, S.; Giordano, C.; De Novellis, V.; Rossi, F.; et al. Stealth liposomes encapsulating zoledronic acid: A new opportunity to treat neuropathic pain. Mol. Pharm. 2013, 10, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, G.; Salzano, G.; Caraglia, M.; Abbruzzese, A. Nanotechnologies: A strategy to overcome blood-brain barrier. Curr. Drug Metab. 2012, 13, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Caraglia, M.; de Rosa, G.; Salzano, G.; Santini, D.; Lamberti, M.; Sperlongano, P.; Lombardi, A.; Abbruzzese, A.; Addeo, R. Nanotech revolution for the anti-cancer drug delivery through blood-brain barrier. Curr. Cancer Drug Targets 2012, 12, 186–196. [Google Scholar] [CrossRef]
- Allen, T.M. Long-circulating (sterically stabilized) liposomes for targeted drug delivery. Trends Pharmacol. Sci. 1994, 15, 215–220. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, P.I.; Wang, C.C. Targeting nevirapine delivery across human brain microvascular endothelial cells using transferrin-grafted poly(lactide-co-glycolide) nanoparticles. Nanomedicine 2011, 6, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Huwyler, J.; Yang, J.; Pardridge, W.M. Receptor mediated delivery of daunomycin using immunoliposomes: Pharmacokinetics and tissue distribution in the rat. J. Pharmacol. Exp. Ther. 1997, 282, 1541–1546. [Google Scholar]
- Gosk, S.; Vermehren, C.; Storm, G.; Moos, T. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J. Cereb. Blood Flow Metab. 2004, 24, 1193–1204. [Google Scholar] [CrossRef]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef]
- Begley, D.J. Delivery of therapeutic agents to the central nervous system: The problems and the possibilities. Pharmacol. Ther. 2004, 104, 29–45. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Zuchero, Y.J.Y.; Chen, X.; Bien-Ly, N.; Bumbaca, D.; Tong, R.K.; Gao, X.; Zhang, S.; Hoyte, K.; Luk, W.; Huntley, M.A.; et al. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron 2016, 89, 70–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, L.B.; Thomsen, M.S.; Moos, T. Targeted drug delivery to the brain using magnetic nanoparticles. Ther. Deliv. 2015, 6, 1145–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begley, D.J.; Brightman, M.W. Structural and functional aspects of the blood-brain barrier. Prog. Drug Res. 2003, 61, 39–78. [Google Scholar]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor, M.A.; Malfroy, B.; Starzyk, R.M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef]
- Roberts, R.L.; Fine, R.E.; Sandra, A. Receptor-mediated endocytosis of transferrin at the blood-brain barrier. J. Cell Sci. 1993, 104, 521–532. [Google Scholar]
- Moos, T.; Morgan, E.H. Restricted transport of anti-transferrin receptor antibody (OX26) through the blood-brain barrier in the rat. J. Neurochem. 2001, 79, 119–129. [Google Scholar] [CrossRef]
- Paris-Robidas, S.; Emond, V.; Tremblay, C.; Soulet, D.; Calon, F. In vivo labeling of brain capillary endothelial cells after intravenous injection of monoclonal antibodies targeting the transferrin receptor. Mol. Pharmacol. 2011, 80, 32–39. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Thomsen, L.B.; Andresen, T.L.; Moos, T. Targeting the transferrin receptor for brain drug delivery. Prog. Neurobiol. 2019, 181, 101665. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef]
- Burkhart, A.; Skjørringe, T.; Johnsen, K.B.; Siupka, P.; Thomsen, L.B.; Nielsen, M.S.; Thomsen, L.L.; Moos, T. Expression of Iron-Related Proteins at the Neurovascular Unit Supports Reduction and Reoxidation of Iron for Transport Through the Blood-Brain Barrier. Mol. Neurobiol. 2016, 53, 7237–7253. [Google Scholar] [CrossRef] [PubMed]
- Skjørringe, T.; Burkhart, A.; Johnsen, K.B.; Moos, T. Divalent metal transporter 1 (DMT1) in the brain: Implications for a role in iron transport at the blood-brain barrier, and neuronal and glial pathology. Front. Mol. Neurosci. 2015, 8, 19. [Google Scholar] [PubMed]
- Johnsen, K.B.; Moos, T. Revisiting nanoparticle technology for blood-brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J. Control. Release 2016, 222, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Alata, W.; Tremblay, C.; Paris-Robidas, S.; Calon, F. Transferrin Receptor-Mediated Uptake at the Blood-Brain Barrier Is Not Impaired by Alzheimer’s Disease Neuropathology. Mol. Pharm. 2019, 16, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Alata, W.; Paris-Robidas, S.; Emond, V.; Bourasset, F.; Calon, F. Brain uptake of a fluorescent vector targeting the transferrin receptor: A novel application of in situ brain perfusion. Mol. Pharm. 2014, 11, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, B.; Kulkarni, S.D.; Villar, P.S.; Smith, R.S.; Eberly, C.; Araneda, R.C.; Depireux, D.A.; Shapiro, B. Movement of magnetic nanoparticles in brain tissue: Mechanisms and impact on normal neuronal function. Nanomedicine 2015, 11, 1821–1829. [Google Scholar] [CrossRef]
- Thomsen, L.B.; Linemann, T.; Pondman, K.M.; Lichota, J.; Kim, K.S.; Pieters, R.J.; Visser, G.M.; Moos, T. Uptake and transport of superparamagnetic iron oxide nanoparticles through human brain capillary endothelial cells. ACS Chem. Neurosci. 2013, 4, 1352–1360. [Google Scholar] [CrossRef]
- Kong, S.D.; Lee, J.; Ramachandran, S.; Eliceiri, B.P.; Shubayev, V.I.; Lal, R.; Jin, S. Magnetic targeting of nanoparticles across the intact blood–brain barrier. J. Control. Release 2012, 164, 49–57. [Google Scholar] [CrossRef]
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic nanoparticle-based approaches to locally target therapy and enhance tissue regeneration in vivo. Nanomedicine 2012, 7, 1425–1442. [Google Scholar] [CrossRef]
- del Burgo, L.S.; Hernández, R.M.; Orive, G.; Pedraz, J.L. Nanotherapeutic approaches for brain cancer management. Nanomedicine 2014, 10, e905–e919. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, T.J.; Yu, K.N.; Kim, B.G.; Park, S.J.; Kim, H.W.; Lee, K.H.; Park, S.B.; Lee, J.K.; Cho, M.H. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol. Sci. 2006, 89, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Oliveira, S.; Rijcken, C.J.F.; Pieters, E.H.E.; Etrych, T.; Ulbrich, K.; van Nostrum, R.C.F.; Storm, G.; Hennink, W.E.; Lammers, T. Intrinsically active nanobody-modified polymeric micelles for tumor-targeted combination therapy. Biomaterials 2013, 34, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chang, J.; Fu, X.; Liang, C.; Liang, S.; Yan, R.; Li, A. Nano-sized cationic polymeric magnetic liposomes significantly improves drug delivery to the brain in rats. J. Drug Target. 2012, 20, 416–421. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, F.; Ruffinatti, F.A.; Boschi, S.; Stura, I.; Rainero, I.; Abollino, O.; Cavalli, R.; Guiot, C. Magnetic Nanoparticles in the Central Nervous System: Targeting Principles, Applications and Safety Issues. Mol. Online 2017, 23, 9. [Google Scholar] [CrossRef]
- Tiebosch, I.A.C.W.; Crielaard, B.J.; Bouts, M.J.R.J.; Zwartbol, R.; Salas-Perdomo, A.; Lammers, T.; Planas, A.M.; Storm, G.; Dijkhuizen, R.M. Combined treatment with recombinant tissue plasminogen activator and dexamethasone phosphate-containing liposomes improves neurological outcome and restricts lesion progression after embolic stroke in rats. J. Neurochem. 2012, 123 (Suppl. 2), 65–74. [Google Scholar] [CrossRef]
- Dandamudi, S.; Campbell, R.B. Development and characterization of magnetic cationic liposomes for targeting tumor microvasculature. Biochim. Biophys. Acta 2007, 1768, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Ishida, T.; Iden, D.L.; Allen, T.M. A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs. FEBS Lett. 1999, 460, 129–133. [Google Scholar] [CrossRef]
- Boutry, S.; Forge, D.; Burtea, C.; Mahieu, I.; Murariu, O.; Laurent, S.; Elst, L.V.; Muller, R.N. How to quantify iron in an aqueous or biological matrix: A technical note. Contrast Media Mol. Imaging 2009, 4, 299–304. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Shigeoka, A.A.; Holscher, T.D.; King, A.J.; Hall, F.W.; Kiosses, W.B.; Tobias, P.S.; Mackman, N.; McKay, D.B. TLR2 is constitutively expressed within the kidney and participates in ischemic renal injury through both MyD88-dependent and -independent pathways. J. Immunol. 2007, 178, 6252–6258. [Google Scholar] [CrossRef]
- Faria, M.R.; Cruz, M.M.; Gonçalves, M.C.; Carvalho, A.; Feio, G.; Martins, M.B.F. Synthesis and characterization of magnetoliposomes for MRI contrast enhancement. Int. J. Pharm. 2013, 446, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Do, T.M.; Noel-Hudson, M.; Ribes, S.; Besengez, C.; Smirnova, M.; Cisternino, S.; Buyse, M.; Calon, F.; Chimini, G.; Chacun, H.; et al. ABCG2-and ABCG4-mediated efflux of amyloid-β peptide 1-40 at the mouse blood-brain barrier. J. Alzheimer’s Dis. 2012, 30, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Lamb, J.E.; Ray, F.; Ward, J.H.; Kushner, J.P.; Kaplan, J. Internalization and subcellular localization of transferrin and transferrin receptors in HeLa cells. J. Biol. Chem. 1983, 258, 8751–8758. [Google Scholar] [PubMed]
- Schnyder, A.; Huwyler, J. Drug transport to brain with targeted liposomes. NeuroRX 2005, 2, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.I. Pharmacological inhibition of endocytic pathways: Is it specific enough to be useful? Methods Mol. Biol. 2008, 440, 15–33. [Google Scholar]
- Linemann, T.; Thomsen, L.B.; Jardin, K.G.D.; Laursen, J.C.; Jensen, J.B.; Lichota, J.; Moos, T. Development of a novel lipophilic, magnetic nanoparticle for in vivo drug delivery. Pharmaceutics 2013, 5, 246–260. [Google Scholar] [CrossRef]
- Senyei, A.; Widder, K.; Czerlinski, G. Magnetic Guidance of Drug-Carrying Microspheres. J. Appl. Phys. 1978, 49, 3578–3583. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Wang, J.C.; Bian, D.Y.; Zhang, X.; Zhang, Q. Targeted delivery of RGD-modified liposomes encapsulating both combretastatin A-4 and doxorubicin for tumor therapy: In vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2010, 74, 467–473. [Google Scholar] [CrossRef]
- Dragicevic-Curic, N.; Scheglmann, D.; Albrecht, V.; Fahr, A. Development of liposomes containing ethanol for skin delivery of temoporfin: Characterization and in vitro penetration studies. Colloids Surf. B Biointerfaces 2009, 74, 114–122. [Google Scholar] [CrossRef]
- Rojas, J.M.; Gavilán, H.; del Dedo, V.; Lorente-Sorolla, E.; Sanz-Ortega, L.; da Silva, G.B.; Costo, R.; Perez-Yagüe, S.; Talelli, M.; Marciello, M.; et al. Time-course assessment of the aggregation and metabolization of magnetic nanoparticles. Acta Biomater. 2017, 58, 181–195. [Google Scholar] [CrossRef]
- Salvati, A.; Pitek, A.S.; Monopoli, M.P.; Prapainop, K.; Bombelli, F.B.; Hristov, D.R.; Kelly, P.M.; Åberg, C.; Mahon, E.; Dawson, K.A. Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat. Nanotechnol. 2013, 8, 137–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, N.; Zhang, Y.; Zhu, C.; Boado, R.J.; Pardridge, W.M. Brain-specific expression of an exogenous gene after i.v. administration. Proc. Natl. Acad. Sci. USA 2001, 98, 12754–12759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, N.; Pardridge, W.M. Noninvasive gene targeting to the brain. Proc. Natl. Acad. Sci. USA 2000, 97, 7567–7572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Schlachetzki, F.; Pardridge, W.M. Global non-viral gene transfer to the primate brain following intravenous administration. Mol. Ther. 2003, 7, 11–18. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef]
- Rivière, C.; Martina, M.S.; Tomita, Y.; Wilhelm, C.; Dinh, A.T.; Ménager, C.; Pinard, E.; Lesieur, S.; Gazeau, F.; Seylaz, J. Magnetic targeting of nanometric magnetic fluid loaded liposomes to specific brain intravascular areas: A dynamic imaging study in mice. Radiology 2007, 244, 439–448. [Google Scholar] [CrossRef]
- Qiao, R.; Jia, Q.; Hüwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.J.; Gao, M. Receptor-mediated delivery of magnetic nanoparticles across the blood-brain barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef]
- Ding, H.; Sagar, V.; Agudelo, M.; Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Raymond, A.; Samikkannu, T.; Nair, M.P. Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology 2014, 25, 055101. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Xie, S.; Yang, B.; Xu, Q.; Tan, J. Superparamagnetic Iron Oxide Nanoparticles Modified with Tween 80 Pass through the Intact Blood-Brain Barrier in Rats under Magnetic Field. ACS Appl. Mater. Interfaces 2016, 8, 11336–11341. [Google Scholar] [CrossRef]
- Lueshen, E.; Venugopal, I.; Soni, T.; Alaraj, A.; Linninger, A. Implant-Assisted Intrathecal Magnetic Drug Targeting to Aid in Therapeutic Nanoparticle Localization for Potential Treatment of Central Nervous System Disorders. J. Biomed. Nanotechnol. 2015, 11, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Pilakka-Kanthikeel, S.; Atluri, V.S.R.; Sagar, V.; Saxena, S.K.; Nair, M. Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: An in vitro study. PLoS ONE 2013, 8, e62241. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, I.; Habib, N.; Linninger, A. Intrathecal magnetic drug targeting for localized delivery of therapeutics in the CNS. Nanomedicine 2017, 12, 865–877. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Hu, J.; Zhang, L.; Zhang, L.; Sun, Y.; Ma, N.; Chen, X.; Gao, Z. Study of amphotericin B magnetic liposomes for brain targeting. Int. J. Pharm. 2014, 475, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Do, T.D.; Amin, F.U.; Noh, Y.; Kim, M.O.; Yoon, J. Guidance of Magnetic Nanocontainers for Treating Alzheimer’s Disease Using an Electromagnetic, Targeted Drug-Delivery Actuator. J. Biomed. Nanotechnol. 2016, 12, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Lichota, J.; Skjørringe, T.; Thomsen, L.B.; Moos, T. Macromolecular drug transport into the brain using targeted therapy. J. Neurochem. 2010, 113, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Koyabu, N.; Yonemitsu, Y.; Shimazoe, T.; Watanabe, S.; Naito, M.; Tsuruo, T.; Ohtani, H.; Sawada, Y. In vivo delivery of glial cell-derived neurotrophic factor across the blood-brain barrier by gene transfer into brain capillary endothelial cells. Hum. Gene Ther. 2003, 14, 1181–1191. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shao, K.; Liu, Y.; Kuang, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; Jiang, C. Tumor-targeting and microenvironment-responsive smart nanoparticles for combination therapy of antiangiogenesis and apoptosis. ACS Nano 2013, 7, 2860–2871. [Google Scholar] [CrossRef] [PubMed]




| Hydrodynamic Size (nm) | PDI | ζ-potential (mV) | |
|---|---|---|---|
| Magnetic Nanoparticles | 117.17 ± 1.17 | 0.17 | −14.83 ± 0.42 |
| Magnetic Liposomes | 150.20 ± 2.44 | 0.22 | 15.66 ± 1.65 |
| OX26-Magnetic Immunoliposomes | 181.90 ± 6.32 | 0.29 | −7.14 ± 3.13 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomsen, L.B.; Linemann, T.; Birkelund, S.; Tarp, G.A.; Moos, T. Evaluation of Targeted Delivery to the Brain Using Magnetic Immunoliposomes and Magnetic Force. Materials 2019, 12, 3576. https://doi.org/10.3390/ma12213576
Thomsen LB, Linemann T, Birkelund S, Tarp GA, Moos T. Evaluation of Targeted Delivery to the Brain Using Magnetic Immunoliposomes and Magnetic Force. Materials. 2019; 12(21):3576. https://doi.org/10.3390/ma12213576
Chicago/Turabian StyleThomsen, Louiza Bohn, Thomas Linemann, Svend Birkelund, Gitte Abildgaard Tarp, and Torben Moos. 2019. "Evaluation of Targeted Delivery to the Brain Using Magnetic Immunoliposomes and Magnetic Force" Materials 12, no. 21: 3576. https://doi.org/10.3390/ma12213576
APA StyleThomsen, L. B., Linemann, T., Birkelund, S., Tarp, G. A., & Moos, T. (2019). Evaluation of Targeted Delivery to the Brain Using Magnetic Immunoliposomes and Magnetic Force. Materials, 12(21), 3576. https://doi.org/10.3390/ma12213576